Abstract
A reef-associated mollusc fauna (gastropods and bivalves) and its facies context are described from latest Triassic (Sevatian–Rhaetian) reef carbonates of Austria (Rötelwand reef at Gaissau and Gosaukamm near Hallstatt). The studied carbonates from the Rötelwand reef consist of mollusc-rich rudstones, partly boundstones, which contain branched corals (Cycliphyllia and Retiophylia, Pinacophyllum), whereas coralline sponges are absent. The rich foraminiferid fauna that is associated with the reef builders consists of 11 genera; eight of these genera became extinct until the end of the Rhaetian. Associated with small patch reefs was a rich mollusc fauna with 19 gastropod species and 8 epifaunal bivalve species. The gastropod fauna is dominated by Microschiza rhaetica, Trochotoma praecursor, and the large growing Purpuroidea moosleitneri. Six gastropod species are new to science: Angulomphalus senowbarii sp. nov., Stuorella zapfei sp. nov., Hologyra callosa sp. nov., Microschiza rhaetica sp. nov., Angularia corallina sp. nov., and Purpuroidea moosleitneri sp. nov. Four Triassic gastropod species are placed in other genera (new combinations): Tylotrochus diversicostatus Wolff, 1967 and Eucycloscala epitoniformis Nützel and Senowbari-Daryan, 1999 are placed in Sadkia, Praelittorina sepkoskii Nützel and Erwin, 2004 in Microschiza, and Purpuroidea? minioi Leonardi, 1935 in Angularia Koken, 1892. Reversal of precedence is proposed for Angularia Koken, 1892 (Gastropoda) and Angularia Busk, 1881 (Bryozoa) under ICZN Art. 23.9. Although reefs suffered a catastrophic decline at the end of the Triassic, most of the studied reef-associated bivalve and gastropod genera survived into the Jurassic, indicating a considerable ecological plasticity of these groups. Only 12 out of 47 reef-associated mollusc genera became extinct (25.5%). This observation is at variance with earlier suggestions that taxa that were associated with reefs and carbonate substrata had a significantly higher extinction risk than level-bottom dwellers. However, extinction at the species level appears more severe; only three bivalve species but no gastropod species recorded in this fauna have records from the Jurassic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical reefs belong to the most diverse marine ecosystems not so much because of the diversity of the reef builders but chiefly due to the high number of reef-associated species. However, most fossil reefs of high geological age are strongly lithified and diagenetically altered, including aragonite dissolution (particularly relevant for molluscs), replacement by calcite and dolomitization. As a result, it is quite common that only few reef-associated species are reported in studies of fossil reefs. Typically, the presence of reef-associated molluscs is mentioned only cursory in such studies, and taxa are often listed in open nomenclature lacking species identification and commonly also illustrations. The present study reports fossiliferous reefal limestones including reef builders (corals) and associated benthic reef dwellers, especially molluscs (gastropods and bivalves), from the “Upper Rhaetian Reef Limestone” (“Oberrhät-Riffkalk”) and the Sevatian-to-Rhaetian “Dachsteinkalk”. The studied material comes from the Rötelwand Reef at Gaissau and the Gosaukamm in Austria (Figs. 1, 2). Gastropods are especially abundant in these coral-reef limestones and some species are rather large (especially Purpuroidea moosleitneri sp. nov., largest specimen ca. 90 mm high, 80 mm wide). The gastropod and bivalve fauna described herein is part of a mainly coarse-grained reefal debris facies with nearby intercalations of small patch reefs (in case of the Rötelwand locality), which are almost exclusively composed of large phaceloid coral colonies (Cycliphyllia cyclica). Gastropods and bivalves from the Upper Rhaetian Reef Limestone are present in various museum and private collections, but have not been studied in detail with the exception of the studies by Zapfe (1963, 1967a). However, Zapfe’s studies provided only illustrations of a few bivalve specimens, whereas most taxa were not illustrated, and many were described in open nomenclature only. In this context, the aims of our study are twofold: first, we document in detail the facies of the carbonate rocks and the biodiversity of the fauna. Second, we use these data to trace the fate of the reef-associated gastropod and bivalve taxa across the Triassic–Jurassic boundary, which coincided with one of the largest global mass extinction events including a major reef crisis (e.g., Hallam and Wignall 1997; Hautmann et al. 2008a; Hautmann 2021; Kiessling 2005; Kiessling et al. 2007). Our main interest here is the question whether the reef-associated taxa suffered above average during this event as a consequence of the loss of their preferred habitats (reefs), as proposed by Kiessling et al. (2007) and Kiessling and Aberhan (2007), or whether they were able to survive the crisis in alternative habitats due to their ecological flexibility.
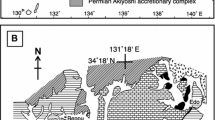
Locality map of the Salzburg and Dachstein area, Austria. a, b Rötelwand reef complex near Gaissau, SE Salzburg, Austria. Geographically, the location is called “Looswand”. The gastropod findings as well as the rock samples stem from the southern part of the reef complex marked with a black dot in b; see also Fig. 2; c Gosaukamm, mountain range west of Hoher Dachstein summit, SE Lake Hallstatt; fossils were collected from the scree of the steep NE slope, mostly at a height between 1500 and 1750 m a.s.l
Materials and methods
Material was obtained from museum, own, and private collections. Specimens were mechanically prepared and most illustrated specimens were coated with ammonium chloride for photography. Thin sections and polished slabs were prepared at the SNSB-BSPG. The studied material is reposited at the Naturhistorisches Museum Wien (NHMW) and the Bayerische Staatssammlung für Paläontologie und Geologie, München (SNSB-BSPG). A few studied gastropod specimens (non-type material) were given back to private collectors.
Geological setting and location
Initially, the present study comprised only the fossil occurrence of the Rötelwand Reef at Gaissau and its microfacies. A visit at the Naturhistorisches Museum Wien revealed that the same taxa of mollusc reef dwellers were present in collections from the slightly older (Sevatian and lower Rhaetian) Dachsteinkalk from the Gosaukamm (Gosau-Ridge). Bivalves and gastropods from this collection are included in this study. The microfacies analysis concerns only the fossiliferous Rötelwand Reef.
The Northern Calcareous Alps, a part of the Austroalpine nappe complex, are the northernmost overthrusted part of the eastern Alps. They form a W-to-E trending, 500-km-long, and 20 to 50-km-wide thrust belt that extends from Bregenz in the west to Vienna in the east. The Calcareous Alps in the Salzburg region are characterized by the overthrust of the Staufen–Höllengebirgs-thrust system (Tirolikum) virtually up to the northern margin of the Northern Calcareous Alps (e.g., Tollmann 1985; Wagreich et al. 1996). The Tirolikum can be subdivided into several tectonic units (Osterhorn-Tirolikum, Sparber thrust sheet, Schafberg-Tirolikum) separated by faults (e.g., Plöchinger 1973, 1982; Tollmann 1985; Wagreich et al. 1996). The sedimentological record of the Tirolikum encompasses Permian, thick Triassic Hauptdolomit and Dachstein limestones, in terms of facies strongly differentiated Jurassic and Lower Cretaceous deposits uncomformably overlain by Upper Cretaceous to Palaeogene sediments. Succeeding tectonic units comprise Hallstatt nappe sheets (Tiefjuvavikum) put in place in the Late Jurassic as well as the Berchtesgaden thrust sheet and the Dachstein thrust sheet (Hochjuvavikum; e.g., Plöchinger 1982; Tollmann 1985) all of which gain ground toward the east in the Salzkammergut. Also, the Berchtesgaden and Dachstein thrust sheets are unconformably overlain by Upper Cretaceous strata of the Gosau group (Wagreich et al. 1996).
Southeast of the Salzburg region, the Dachstein massif and the Gosaukamm near Hallstatt with their thick Upper Triassic platform carbonate succession (Dachstein limestone) belong to the Dachstein nappe, a major component of the Juvavikum thrust sheet complex (e.g., Mandl 2000; Krystyn et al. 2009).
