Abstract
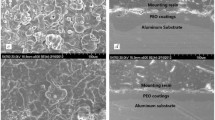
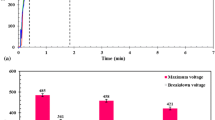
Titanium deposits were prepared on 304 stainless steel substrates by both constant current and pulse current methods in molten KCl-NaCl-LiCl eutectic salts with 2 mol % potassium hexafluorotitanate. The influence of electrolysis conditions on the crystal orientation and morphology of the titanium deposits was investigated by using X-ray diffraction and scanning electron microscopy, respectively. It was found that the orientation index of the titanium deposits varied with temperature, electrolysis time, current density and pulse shape during electrolysis. The deposits showed a granular structure, in which the grains became larger with increase in temperature. Titanium deposits with pulse current above 873 K had a better corrosion resistance than pure titanium sheet.
Similar content being viewed by others
References
G. P. Capsimalis, E. S. Chen and R. E. Peterson, J. Appl. Electrochem. 17 (1987) 253.
G. W. Mellors and S. Senderof, J. Electrochem. Soc. 112 (1965) 266.
P. Taxil and J. Mahenc, J. Appl. Electrochem. 17 (1987) 261.
A. M. Emsley and M. P. Hill, ibid. 17 (1987) 283.
S. H. White and U. M. Twardoch, ibid. 17 (1987) 225.
D. M. Ferry, G. S. Picard and B. L. Tremillon, J. Electrochem. Soc. 135 (1988) 1443.
Frederic Lantelme, Abdeslam Barhoun, G. Li and J. -P. Besse, ibid. 139 (1992) 1249.
J. De Lepinay, J. Bouteillon, S. Traore, D. Renaud and M. J. Barbier, J. Appl. Electrochem. 17 (1987) 294.
Chen Guang-sen, M. Okido and T. Oki, ibid. 17 (1987) 849.
Idemibid. 18 (1988) 80.
IdemElectrochim. Acta. 32 (1987) 1637.
J. De Lapinay and P. Paillere, ibid. 29 (1984) 1243.
L. P. Polykova, P. T. Stangrit and E. G. Polyakov, ibid. 31 (1986) 159.
S. Mori, E. Kuroda and K. Kawamura, Denki Kagaku 42 (1974) 175.
M. Nardin and G. Lorthioir, J. Less-Common Metals 56 (1977) 269.
D. M. Ferry and G. S. Picard, J. Appl. Electrochem. 20 (1990) 125.
F. R. Clayton, G. Mamantov and D. L. Manning, J. Electrochem. Soc. 120 (1973) 1193.
Idem, G. Mamantov and D. L. Manning, ibid. 120 (1973) 1199.
B. N. Popov, M. C. Kimble, R. E. White and H. Wendt, J. Appl. Electrochem. 21 (1991) 351.
T. Vargas and D. Inman, ibid. 17 (1987) 270.
K. S. Wilson and J. A. Roger, Tech. Proc. Amer. Electroplaters Soc. 51 (1964) 92.
A. Robin, J. De Lepinay and M. J. Barbier, J. Electroanal. Chem. 230 (1987) 125.
D. Wei, R. Ichino, M. Okido and T. Oki, J. Surf. Finish. Soc. Japan, 44 (1993) 33.
G. H. Gilmer and K. A. Jackson, ‘Current Topics in Materials Science’, Vol. 2, (edited by E. Ealdis), North-Holland, Amsterdam (1977), Chap. 2.
M. Y. Abyaneh and M. Fleischmann, J. Electroanal. Chem. 119 (1981) 187.
D. D. Wang and T. Old, J. Vac. Sci. Technol. A8 (1990) 3163.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wei, D., Okido, M. & Oki, T. Characteristics of titanium deposits by electrolysis in molten chloride-fluoride mixture. JOURNAL OF APPLIED ELECTROCHEMISTRY 24, 923–929 (1994). https://doi.org/10.1007/BF00348783
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00348783