Abstract
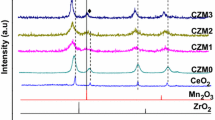
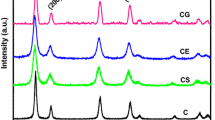
Cerium and cerium-based oxides are found to be an important element in three-way catalytic converter (TWC). The effective utilization of TWC is found to be reduced due to thermal loading which results in structural deformation of ceria. Doping Zr4+ into the rare earth element can increase the oxygen storage capacity and thermal stability. Hence, an attempt was made to study the oxygen storage capacity and thermal stability of ceria by doping Zr4+ and Nd3+. Cerium-based nanocrystallite in the composition of Ce0.6Zr0.4−xNd1.3xO2 (0 ≤ x ≤ 0.4) was prepared by sol–gel synthesize technique with citric acid as a gel-forming agent. X-ray diffraction (XRD) result shows that doping Nd3+ into ceria lattice forms homogenous solid solution of cubic fluorite structure up to 25 % of substitute only. Doping higher amount of Nd3+ into ceria lattice leads to the formation of Nd2O3. Raman spectrum study confirms that oxygen storage capacity band is present in Ce0.6Zr0.4O2 and Ce0.6Zr0.3Nd0.13O2. The oxygen storage capacity was calculated through weight loss of the sample during the second heating cycle with cyclic heating from 30 to 800 °C in thermogravimetric analysis (TGA). The TGA study reveals that the oxygen storage capacity of Ce0.6Zr0.4O2 decreases after the substitution of Nd3+, which is due to the larger ionic radius of Nd3+ compared with that of Zr4+ and CeO2.





Similar content being viewed by others
References
Park S, Vohs JM, Gorte RJ. Direct oxidation of hydrocarbons in a solid-oxide fuel cell. Lett Nat. 2000;404(6775):265.
Jasinski P, Suzuki T, Anderson HU. Nanocrystalline undoped ceria oxygen sensor. Sens Actuators B. 2003;95(1–3):73.
Yabe S, Yamashita M, Momose S, Tahira K, Yoshida S, Li R, Yin S, Sato T. Synthesis and UV-shielding properties of metal oxide doped ceria via soft solution chemical processes. Int J Inorg Mater. 2001;3(7):1003.
Si R, Zhang YW, You LP, Yan CH. Self-organized monolayer of nanosized ceria colloids stabilized by poly(vinylpyrrolidone). J Phys Chem B. 2006;110(12):5994.
Yamamura K, Takiguchi T, Ueda M, Deng H, Hattori AN, Zettsu N. Plasma assisted polishing of single crystal SiC for obtaining atomically flat strain-free surface. CIRP Ann Manuf Technol. 2011;60(1):571.
Zhang F, Chen CH, Raitano JM, Hanson JC, Caliebe WA, Khalid S, Chan SW. Phase stability in ceria-zirconia binary oxide nanoparticles: the effect of the Ce3+ concentration and the redox environment. J Appl Phys. 2006;99(8):084313.
Aneggi E, Boaro M, Leitenburg CD, Dolcetti G, Trovarelli A. Insights into the redox properties of ceria-based oxides and their implications in catalysis. J Alloy Compd. 2006;408:1096.
Fornasiero P, Monte RD, Rao GR, Kaspar J, Meriani S, Trovarelli A, Grazinani M. Rh-loaded CeO2–ZrO2 solid solution as highly efficient oxygen exchangers: dependence of the reduction behavior and the oxygen storage capacity on the structural properties. J Catal. 1995;151(1):168.
Kawamoto JI, Yagi Y, Saito M, Yamamura H. Oxide-ion conduction and dielectric relaxation in the fluorite-type Zr0.8Ln0.2O1.9 (Ln = Nd, Sm, Eu, Gd, Dy, Ho, Er, Tm, Yb, Lu) system. IOP Conf Ser Mater Sci Eng. 2011;18(13):132010.
Suda A, Ukyo Y, Sobukawa H, Sugiura M. Improvement of oxygen storage capacity of CeO2–ZrO2 solid solution by heat treatment in reducing atmosphere. J Ceram Soc Jpn. 2002;110(2):126.
Zhang Z, Zhang Y, Mu Z, Yu P, Ni X, Wang S, Zheng L. Synthesis and catalytic properties of Ce0.6Zr0.4O2 solid solutions in the oxidation of soluble organic fraction from diesel engines. Appl Catal B. 2007;76(3–4):335.