In Late Triassic times, the Northern Calcareous Alps were part of a huge, up to 300-km-wide shelf at the passive continental margin of the northwestern Neotethys, located about 30º north of the equator. Tropical shallow-water conditions favored the formation of giant, up to 1200-m-thick carbonate platforms composed basically of Hauptdolomit and Dachstein Limestone. The southern and south-western, ocean-facing margins of the carbonate platforms (e.g., Hoher Göll, Hochkönig, and Gosaukamm) were characterized by the development of thick reefal carbonates formed by massive Dachstein Limestone. The transition into the pelagic realm of the Hallstatt basin is often formed by steep slopes. With the beginning of the Rhaetian, the Kössen Basin was formed in the northern part of the Hauptdolomit/Dachstein carbonate shelf. Patch reefs and reef-like structures (“Upper Rhaetian Reef Limestone”) developed in ramp position, adjacent to the Dachstein platform (Steinplatte; Stanton and Flügel 1995), on the Dachstein platform (Adnet; Schäfer 1979) as well as within the Kössen basin (e.g., Rötelwand, Feichtenstein, and Gruber reef structures; Schäfer 1979; Senowbari-Daryan 1980; Flügel 1981; Stanton and Flügel 1987; cf. Bernecker 2005).
The Rötelwand Reef, yielding part of the gastropod fauna described herein, underlain and surrounded by Kössen beds, is about 100 m thick and shows both a lateral and a vertical facies zonation pattern. According to Schäfer (1979), the Rötelwand reef complex exhibits four developmental stages. It started with a preexisting relief in the Kössen beds formed by mechanical accumulation of sandy shoals (oolitic or bivalve shell beds) (Stage 1). In a deeper water stage, the formation of a carbonate mud-mound was caused by sporadic reef growth on the underlying shoals and by mechanical–biological baffling of lime mud between reef-building corals (Stage 2). Growing actively into the wave base, the mud-mound was capped by thick bivalve shell beds (Stage 3). Thus, the reef structure had developed into a shallow-water zone providing environmental conditions comparable to those of the Dachstein platform. Then, a small platform was formed in shallow water, with coral-coralline sponge-reef growth on its northern slope, and with the deposition of carbonate sands (oncolithic, algal-foraminiferal facies) with coral-reef patches under high-energy, wind exposed conditions in the south, which correspond with subtidal lagoonal facies types of the Dachstein platform (Stage 4). The gastropod mollusc fauna described herein stems from stage 4, the high-energy wind and current exposed shallow-water reef zone at the southern margin of the reef complex.
The outcrop of the Rötelwand Reef (“Looswand”) is a steep slope and cliff surface cutting across the reef complex (Schäfer 1979; Schäfer and Senowbari-Daryan 1981; Stanton and Flügel 1987). It is located east of Gaissau near Hallein south of Salzburg. The sampling site is located in the southern part of the upper Rötelwand Reef complex close to the “middle” driveway above the escarpment of the “Looswand” (coordinates: 47° 41′ 50.2″ N, 13° 14′ 53.6″ E, altitude: 1176 m a.s.l; Figs. 1, 2). The rock samples yield a rich foraminifer fauna composed of common duostomids, miliolids, involutinids, and nodosariids (see facies description below). Especially, the occurrence of duostomids and Trocholina turris confirms the Rhaetian age of the Rötelwand Reef as generally supposed in the previous studies (e.g., Schäfer and Senowbari-Daryan 1981; Schäfer 1984; Flügel 2002; cf. Gale et al. 2012).
Molluscs from the Gosaukamm (Dachstein limestone)
The mollusc material from the Gosaukamm and also part of the material from the Rötelwand Reef studied herein comes from a collection present at the Naturhistorisches Museum Wien (NHMW) that was collected in the 1960s by Helmut Zapfe, Heinz Kollmann, Erik Flügel, and Herbert Summersberger (Zapfe 1967a). The material represents the same facies with the same corals and mollusc species as those present at the Rötelwand Reef. Like in the Rötelwand Reef, the diversity of the reef builders is high in the Gosaukamm reefs (Flügel 2002, fig. 21). According to Flügel (2002), the Dachstein Limestone of the Gosaukamm is slightly older (Sevatian-to-lower Rhaetian) than the Upper Rhaetian Reef Limestone (“Oberrhät-Riffkalk”), but, as said above, the faunal content is almost identical. Most molluscs studied herein are present in both complexes. The phenomenon of shared presence of species in the Dachstein Limestone and the Upper Rhaetian Reef Limestone was called mixed fauna (“Mischfaunen”) by Zapfe (1967a), who noted that most species had a Norian (Sevatian) age but some are Rhaetian. Zapfe (1967a) noted that the fossils from the Gosaukamm were collected from the scree of the steep NE slope of the Gosaukamm, mostly at a height between 1,500 and 1,750 m. According to Krystyn et al. (2009), reef growth of the Dachstein limestone started in the Early Norian and reached a peak in the early Rhaetian.
Facies of the fossiliferous, mollusc-rich carbonates of the Rötelwand Reef
General facies architecture and previous studies
The Rötelwand Reef complex that yielded part of the mollusc material studied herein was comprehensively investigated by Schäfer (1979, 1984), Schäfer and Senowbari-Daryan (1981), and Stanton and Flügel (1987) in terms of faunal composition and facies development and in comparison with other well-known Rhaetian reef complexes (e.g., Gruber Reef, Adnet Reef, Feichtenstein Reef). Further studies focused on particular faunal aspects like the taxonomy and distribution of corals and foraminifers within the Rötelwand reef complex (Schäfer and Senowbari-Daryan 1978a, b). Flügel (2002, fig. 21) found that the diversity of reef builders in the Rötelwand Reef is among the highest of ones recorded from Triassic reefs.
The facies described here developed in the upper part of the Rötelwand reef complex corresponding to stage 4 of the overall sequential development of the reef complex. This stage of reef development exhibits a facies zonation in broad, interfingering ecologic belts. From north to south, a detrital mud facies, shell beds, and coral-sponge boundstone facies (i.e., the reef core facies) graded into a coarse-grained high-energy coral-reef debris facies followed by algal foraminifera facies and again detrital mud facies. According to Schäfer (1979), the zonation with a high-energy reefal debris zone at the southern margin of the reef is due to a southerly wind direction. This zone is inhabited by the mollusc fauna described herein (Fig. 3).
Simplified facies model of the isolated Rötelwand patch reef complex with the facies zones defined by Schäfer (1979, 1984) for the upper growth stage; slightly modified by own data. The new gastropod fauna lived in sheltered areas of the high-energy reef crest zone and the upper slope at the southern wind exposed margin of the patch reef complex. Robust phaceloid coral colonies of Cycliphyllia cyclica are capable of living in this high-energy zone
Facies description and associated biota
Polished slabs and rock samples revealing the two largely prevailing facies types within the high-energy gastropod-bearing facies zone (see also Fig. 3). a–c Coarse-grained branching coral rudstone; Gas = gastropod, Cyc = Cycliphyllia cyclica, Ret = Retiophyllia spp., In = intraclast, Br = brachiopod, Rfc = isopachous radial fibrous calcite; d branching coral bafflestone (weathered surface); Cycliphyllia cyclica branches (emphasized by dashed lines). Note micritic geopetal infillings with red mud at the top probably derived from the Hallstatt basin with its red deeper water muddy sediments swept in by north directed currents (Schäfer 1979; Flügel 1963; Flügel and Flügel-Kahler 1963). Scale bar = 1 cm. a SNSB-BSPG 2021 XVI 1; b SNSB-BSPG 2021 XVI 2; c SNSB-BSPG 2021 XVI 3; d SNSB-BSPG 2021 XVI 4
Prepared rock sample from the high-energy gastropod-coral rudstone facies, Rötelwand Reef (Gaissau); Pur = Purpuroidea moosleitneri sp. nov., Mic = Microschiza rhaetica sp. nov., Tr = Trochotoma praecursor, Cr = Cryptaulax sp., Cyc = Cycliphyllia cyclica branches. Scale bar = 2 cm. SNSB-BSPG 2009 IV 41
Basically, we deal with a coarse-grained bioclast-intraclast-rudstone, with local transition to floatstone. Locally, autochthonous phaceloid coral boundstone patches occur, mainly composed of robust Cycliphyllia bafflestones. However, large, well-preserved gastropods (Purpuroidea and Microschiza) are the most prominent constituents in the rudstone/floatstone facies, contrasting the fragmented biota including branched corals (Cycliphyllia, Retiophylia, and Pinacophyllum), echinoderms, small molluscs, bryozoans, and porostromate cyanobacteria (e.g., Cayeuxia). Remarkably, coralline sponges, common in other reef zones of the Rötelwand reef complex, are completely missing at the studied site. Non-skeletal constituents include bioclastic or micritic intraclasts and lumps (mainly composed of Lithocodium and Bacinella). The groundmass is either micritic (matrix in the strict sense) or predominantly sparitic, and the latter composed of thick radial fibrous cement crusts.
