Abstract
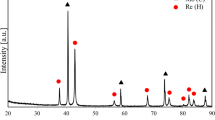
The purity, preferred orientation, microstructure, microhardness, bonding strength, thickness uniformity and thermal stability of rhenium (Re) coatings prepared on graphite wafers by chemical vapor deposition (CVD) and electrodeposition (ED) in molten salts were comparatively studied in this paper. It was found that carbon (0.0140 wt%) and oxygen (0.0067 wt%) were the primary impurities for CVD and ED Re coatings, respectively. The diffusion of carbon into CVD Re coating caused higher microhardness near the substrate and helped to improve the bonding strength at the same time. The preferred orientation, microstructure and microhardness of ED Re coating were all susceptible to oxygen. The coating deposition uniformity of ED Re is obviously better than that of CVD Re coating, due to its intrinsic characteristics. The 〈002〉-oriented, coarse columnar CVD Re coating exhibited better thermal stability compared with that of the 〈110〉-oriented, fiber-like columnar ED Re coating, while the ED Re grains grew remarkably and the microstructure evolved toward the similar structure of CVD Re after annealing treatment. The diversity of Re coatings in microstructure could be attributed to the mobility of grain boundaries (affected by temperature and impurity) during deposition processes.











Similar content being viewed by others
References
Carlen JC, Bryskin BD. Rhenium a unique rare metal. Mater Manuf Process. 1994;9(6):1087.
Bryskin BD. Rhenium and its alloys. Adv Mater Process. 1992;142:22.
Naor A, Eliaz N. Properties and applications of rhenium and its alloys. Ammtiac Q. 2010;5(1):11.
Dobrzańska-Danikiewicz AD, Wolany W. A rhenium review-from discovery to novel applications. Arch Mater Sci Eng. 2016;82(2):70.
Lambert JB, Rausch JJ. ASM Metals Handbook, Volume 2, Properties and Section: Nonferrous Alloys and Special Purpose Materials. 10th ed. Ohio: ASM International; 1990. 557.
Leonhardt T, Hamister M, Carlen J, Biaglow J, Reed B. Near-net shape powder metallurgy rhenium thruster. In: 36th AIAA/ASME/SAE/ASEE Jt. Propuls. Conf. Exhib., Huntsville; 2000. https://doi.org/10.2514/6.2000-3132.
Mckechnie T, Shchetkovskiy A. High temperature combustion chambers produced by electroforming. In: 4th Space. Propuls. Conf., Cologne, Germany; 2014. 1.
Toenshoff D, Lanam R, Ragaini J, Shchetkovskiy A, Smirnov A. Iridium coated rhenium rocket chambers produced by electroforming. In: 36th AIAA/ASME/SAE/ASEE Jt. Propuls. Conf. Exhib., Huntsville; 2000. https://doi.org/10.2514/6.2000-3166.
Lanam R, Shchetkovskiy A, Smirnov A, Boland E. Properties and enhanced capabilities for EL-Form rhenium. IZn: 37th Jt. Propuls. Conf. Exhib., Salt Lake City; 2001. https://doi.org/10.2514/6.2001-3697.
Vinogradov-Zhabrov ON, Minchenko LM, Esina NO, Pankratov AA. Electrodeposition of rhenium from chloride melts-electrochemical nature, structure and applied aspects. J Min Metall Sect B. 2003;39(1–2):149.
Zhulikov VV, Gamburg YD. Electrodeposition of rhenium and its alloys. Russ J Electrochem. 2016;52(9):847.
Zhu L, Bai SX, Chen K. Chemical vapor deposition of rhenium on a gourd shaped graphite substrate. Surf Coat Technol. 2012;206(23):4940.
Tong YG, Bai SX, Zhang H, Ye YC. Rhenium coating prepared on carbon substrate by chemical vapor deposition. Appl Surf Sci. 2012;261:390.
Singh J, Wolfe DE. Nano-grained net-shaped fabrication of Re components by EB-PVD. Mater Manuf Process. 2003;18(6):915.
Mittendorf D. The effect of manufacturing processes on the mechanical integrity of rhenium. In: 33rd Jt. Propuls. Conf. Exhib., Seattle; 1997. https://doi.org/10.2514/6.1997-2675.
Biaglow J. High temperature rhenium material properties. In: 34th AIAA/ASME/SAE/ASEE Jt. Propuls. Conf. Exhib., Cleveland; 1998. https://doi.org/10.2514/6.1998-3354.