Priya NS, Somayaji C, Kanagaraj S. Oxygen storage capacity of CexZr 1−xO 2 (0.4 ≤ x ≤ 0.8) solid solution using thermogravimetric analysis. Adv Mater Res. 2013;747:579.
Giménez AMH, Xavier LPS, López AB. Improving ceria-zirconia soot combustion catalysts by neodymium doping. Appl Catal A. 2013;100:462.
Jia L, Shen M, Hao J, Rao T, Wang J. Dynamic oxygen storage and release over Mn0.1Ce0.9O x and Mn0.1Ce0.6Zr 0.3Ox complex compounds and structural characterization. J Alloy Compd. 2008;454(1–2):321.
Rui R, Hongwei Z, Xiaodong WU, Jun F, Duan W. Structure and oxygen storage capacity of Pd/Pr/CeO2–ZrO2 catalyst: effects of impregnated praseodymia. J Rare Earths. 2014;32(2):108.
Mikulova J, Rossignol S, Gerardy F, Mesnard D, Charles K, Duprez D. Properties of cerium–zirconium mixed oxides partially substituted by neodymium: comparison with Zr–Ce–Pr–O ternary oxides. J. Solid State Chem. 2006;179(8):2511.
Kaspar J, Fornasiero P, Graziani M. Use of CeO2-based oxides in the three-way catalysis. Catal Today. 1999;50(2):285.
Robert CL, Long JW, Lucas EM, Pettigrew KA, Stroud RM, Doesche MS, Rolison DR. Sol-gel-derived ceria nanoarchitectures: synthesis, characterization, and electrical properties. Chem Mater. 2006;18(1):50.
Das M, Patil S, Bhargava N, Kanga JF, Riedel LM, Seal S, Hickman JJ. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials. 2007;28(10):1918.
Tu YB, Luo JY, Meng M, Wang G, He JJ. Ultrasonic-assisted synthesis of highly active catalyst Au/MnOx–CeO 2 used for the preferential oxidation of CO in H2-rich stream. Int J Hydrogen Energy. 2009;34(9):3743.
Pengpanich S, Meeyoo V, Rirksomboon T, Bunyakiat K. Catalytic oxidation of methane over CeO2–ZrO2 mixed oxide solid solution catalysts prepared via urea hydrolysis. Appl Catal A. 2002;234(1–2):221.
Rupp JLM, Scherrer B, Harvey AS, Gauckler LJ. Crystallization and grain growth kinetics for precipitation-based ceramics: a case study on amorphous ceria thin films from spray pyrolysis. Adv Funct Mater. 2009;19(17):2790.
Wang R, Crozier PA, Sharma R, Adams JB. Nanoscale heterogeneity in ceria zirconia with low-temperature redox properties. Journal of Physical Chemistry B. 2006;110(18):278.
Vantomme A, Yuan ZY, Du G, Bl S. Surfactant-assisted large-scale preparation of crystalline CeO2 nanorods. Langmuir. 2005;21(3):1132.
Tani T, Watanabe N, Takator K. Morphology of oxide particles made by the emulsion combustion method. J Ceramic Soc. 2003;86(6):898.
Chervin CN, Clapsaddle BJ, Chiu HW, Gash AE, Satcher JH, Kauzlarich SM. Aerogel synthesis of yttria-stabilized zirconia by a non-alkoxide sol-gel route. Chem Mater. 2005;17(13):3345.
Priya NS, Somayaji C, Kanagaraj S. Optimization of ceria–zirconia solid solution based on OSC measurement by cyclic heating process. Procedia Eng. 2013;64:1235.
Ozawa M, Matuda K, Suzuki S. Microstructure and oxygen release properties of catalytic alumina-supported CeO2–ZrO2 powders. J Alloys Compd. 2000;303:56.
Wu X, Wu X, Liang Q, Fan J, Weng D, Xie Z, Wei S. Structure and oxygen storage capacity of Pr/Nd doped CeO2–ZrO2 mixed oxides. Solid State Sci. 2007;9(7):636.
Wang Q, Li Z, Zhao B, Li G, Zhou R. Effect of synthesis method on the properties of ceria–zirconia modified alumina and the catalytic performance of its supported Pd-only three-way catalyst. J Mol Catal A: Chem. 2011;344(1–2):132.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Priya, N.S., Somayaji, C. & Kanagaraj, S. Synthesis and characterization of Nd3+-doped Ce0.6Zr0.4O2 and its doping significance on oxygen storage capacity. Rare Met. 40, 231–236 (2021). https://doi.org/10.1007/s12598-016-0698-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-016-0698-3