Gravitational geopetal infillings include peloidal sediment, partly in stromatactoid cavities, as well as infiltrations of red mud probably derived from the Hallstatt basin with its red deeper water muddy sediments swept in by north directed currents (Schäfer 1979; Flügel 1963; Flügel and Flügel-Kahler 1963). These phenomena can be best seen in polished slabs (Fig. 4a–c).
Corals
(Fig. 7).
Branching scleractinian corals from the high-energy gastropod-bearing facies zone. a Cyclicphyllia cyclica, b C. cyclica and Retiophyllia cf. clathrata, c C. cyclica, d Pinacophyllum sp.; e, f Retiophyllia sp., g R. cf. gracilis, and h Cycliphyllia cyclica. All scale bars = 1 mm. a, f SNSB-BSPG 2021 XVI 5, b SNSB-BSPG 2021 XVI 6, c SNSB-BSPG 2021 XVI 7, d, h SNSB-BSPG 2021 XVI 8, and e, g SNSB-BSPG 2021 XVI 9
The scleractinian coral fauna is solely composed of branched forms, with a dominance of robust Cycliphyllia cyclica (Schäfer and Senowbari-Daryan, 1978a). Cycliphyllia cyclica is typically characterized by large calice diameters of about 1 cm, distinct dissepimental rings within the calices as well as lack of costae (Fig. 7a–c). Rarely, several calices arranged together in an almost cerioid pattern (Fig. 7h). Other branching scleractinians include various poorly preserved species of Retiophyllia spp. and rare Pinacophyllum sp. The retiophyllids exhibit calice diameters of 3–6 mm, costate epithecs with common pellicular coverings, endothecs with common dissepiments, as well as granulated septal flanges (Fig. 7b, e–g). Pinacophyllum is typically characterized by thick walls and strongly reduced septal spines, diameter of the calices range between 3 and 4 mm (Fig. 7d).
Skeletal cyanobacteria
Small nodules of erect fan-like growing filaments lacking cross partitions can be attributed to the porostromate cyanobacteria Cayeuxia. Diameters of the filaments (lumen) range from 0.02 to 0.03 mm. Cayeuxia occurs scattered in the rudstone gastropod facies (Fig. 9b).
Foraminifers
(Fig. 8).
Foraminifers from the Rhaetian reefal limestone at “Looswand” (Rötelwand reef complex), Salzburg, Austria. a Duostominid (Duostomina? sp.), scale bar: 0.25 mm, b duostominid (Duostomina? sp.), scale bar: 0.25 mm, c duostominid (Duostomina sp.), scale bar: 0.2 mm, d Trocholina turris (Frentzen), scale bar: 0.15 mm, e duostominid Diplotremina placklesiana Kristan-Tollmann, scale bar: 0.25 mm, f duostominid Diplotremina sp., scale bar: 0.25 mm, g duostominid Duostomina cf. turboidea Kristan-Tollmann, scale bar: 0.25 mm, h miliolid Galeanella cf. tollmanni (Kristan), scale bar: 0.2 mm, i miliolid Galeanella? sp., scale bar: 0.2 mm, j miliolid Galeanella sp., scale bar: 0.1 mm, k duostominid Variostoma sp., scale bar: 0.25 mm, l duostominid Variostoma sp., scale bar: 0.25 mm, m fusulinid “Tetrataxis” sp., scale bar: 0.25 mm, n miliolid Decapoalina cf. schaeferae (Zaninetti, Altiner, Dager and Ducret), scale bar: 0.2 mm, o Densophtalmidium? sp., scale bar: 0.1 mm, p nodosariid Austrocolomia canaliculata (Kristan-Tollmann), scale bar: 0.1 mm, q Planiinvoluta sp., scale bar: 0.2 mm, r Planiinvoluta carinata Leischner, 1961, scale bar: 0.1 mm, s agglutinated foraminifer indet., scale bar: 0.25 mm, and t sessile foraminifer with a ?hyalin spinose test associated with a dark and dense micritic ?microbial crust (cf. Senowbari-Daryan 1980, Plate 17, Fig. 3), scale bar: 0.25 mm. a, b SNSB-BSPG 2021 XVI 11, c SNSB-BSPG 2021 XVI 12, d SNSB-BSPG 2021 XVI 13, e, g, q, s SNSB-BSPG 2021 XVI 14, f SNSB-BSPG 2021 XVI 15, i, r SNSB-BSPG 2021 XVI 16, j SNSB-BSPG 2021 XVI 7, k SNSB-BSPG 2021 XVI 17, l, m SNSB-BSPG 2021 XVI 6, n, p SNSB-BSPG 2021 XVI 7, and h, o, t SNSB-BSPG 2021 XVI 10
The different facies yield a diverse foraminifer fauna with several groups occurring at varying abundances. Duostominids are by far the most abundant group that are present with various taxa. Duostominids are restricted to the Triassic. Typically, their hyaline shell is diagenetically altered, and the overall preservation is unsuitable for determination in many cases. However, identifiable taxa include Duostomina? turboidea Kristan-Tollmann, 1960, Duostomina sp., Diplotremina placklesiana Kristan-Tollmann, 1960, D. subangulata Kristan-Tollmann, 1960, and Variostoma sp. Miliolids are represented by, e.g., Galeanella cf. tollmanni (Kristan, 1957), Miliolipora cuvilieri Brönnimann and Zaninetti (in Brönnimann et al. 1971), and Decapoalina cf. schaeferae (Zaninetti et al. 1982). In addition, but rare groups are lagenids (Austrocolomia canaliculata (Kristan-Tollmann, 1964)), nodosariids (Nodosaria sp.), vaginulinids (Lenticulina sp.), and involutinids (Trocholina turris Frentzen, 1941). Apart from benthic, free-living forms, encrusting forms frequently occur on skeletal constituents, mainly corals and molluscs, belonging mainly to the miliolids (Planiinvoluta sp., Planiinvoluta carinata Leischner, 1961) and spirillinids (Tolypammina? sp.). Within the intraskeletal space of corals, encrusting, irregularly growing, allusively chambered, serial foraminifera revealing a hyalin? spinose test occasionally occur. Remarkably, they are always associated with a dark and dense micritic, possibly microbial “envelope”. This locally abundant form is equivalent to the sessile foraminifer “fam. and gen. indet.” from the Rhaetian Gruber Reef illustrated by Senowbari-Daryan (1980: p l. 17, fig. 3).