Chazen M. Materials property test results of rhenium. In: 31st Jt. Propuls. Conf. Exhib., San Diego; 1995. https://doi.org/10.2514/6.1995-2938.
Wang JF, Zhu L, Ye YC, Zhang H, Bai SX. Black rhenium coating prepared on graphite substrate by electrodeposition in NaCl–KCl–CsCl–K2ReCl6 molten salts. Int J Refract Met Hard Mater. 2017;68:54.
Wang JF, Bai SX, Ye YC, Zhang H, Zhu L. Microstructure and mechanical properties of rhenium prepared by electroforming in NaCl–KCl–CsCl–K2ReCl6 molten salts. Int J Refract Met Hard Mater. 2018;72:263.
Wang D, Gao C, Luo HY, Yang YH, Ma Y. Texture evolution behavior and anisotropy of 2A97 Al–Li alloy during recrystallization at elevated temperature. Rare Met. 2018. https://doi.org/10.1007/s12598-018-0997-y.
Thompson CV. Structure evolution during processing of polycrystalline films. Annu Rev Mater Sci. 2000;30(1):159.
Gasmburg YD, Zangari G. Structure and microstructure of electrodeposited metals and alloys. In: Gamburg YD, Zangari G, editors. Theory and Practice of Metal Electrodeposition. New York: Springer; 2011. 317.
Nielsen CB, Horsewell A, Stergard ML. On texture formation of nickel electrodeposits. J Appl Electrochem. 1997;27(7):839.
Schmelzer JW. Kinetic and thermodynamic theories of nucleation. Mater Phys Mech. 2003;6:21.
Vazquez-Arenas J, Cruz R, Mendoza-Huizar LH. The role of temperature in copper electrocrystallization in ammonia–chloride solutions. Electrochim Acta. 2006;52(3):892.
Raeissi K, Saatchi A, Golozar MA, Szpunar JA. Texture and surface morphology in zinc electrodeposits. J Appl Electrochem. 2004;34(12):1249.
Itoh S, Yamazoe N, Seiyama T. Electrocrystallization of various metals onto copper single crystal substrates. Surf Technol. 1977;5(1):27.
Budevski E, Staikov G, Lorenz WJ. Electrocrystallization: nucleation and growth phenomena. Electrochim Acta. 2000;45(15–16):2559.
Fischer H. Aspects of inhibition in electrodeposition of compact metals II. Effects of morphological interface inhibition. Electrodepos Surf Treat. 1973;1(4):319.
Pangarov NA. Preferred orientations in electro-deposited metals. J Electroanal Chem. 1965;9(1):70.
Ralph HZ, Li JL. Diffusion analysis of rhenium in graphite using rutherford backscattering spectroscopy. Defect Diffus Forum. 2001;194–199:85.
Gottstein G, Shvindlerman LS. Grain Boundary Migration in Metals: Thermodynamics, Kinetics, Applications. Boca Raton: CRC Press; 1999. 144.
Frost HJ, Hayashi Y, Thompson CV, Walton DT. The effect of variability among grain boundary energies on grain growth in thin film strips. Mater Res Soc Symp Proc. 1994;317:431.
Lucke K, Stuwe HP. Recovery and recrystallization of metals. In: Himmel L, editor. New York: Interscience; 1963. 171.
Mendelev MI, Srolovitz DJ. Impurity effects on grain boundary migration. Model Simul Mater Sci Eng. 2002;10(6):R79.
Guo Y, Xie H, Jiang Z, Xia Z. Mechanical properties and thermal shock resistance of rhenium coating in iridium/rhenium/carbon–carbon composites. Procedia Eng. 2015;99:1407.
Isobe Y, Tanaka M, Yamanaka S, Miyake M. Chemical vapour deposition of rhenium on graphite. J Less Common Met. 1989;152(1):177.
Bengoa LN, SeréP R, Conconi MS, Egli WA. Morphology and texture of zinc deposits formed at the edge of a rotating washer electrode. J Mater Eng Perform. 2016;25(7):2936.
Wei D, Okido M, Oki T. Characteristics of titanium deposits by electrolysis in molten chloride–fluoride mixture. J Appl Electrochem. 1994;24(9):923.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 51501224).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, JF., Bai, SX., Ye, YC. et al. A comparative study of rhenium coatings prepared on graphite wafers by chemical vapor deposition and electrodeposition in molten salts. Rare Met. 40, 202–211 (2021). https://doi.org/10.1007/s12598-019-01359-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-019-01359-3