Microproblematica
Bioclasts and biomorphs, especially corals, are heavily encrusted by Lithocodium and Bacinella (Fig. 9f), formerly interpreted as loftusiid foraminifera and putative cyanobacterial filaments, respectively (e.g., Schmid and Leinfelder 1996). According to studies by Cherchi and Schroeder (2010) and Schlagintweit (2011), Lithocodium probably represents Entobia-like sponge borings within dense leiolitic microbial crusts and bioclasts, and Bacinella is a network of anastomosing filaments originated by pioneering boring activity of the sponge. However, the lack of spicula and typical microbial fabrics (i.e., microbial peloids or thrombolytic/stromatolitic structures) as well as the occurrence of encrusting Bacinella threads in the interseptal space of retiophyllid corals render the entobian origin at least debatable (Fig. 9e, f). Interestingly, the observed Bacinella threads resemble the “chasmoendolithic basal stage” of Lithocodium, the diagnosis of which was emended by Schlagintweit et al. (2010). These authors reinterpreted Lithocodium aggregatum, including the type material of Elliott (1956), as well as Bacinella irregularis from the Cretaceous, as filamentous septate ulvophycean algae. Possibly, the Lithocodium/Bacinella consortium briefly described herein represents the first occurrence of Lithocodium in the revised sense of Schlagintweit et al. (2010) in the Triassic, but this assumption needs to be confirmed.
Microfacies. a Micritic rudstone rich in branching coral fragments, and b longitudinal sections of large phaceloid coral branches embedded in a bioclastic peloidal groundmass. Note porostromate cyanobacterial nodule (Cayeuxia) at the upper margin, c bioclastic floatstone with corals (co), echinods (e), molluscs (m); note Lithocodium crust (Li, lower left) and Thaumatoporella (th, lower right), d large intraclast (bioclastic floatstone) locally encrusted by foraminifers (cf. Planinvoluta sp.) and microbialites, e bioclast floatstone/rudstone, note textulariid, duostominid and lenticulid foraminifers in the groundmass as well as chasmoendolithic Bacinella threads in interseptal spaces within coral branches, and f Lithocodium encrusting coral branches. Chasmoendoltihic Bacinella threads grown in interseptal space of corals (lower margin) render general entobian origin of Triassic Lithocodium supposed by Schlagintweit (2011) questionable. All scale bars = 1 mm. a SNSB-BSPG 2021 XVI 5, b, d SNSB-BSPG 2021 XVI 10, and c, e, f SNSB-BSPG 2021 XVI 7
Tubiphytes sp. rarely occurs in the coral-rich debris facies. The sparite-filled internal cavity is surrounded by a conspicuous dense micritic envelope. Tubiphytes occurs isolated in the groundmass or attached to skeletal remains like coral septa. The systematic position of Tubiphytes is still a matter of debate. However, many authors (e.g., Schmid 1995; Senowbari-Daryan et al. 2008) suppose Tubiphytes to be a foraminifer or a combination of foraminifer, tubes of uncertain affinity, and cyanophyceans. For a review of interpretations and the systematic position of Triassic Tubiphytes, see Senowbari-Daryan (2013).
Non-skeletal constituents
Abundant poorly sorted micritic intraclasts include small sub-rounded-to-sub-angular particles (0.025–0.050 mm) as well as large sub-rounded-to-irregular particles (0.5–several mm), the latter sometimes revealing internal structures (e.g., sparitic spheres, small bioclasts). Particularly large intraclasts (up to 7 × 5 mm) even show floatstone fabrics, and contain mollusc and coral bioclasts as well as foraminifers. The surfaces of these intraclasts are frequently encrusted by miliolid foraminifers like Planiinvoluta sp. (Fig. 9d).
Lumps are also common constituents, mainly formed by Lithocodium sp. and Bacinella sp. Generally, they are small- to medium-sized (2–4 mm), although very large-sized Lithocodium/Bacinella lumps of up to 10 cm were described by Schäfer (1979).
In our understanding, irregular crusts of Lithocodium/Bacinella or microbial coatings on larger bioclasts (mainly branching corals) cannot be classified as oncoids as Schäfer (1979) did. Pronounced irregularity as well as often minor thicknesses contradicts her interpretation. Many larger bioclasts even show destructive cortoid coatings.
Cementation
The bioclastic rudstones and partly boundstones exhibit thick early diagenetic gray and cloudy isopachous fringes of radiaxial fibrous cements (RFC) forming rims in intergranular pores. They are up to 3 mm thick, and are characterized by elongated calcite crystals with subcrystals and upward concave curvature of twin lamellae and cleavage (cf. Kendall 1985). Within each subcrystal that diverges from the substrate, an opposing pattern of distally convergent optic axes occurs, caused by the upward concave curvature of cleavage and twin lamellae. The early diagenetic or marine phreatic origin of the RFC cement crusts is evidenced by the following observations: RFC is the first cement generation, it developed in marine skeletal sands, it forms isopachous rims around pores, and it developed in uncompacted shelter pore cavities (cf. Flügel 2004). Current interpretations focus on neomorphic replacement by calcite of radiating bundles of acicular Mg-calcite that precipitated as early submarine cement (Davies 1977; Davies and Nassichuk 1990). The remaining pore space is filled with late-diagenetic clear blocky calcite.
Facies interpretation
The facies described above developed within a high-energy regime subjected to strong currents in the front of the windward edge of the actual reef as evidenced by, e.g., the winnowed coarse-grained mainly rudstone fabric, the robust phaceloid corals, as well as the thick isopachous radial fibrous early diagenetic cements. The facies corresponds to the “oncolitic reef detritus facies (oncolitic facies)” described by Schäfer (1979), although she did not report the large gastropod fauna including purpuroids. However, she mentioned small pockets with gastropod accumulations within the Cycliphyllia cyclica dominated patch reefs (“Riffknospen”). According to Schäfer (1979), the coarse-grained bioclastic limestones can be correlated with facies zone 6 (“winnowed edge sands”) of Wilson (1975). The common retiophyllid coral Cycliphyllia cyclica appeared to be well adapted to strong currents and high water energy by its thick-branched and thick-walled calices that are additionally stabilized by intracalical dissepimental rings. Cycliphyllia formed robust bafflestone patches of more than 1 m in height and width within the debris-rich facies zone (cf. Schäfer 1979).
The mostly unfragmented preservation of the well-preserved large gastropods, especially of Purpuroidea and Microschiza, indicates that the shells were not transported very far and are more or less autochthonous. Most of them were probably associated with the small Cycliphyllia patch reefs and are still found in close association with these corals. Episodic storms together with erosion and resedimentation of dead gastropod shells might account for the fragmented bioclastic mollusc fraction of the rudstones (floatstones) as found in thin sections and polished slabs. The thick sculptured shells are indicative of an epifaunal lifestyle.
Systematic paleontology of gastropods and bivalves
Class Gastropoda Cuvier, 1795
Subclass Vetigastropoda Salvini-Plawen, 1980
Family Symmetrocapulidae Wenz, 1938
Remarks. The family Symmetrocapulidae was placed in Neritimorpha (Neritopsoidea) by Bouchet et al. (2005, 2017) and Monari et al. (2018). However, the protoconch of the Middle Jurassic Symmetrocapulus cancellatina Gründel, 1998 is clearly of the vetigastropod type and unlike the protoconchs found in Neritimorpha (Gründel 1998, 2000). The type species of Symmetrocapulus closely resembles Symmetrocapulus cancellatina in shape and in having a dextrally coiled early shell—therefore, the assumption that both are congeneric is justified at this point. However, the protoconch of the type species of Symmetrocapulus has to be studied in detail to corroborate this. According to the current state of knowledge, Symmetrocapulus is a limpet-shaped vetigastropod or a cocculiniform. The latter, however, have bilateral symmetrical protoconchs (e.g., McLean and Harasewych 1995) which seems not to be the case in Symmetrocapulus.
Genus Symmetrocapulus Dacqué, 1934 in Gürich
Type species. Patella rugosa Sowerby, 1816 (non Röding 1798), Bathonian, Gloucestershire, south-western England, by original designation.
Remarks. Symmetrocapulus is not available from Haber (1932) [as Symetrocapulus] because of lacking diagnosis. Patella rugosa J. Sowerby, 1816, is a junior homonym of Patella rugosa Röding, 1798.
Range. Rhaetian (herein)–Early Cretaceous (Tracey et al. 1993).
Symmetrocapulus sp.
Figure 10a, b
a, b Symmetrocapulus sp. a NHMW 2021/0121/0001(Gosaukamm). b NHMW 2021/0122/0001 (Rötelwand). c Pleurotomaria turbo Stoppani, 1860, specimen from Krispler collection, arrows in c2 indicate the presumed position of the selenizone
Material. Two specimens, one from Gosaukamm (NHMW 2021/0121/0001) and one from the Rötelwand Reef at Gaissau (NHMW 2021/0122/0001).
Description. Shell cap-shaped, bilateral symmetrical, longer than wide (28 mm long, 23 mm wide, 13 mm high) or approximately as long as wide; outline of peristome oval with apical portion narrower; apex median (lying on dorsal length axis), close to shell edge, distinctly bent; shell in lateral view with steep, concave outline at apical portion, evenly arched, convex outline in abapical portion; shell ornamented with fine, dense mesh-work of radial and co-marginal riblets.
Remarks. Limpet-shaped molluscs from the Triassic were placed in the genera Patella, Scurria, and Scurriopsis. Most of these species are poorly known, i.e., muscle scars are usually unknown and protoconchs of Triassic limpets have never been reported to our knowledge. Even the shell ornament is unknown or poorly known for many of the taxa. Without knowledge of the muscle scars, the orientation of limpets is unclear, i.e., whether the apex is situated anteriorly or posteriorly. The present shells differ from Scurriopsis Gemmellaro, 1879 and Hennocquia Dacqué, 1934 (in Gürich 1934) by having a distinctly bent apex and hence a concave posterior flank in lateral view.
Order Pleurotomariida Cox and Knight, 1960 in Knight et al. 1960
Family Pleurotomariidae Swainson, 1840
Genus Pleurotomaria Defrance, 1826
Type species. Trochus anglicus Sowerby, 1818, subsequent designation by Woodward (1851); neotype from the Middle Lias of South Petherton near Ilminster, Somerset, England; ICZN Official List, Opinion 582, 1960: 276 (Cox 1955).
Range. Carnian/Norian (Begg and Grant-Mackie 2003)–Early Cretaceous (Harasewych and Kiel 2007).
Pleurotomaria turbo Stoppani, 1860
Figure 10c
*1860Pleurotomaria? turbo Stopp.—Stoppani: p. 41 pl. 2, figs. 22–24.
1897Pleurotomaria marmoraea—Koken: p. 22, pl. 7, fig. 1 (non fig. 2).
1930Sisenna turbo Stopp.—Osswald: 763, pl. 53, fig. 24.
1937Worthenia turbo (Stoppani)—Kutassy: 440, pl. 1, figs. 65, 66.
1942Sisenna turbo (Stoppani) Oßwald—Kühn: 136, 150.
1967Worthenia turbo (Stoppani)—Zapfe: 440, pl. 4, figs. 2a, b.
Material. One specimen from Rötelwand Reef (Gaissau), Krispler collection.
Description. Shell turbiniform, low-spired, with gradate spire, about as high as wide; whorl face with bulge-like shoulder, approximately parallel to shell axis below shoulder, concave below shoulder, slightly convex at mid-whorl, concave below mid-whorl, with abapical spiral bulge forming transition to base; bulge-like shoulder ornamented with strong, broad, blunt, axially elongated nodes, numbering ca. 30 on last whorl; nodes separated by narrow interspaces; nodes crossed by at least 5 weaker spiral lirae; whorl face smooth or with very faint spiral ornamentation; two faint threads bordering smooth, convex area at mid-whorl, probably representing selenizone; base not exposed, seemingly flatly convex.
Remarks. The nodular shoulder with the spiral lirae characterizes this species. Stoppani’s (1860) illustration is technically insufficient, but it seems to be likely that the present specimen belongs to the same species. The present specimen is probably also conspecific with the specimen from the Rhaetian Zlambach Marls of the Fischerwiese (Austria) illustrated by Zapfe (1967b, pl. 4, fig. 2) and also one of the specimens from the Fischerwiese that was illustrated by Koken (1897, pl. 7, fig. 1, not fig. 2) as Pleurotomaria marmoraea Koken, 1897. A selenizone is faintly visible in the studied specimen, and hence, its assignment to Pleurotomaria seems to be justified. Based on the studied specimen, an assignment to Sisenna (Osswald 1930) or Worthenia (Zapfe 1967b) can be rejected, because the whorl angulation clearly lacks a selenizone as would be the case in the mentioned genera (Karapunar and Nützel 2021). If the selenizone is really present at mid-whorl, below the angulation, Pleurotomaria? turbo would be the earliest certain representative of the genus Pleurotomaria in the Northwestern Tethys (Paleotethys). Begg and Grant-Mackie (2003) reported Pleurotomaria from the Norian of New Zealand and New Caledonia (Southeastern Tethys-Neotethys). Other assignments of Triassic species to Pleurotomaria are outdated.
Family Trochotomidae Cox, 1960 in Knight et al.
Genus Trochotoma Eudes-Deslongchamps, 1842
Type species. Trochotoma conuloides Eudes−Deslongchamps, 1842, subsequent designation by Woodward (1851); France, Calvados, Middle Jurassic, Bathonian.
Remarks. Ferrari et al. (2015) gave an overview of trochotomids which encompass a considerable number of mainly Jurassic species representing the type genus Trochotoma. These species are mostly quite similar to each other. Ferrari et al. (2015) stated that Jurassic Trochotoma species are frequently related to reef environments of the Tethyan region (Europe, northern Africa).
A few Triassic species representing Trochotoma have been reported, among it Trochotoma praecursor (Stoppani, 1860) that is present in the Rhaetian assemblages studied herein. Furthermore, T. carpathica Kollarova-Andrusova and Kochanova, 1973 (Norian, Slovakia), T. fistulata Sachariewa-Kowatschewa, 1962 (Carnian, Bulgaria), T.? orbita Pan, 1982 (Early Triassic, China), and T. planoconvexa Yu, Pan and Wang, 1974 have been reported, all of which are doubtful members of Trochotoma according to Ferrari et al. (2015). Trochotoma carpatica is untypical for the genus in having a pronounced axial ornament on the ramp and a vertical outer whorl face instead of an inclining one; this species needs to be re-studied. Furthermore, Trochotoma sp. reported by Kollarova-Andrusova and Kochanova (1973) also has axial ribs. Trochotoma? orbita was only tentatively assigned to Trochotoma and seems to have evenly rounded whorls which is untypical for this genus. The type material of this species seems to be poorly preserved. Trochotoma fistulata as figured by Sachariewa-Kowatschewa (1962) seems to be correctly assigned to this genus. Trochotoma fallax (Kittl, 1891) from the early Carnian St. Cassian Formation is the oldest known representative of the genus (Karapunar and Nützel 2021). The fragmentary specimen reported by Nützel and Erwin (2004, fig. 12) from the Norian of Idaho as Tretospira sp. could represent a Trochotoma species.
Range. Carnian (Sachariewa-Kowatschewa 1962; Karapunar and Nützel 2021)–Late Jurassic (Ferrari et al. 2015).
Trochotoma praecursor (Stoppani, 1861)
Figure 11
Trochotoma praecursor (Stoppani, 1861). a SNSB-BSPG 2009 IV 42b. b SNSB-BSPG 2021 XVI 18. c SNSB-BSPG 2021 XVI 19a. d NHMW 2021/0122/0002. e NHMW 2021/0122/0003. f NHMW 2021/0122/0004. g NHMW 2021/0122/0005. h Specimen from Krispler collection. i Specimen from Krispler collection. All from Rötelwand Reef (Gaissau)
*1861Ditremaria praecursor Stopp.―Stoppani: 41, pl. 2, figs. 17–19.
1893Pleurotomaria scansilis―Ammon: 189, fig. 20.
1963Ditremaria praecursor (Stoppani)―Zapfe: 41.
? 1967Trochotoma cf. vetusta Terquem―Zapfe: 442, pl. 4, fig. 4 a–c
Material. Eleven specimens from the Rötelwand (Gaissau), NHMW 2021/0122/0002-2021/0122/0005, SNSB-BSPG 2009 IV 41a, 42b, SNSB-BSPG 2011 XXXVIII 1a, SNSB-BSPG 2021 XVI 18, 19a; two specimens from the Krispler collection.
Description. Shell trochiform with gradate spire; spire angle variable (70°–90°); whorl face angulated at one-third to one-fifth of whorl face of spire whorls below adapical suture; ramp straight-to-slightly concave, weakly inclined; whorl face below angulation more strongly inclined, straight-to-slightly concave, with more or less developed bulge at transition to base which forms whorl periphery; whorl face ornamented with spiral cords of variable strength and at variable distance to each other; up to 14 spiral cords below angulation, up to 4 on ramp; spiral cords strongest at middle of whorl face, weaker on ramp, near angulations and near abapical suture; whorls ornamented with strengthened growth lines, prososcline prososcyrt below angulation and on ramp but more oblique on ramp; growth lines curving backward toward angulation where selenizone lies (no trema was observed); selenizone narrow, with sharp lunulae, bordered by spiral cords, upper one forming whorl angulation; base excavate, concave, ornamented with spiral cords in the same way as on whorl face.
Remarks. The present material is quite variable in spiral ornamentation and apical angle. It agrees generally well with the illustration of the type specimen of T. praecursor from the Rhaetian of Lombardy (beds with Rhaetavicula contorta, accordingly frequent near l ‘Azzaròla, N Italy) given by Stoppani (1860) regarding general shape, spiral ornamentation and the excavate, funnel-like base. However, the ramp and the whorl face below the carination are seemingly slightly convex instead of straight to concave as in the present material. Although the species identity of our material seems to be correct, a study of Stoppani’s (1860) type material is needed for corroboration. Stoppani (1860) provided no information about the growth-line pattern and the selenizone. He stated that he had no specimen that was well enough preserved to show the trema and the same is also true for the present material (see also Zapfe 1963). A trema is only formed in the last part of the last whorl and thus is not preserved if this part is broken off. If T. praecursor lacks a trema, it could represent a different genus, for instance Bathrotomaria, but this genus does not have a funnel-like base.
Zapfe (1963) reported this species from Adnet-Kirchholz (Deißlbruch, Austria); he gave a short description but no illustration. This author pointed out that Pleurotomaria scansilis Ammon, 1893 is very similar or identical with T. praecursor and that Ammon (1893) erroneously gave an Early Jurassic age for Pleurotomaria scansilis which actually is from the Rhaetian reef limestone. We examined the holotype of Pleurotomaria scansilis (SNSB-BSPG 1929 XI 623). It is clearly conspecific with our material and has exactly the same color and type of preservation. An old label says “Pleurotomaria? Dachsteinkalk aus dem Adneter Tropfmarmorbruch” and therefore Zapfe's (1963) assumption that this taxon is Rhaetian is correct.
Trochotoma praecursor closely resembles T. vetusta Terquem, 1855 from the Early Jurassic (late Hettangian) of Brouch (Grand Douchy of Luxembourg) as reported by Meier and Meiers (1988) and Monari et al. (2011). However, T. vetusta has generally a wider apical angle and lower whorls.
Family Ptychomphalidae Wenz, 1938
Genus Angulomphalus Gründel, 2011
Type species. Helicina expansa Sowerby, 1821, original designation; France; Pliensbachian, Early Jurassic.
Remarks. Well-preserved specimens representing the type species of the Carboniferous genus Trepospira Ulrich in Ulrich and Scofield (1897) were studied by Karapunar et al. (2022). There seem to be no major differences of Trepospira (family Eotomariidae) and the Jurassic genera Angulomphalus Gründel, 2011 and Ptychomphalus (family Ptychomphalidae Wenz, 1938). Angulomphalus differs from Ptychomphalus in having an angular periphery instead of having a rounded one. As to whether this slight difference is sufficient to separate these genera or whether they are synonymous needs further study. This is the first report of the genus Angulomphalus from the Triassic.
Range. Rhaetian (herein)–Toarcian (Gründel 2011).
Angulomphalus senowbarii sp. nov.
Figure 12
urn:lsid:zoobank.org:act:53C50C30-B38E-4BFC-BBC8-DFA9D12043DB.
Material. Six specimens; holotype NHMW 2021/0122/0006 (Rötelwand); two paratypes from Gosaukamm NHMW 2021/0121/0002, 2021/0121/0003; two paratypes from Rötelwand (Gaissau) NHMW 2021/0122/0007, SNSB-BSPG 2021 XVI 20; one specimen collection Krispler.
Type locality and stratum. Rötelwand, Austria, Oberrhät Formation, Late Triassic, Rhaetian.
Etymology. In honor of Baba Senowbari-Daryan for his excellent work on Mesozoic reefs and sponges.
Description. Shell low-spired, sublenticular; largest specimen 27 mm wide, 19 mm high; spire low but clearly elevated; whorls convex, angulated at borders of selenizone at periphery; whorls with subsutural shoulder followed by nodular bulge; whorl face between nodular bulge and selenizone concave; nodes axially elongated, prosocline; selenizone concave bordered by spiral cords; adapical spiral cord with numerous nodes; selenizone covered by succeeding whorls, only exposed in last whorls when whorl is deflected downward; base convex, anomphalous with callus in umbilical region; whorls covered with fine spiral striation (commonly obscured by preservation).
Remarks. Angulomphalus senowbarii sp. nov. has a much wider selenizone than the other known members of the family Ptychomphalidae. In this respect, it resembles the Carboniferous genus Trepospira. However, Trepospira does not develop a convex subsutural bulge and concave ramp near the selenizone. This feature is known from the type species Angulomphalus expansus. The presence of an umbilical callus and the slightly elevated selenizone with distinct borders also suggest a placement in Angulomphalus. Angulomphalus wehenkeli (Terquem and Piette 1865) from the Early Jurassic (Upper Hettangian) of Brouch, Luxembourg, is very similar (Monari et al. 2011; alias Ptychomphalus caepa in Meier and Meiers 1988; Gründel 2011), but the selenizone of the mature whorls is not bordered by spiral cords, it lacks a spiral striation, and the subsutural knobs are not as elongated.
Family Stuorellidae Bandel, 2009
Genus Stuorella Kittl, 1891
Type species. Trochus subconcavus Münster, 1841, St. Cassian Formation, Carnian, Italy; by monotypy.
Remarks. Apart from two occurrences of Stuorella from Jurassic deposits of Sicily (Szabó et al. 2021) (but see Karapunar and Nützel 2021) and Cretaceous deposits of Spain (Kiel and Bandel 2000), the family is restricted to the Triassic. An Anisian reference to Stuorella (Schnetzer 1934) seems to be doubtful. The oldest certain members of Stuorella have a Ladinian age (Kittl 1894).
Range. Ladinian (Kittl 1894)–Campanian (Kiel and Bandel 2000).
Stuorella zapfei sp. nov.
Figure 13
urn:lsid:zoobank.org:act:E96F8ED9-0CB9-4642-828A-D81FA025BB08.
Material. Three specimens from Rötelwand (Gaissau); holotype SNSB-BSPG 2009 IV 45a, paratype NHMW 2021/0122/0008; SNSB-BSPG 2009 IV 46a.
Type locality and stratum. Rötelwand, Austria, Oberrhät Formation, Late Triassic Rhaetian.
Etymology. After Helmut Zapfe for his work on the Late Triassic faunas of the Alps.
Description. Shell trochiform, conical, with straight flanks; apical angle ca. 60°; whorls very low, largely smooth with weak spiral striation of threads and furrows; whorl face straight, slightly imbricate, narrowly exposing basal edge; basal edge forms slightly nodular bulge; possible selenizone low on whorls, covered by spiral striae, flush with whorl face; transition to base at angular edge; base flat, slightly concave, with remains of spiral striation.
Remarks. This species likely has a selenizone low on the whorls which is, however, obscured by preservation. It is placed in the slit-band gastropod genus Stuorella. If it had no selenizone, a placement in the trochoid genus Epulotrochus Cossmann, 1918 would be possible. For instance, Epulotrochus strobiliformis (Hoernes) from the Norian Hallstätterkalke of Austria and Timor is similar but lacks a selenizone, spiral striation is weaker or absent, and it has a lower apical angle (see Tichy 1979). Stuorella tofanae Leonardi and Fiscon, 1959 from the St. Cassian Formation is the most similar species. This species has a distinct axial ornament on its early whorls that is later reduced. It differs from Stuorella zapfei in having distinct lunulae on the selenizone and a weaker spiral striation (Karapunar and Nützel 2021).
Some species of the Jurassic genus Pyrgotrochus P. Fischer, 1880 closely resemble the present species for instance P. punctatus (Sowerby, 1818) (Monari et al. 2018) or P. elongatus (Sowerby, 1818) (Lindström and Peel 2010). However, the Early Jurassic (Pliensbachian) type species P. bitorquatus (Eudes-Deslongchamps 1849) has a median selenizone and strong nodes at both sutures according to the diagnosis and illustration given by Wenz (1938, p. 147, fig. 213). Nevertheless, Knight et al. (1960) and Harasewych and Kiel (2007, Table1) gave a submedian selenizone as diagnostic. In contrast to the type species of Pyrgotrochus, Stuorella zapfei has the selenizone low on the whorls and lacks a strong nodular ornament. However, many other species assigned to Pyrgotrochus also have a submedian selenizone and only a weak ornamentation. Those species are close to Stuorella zapfei, but taken together, this species is closer to the type species of Stuorella than to that of Pyrgotrochus, especially regarding the very low whorls and the submedian position of the selenizone. The genera Pyrgotrochus and Stuorella are closely related according to Harasewych (2002, fig. 9) who treated them a sister groups. However, this proposal needs corroboration. According to Monari (written communication 2021), Pyrgotrochus has an early shell with sharply reticulate ornament which is typical of the family Pleurotomariidae (Szabó et al. 2021; Karapunar and Nützel 2021) whereas in Stuorella, the spiral ornament is dominant. Although poorly preserved, the early whorls of the present new species seem to lack this ornament pattern.
Stuorella pappi Kutassy, 1937 (Norian, Hungary) is similar but the selenizone is bordered by distinct spiral cords and the whorls lack a spiral striation.
Family Zygitidae Cox, 1960 in Knight et al.
Genus Kokenella Kittl, 1891
Type species. Porcellia fischeri Hörnes, 1855, Hallstatt Limestone, Norian, Austria; original designation.
Range. Anisian (Kittl 1908; Tichy 1980)–Rhaetian (Gugenberger 1933; Zapfe 1967b; Nützel and Senowbari-Daryan 1999).
Kokenella sp.
Figure 14
Material. Two specimens, Rötelwand (Gaissau), collection Krispler.
Description. Shell planispiral, widely phaneromphalous, 31 mm wide, 11 mm high; whorls convex with rounded periphery; whorls ornamented with broad, wavy radial ribs separated by wide interspaces, ca. 10–12 ribs per whorl; ribs do not continue onto periphery; whorls with traces of weak spiral cords obscured by preservation; selenizone on periphery also obscured by preservation.
Remarks. This is a typical but rather large representative of the Middle and Late Triassic genus Kokenella. The preservation is too poor for identification or characterization of a new taxon. The present specimens closely resembles the K. costata (Münster, 1841) from the Late Triassic (Carnian) St. Cassian Formation (see Karapunar and Nützel 2021) that has also been reported from the Rhaetian of Iran (Nützel and Senowbari-Daryan 1999).
Genus Sadkia Conti and Monari, 2001
Type species. Sadkia richensis Conti and Monari, 2001, Middle Jurassic, Early Bajocian, Central High Atlas, Morocco, original designation.
Range. Rhaetian (herein)–Bajocian (Conti and Monari 2001).
Sadkia diversicostata (Wolff, 1967) comb. nov.
Figure 15
Sadkia diversicostatus (Wolff, 1967). a Holotype SNSB-BSPG 1967 XXIII 1. b SNSB-BSPG 1967 XXIII 2. c Collection Krispler (Rötelwand, Gaissau)
*1967 Tylotrochus diversicostatus sp. nov.—Wolff: 237, pl. 9, figs. 2a, b,3.
Material. Four specimens; holotype (SNSB-BSPG 1966 XXIII 1 from the Wendelstein, Bavaria, illustrated here Fig. 15a) and paratype (SNSB-BSPG 1966 XXIII 2, illustrated here Fig. 15b), two specimens from the Krispler collection, both from Rötelwand Reef (Gaissau).
Description. Shell turbinifom; whorls strongly convex; sutures deep; largest specimen 25 mm high, 18 mm wide; whorls ornamented with pronounced, straight, prosocline axial ribs separated by much wider interspaces and 5–8 much weaker, equally spaced spirals cords; spiral cords pass over axial ribs without forming nodes at intersections; base convex, ornamented with weaker continuation of axial ribs and spiral cords.
Remarks. The present specimens closely resemble the type specimens of Tylotrochus diversicostatus Wolff, 1967 from the Rhaetian of the Bavarian Alps (Wendelstein) (Fig. 15a,b) which are, however, much smaller and may represent juvenile specimens of this species. Sadkia diversicostata species resembles Eucycloscala epitoniformis Nützel and Senowbari-Daryan, 1999 from the Late Triassic (Norian/Rhaetian) of Iran; we remove this species from Eucycloscala and place it in Sadkia: Sadkia epitoniformis comb. nov. Sadkia epitoniformis has fewer spiral cords and strengthened growth lines. A similar species was reported from the Early Jurassic (Pliensbachian) of Germany (Eucycloidea gen. et sp. indet. in Nützel and Gründel 2015) which has a much finer ornament and more spiral cords. This specimen also represents the genus Sadkia. We take the opportunity to re-illustrate Wolff’s (1967) type specimens. Wolff’s (1967) assignment to Tylotrochus Koken, 1896 cannot be maintained, because the whorls of this genus are not strongly convex and its axial ribs are much weaker. Phasianella cancellata Dittmar, 1864 from the Rhaetian of the North Alps is similar, but its type material needs to be studied to evaluate a possible synonymy with S. diversicotata. Phasianella crassecostata Stoppani, 1863 (Paracerithium?) from the Rhaetian of the South Alps is similar, but has straight axial ribs instead of oblique ones.
Subclass Neritimorpha Koken, 1896
Family Neritopsidae Gray, 1847
Genus Cassianopsis Bandel, 2007
Type species. Naticella armata Münster, 1841, Late Triassic (Carnian), St. Cassian Formation, original designation.
Range. Ladinian (Kittl 1899)–Kimmeridgian (Gründel et al. 2015).
Cassianopsis sp.
Figure 16a, b
Material. Two specimens, Rötelwand (Gaissau), NHMW 2021/0122/0009, SNSB-BSPG 2021 XVI 21.
Description. Shell neritiform with rapidly increasing whorls, broader than high, 20 mm wide, 16 mm high; spire small, gradate; whorls convex with periphery at about mid-whorl; whorls ornamented with pronounced, straight to prosocyrt, prosocline axial ribs separated by wide interspaces; whorls ornamented with numerous spiral cords, much weaker than axial ribs, forming weak nodes at shoulder of whorls; base convex, axial ribs seem to continue onto base.
Remarks. Neritimorphs with strong axial ribs and weaker spiral cords resembling the type species of Cassianopsis from the Carnian St. Cassian Formation have been repeatedly reported from Late Triassic strata. The preservation of the present species is too poor for a safe identification.
Genus Hologyra Koken, 1892
Type species. Hologyra alpina Koken, 1892; subsequent designation by Kittl (1899).
Remarks. Koken (1892) gave a preliminary diagnosis; a full description was provided in Wöhrmann and Koken (1892); Bandel (2007) cited Natica cassiana Wissmann [in Münster], 1841 from the Late Triassic (Carnian) St. Cassian Formation as type species which is, however, not an originally included species.
Range. Anisian (e.g., Koken 1898; Yin Hong-fu and Yochelson 1983)–Rhaetian (herein).
Hologyra callosa sp. nov.
Figure 16d
urn:lsid:zoobank.org:act:54F89DE2-32C7-45C6-85C3-DD7655D4A2E1.
Material. One specimen, Rötelwand (Gaissau), SNSB-BSPG 2009 IV 44.
Type locality and stratum. Gaissau, Austria, Oberrhät Formation, Late Triassic, Rhaetian.
Etymology. Latin for the callous aperture.
Description. Shell globular, 28 mm wide, 24 mm high, with flat-to-slightly immersed spire; whorls evenly rounded, strongly embracing each other, so that the spire whorls can only be seen in small area encircled by the last preserved whorl; sutures shallow; whorls smooth; aperture with strong parietal and columellar callus also covering umbilical area, with callous bulge on columellar part; inner lip oblique, slightly arched.
Remarks. Hologyra involuta (Kittl, 1892) from the Carnian St. Cassian Formation is similar, especially regarding the morphology of the apex. However, Hologyra involuta lacks the callous bulge on the columellar lip and its spire is slightly more elevated. Hologyra alpina Koken, 1892 is broader, and the spire is somewhat more elevated and lacks the callous bulge on the columellar lip. Hologyra timorensis (Krumbeck, 1924) from the Norian of Timor is similar, but is not as broad, lacks a callous bulge on the columellar lip and a greater portion of the apical whorls is exposed than in Hologyra involuta. Species of the genus Marmolatella are evolute instead of involute. Most other neritimorphs of the Triassic have a more elevated spire.
Family Delphinulopsidae Blodgett et al., 2001
Genus Paradelphinulopsis Blodgett et al., 2001
Type species. Paradelphinulopsis vallieri Blodgett et al., 2001, Upper Triassic, Western US, original designation.
Range. Norian (Blodgett et al. 2001)–Rhaetian (herein).
Paradelphinulopsis sp.
Figure 16c
Material. Two specimens, one from Gosaukamm NHMW 2021/0121/0004, one from Rötelwand (Gaissau) SNSB-BSPG 2021 XVI 22.
Description. Shell neritiform, low-spired, with rapidly increasing whorls, 29 mm high, 24 mm wide; whorls with two angulations; upper whorl face flat, demarcated by nodular angulation; whorl face between upper and second angulation straight-to-slightly convex, parallel to shell axis; second angulation slightly nodular; shell ornamented with numerous densely spaced spiral cords; aperture not visible.
Remarks. This specimen is similar to the Late Triassic type species Paradelphinulopsis vallieri from the Western US as documented by Blodgett et al. (2001) and Nützel and Erwin (2004) regarding its ornamentation. However, Paradelphinulopsis vallieri has detached, openly coiled whorls which is not the case in the present specimen.
Subclass Caenogastropoda Cox, 1960 in Knight et al.
Family Purpurinidae Zittel, 1895
Genus Microschiza Gemmellaro, 1878
Type species. Turbo philenor d'Orbigny, 1853; Early Jurassic (Hettangian/Sinemurian), France, Luxembourg, subsequent designation by Cossmann (1909).
Remarks. Meier and Meiers (1988) listed the type species Turbo philenor d'Orbigny, 1853 as a synonym of Microschiza clathrata (Deshayes, 1855) (in Terquem), but it is unclear why they have used the junior name for this species. Fischer and Weber (1997, pl. 23, figs. 22, 23) illustrated the type species. Gründel (2003) treated Turbo philenor d'Orbigny and Microschiza clathrata as synoynms of M. semiornata (Münster, 1844) (in Goldfuss). Wenz (1938) placed Microschiza in Pseudomelaniidae; Fischer and Weber (1997) placed it in Purpurinidae and Gründel (2003) questionably in Purpurinidae.
So far, the only Triassic species that has been assigned to Microschiza is M. arguta Böhm, 1895 from the Ladinian Marmolata Limestone. We studied the type specimen of that species (SNSB-BSPG 1887 XI 205) and are not convinced that this generic assignment is correct. Microschiza arguta is too slender and has only a fine spiral striation instead of distinct spiral cords. Praelittorina sepkoskii Nützel and Erwin, 2004 from the Norian of Idaho is actually better placed in Microschiza (Microschiza sepkoskii comb. nov.) than in the almost globular genus Praelittorina Kutassy, 1937).
Range. Norian (Nützel and Erwin 2004)–Cretaceous, Turonian (Allison 1955; Wenz 1938).
Microschiza rhaetica sp. nov.
Figure 17
Microschiza rhaetica sp. nov. a Holotype NHMW 2021/0121/0005 (Gosaukamm). b Paratype NHMW 2021/0121/0006 (Gosaukamm). c Paratype NHMW 2021/0121/0007 (Gosaukamm). d Paratype NHMW 2021/0122/0010 (Rötelwand). e Paratype NHMW 2021/0122/0011 (Rötelwand). f Paratype NHMW 2021/0122/0013 (Rötelwand). g Paratype NHMW 2021/0122/0014 (Rötelwand). h Paratype NHMW 2021/0122/0015 (Rötelwand)
urn:lsid:zoobank.org:act:77CB523C-8104-45B9-A3CF-A1E5D572E1F3.
Material. 99 specimens; five specimens from Gosaukamm, 55 specimens from Rötelwand (Gaissau); holotype from Gosaukamm NHMW 2021/0121/0005; two paratypes from Gosaukamm NHMW 2021/0121/0006, 2021/0121/0007; five paratypes from Rötelwand NHMW 2021/0122/0010, 2021/0122/0011, 2021/0122/0013, 2021/0122/0014, 2021/0122/0015; NHMW 2021/0121/0008, 2021/0121/0009; SNSB-BSPG 2009 IV 41c–g, SNSB-BSPG 2009 IV 42c, 43b, 45b, 46b–c, 51, 52, SNSB-BSPG 2008 I 90a, SNSB-BSPG 2021 XVI 23–37, 38a, 38b, 39–47, 48a, 49a, 50a, 51a–b, 52a–b, 53, 54a–b; 39 additional unnumbered specimens from NHMW.
Type locality and stratum. Rötelwand, Austria, Oberrhät Formation, Late Triassic, Rhaetian.
Etymology. After the Rhaetian stage.
Description. Shell littoriniform, higher than wide; largest specimen 44 mm high, 33 mm wide; last whorl distinctly higher than spire; whorls embrace at periphery of evenly rounded, convex whorls; sutures shallow, whorls ornamented with alternating weak and strong spiral cords; up to ca. ten spiral cords present on whorl face; ornament indistinct in early whorls (probably due to preservation), strong spiral cords nodular, weak spiral cords not nodular; base convex, with evenly rounded transition from whorl face, covered with spiral cords; aperture oblique, higher than wide; inner lip almost straight with parietal inductura, outer lip evenly arched; aperture with posterior siphonal canal and anterior outlet.
Remarks. Microschiza rhaetica sp. nov. resembles the Early Jurassic (Hettangian) Microschiza semiornata (Münster, 1844 in Goldfuss) (= M. philenor = M. clathrata Deshayes, 1855 in Terquem) from France and Luxembourg (see Meier and Meiers 1988; Fischer and Weber 1997). However, Microschiza semiornata lacks the regular alternation of weak and strong spiral cords, and has a distinct shoulder, and the whorl face is concave below the shoulder.
Family Purpuroideidae Guzhov, 2004
Genus Purpuroidea Lycett, 1848
Type species. Cossmann (1903) cited Purpura moreausia Buvignier, 1843 as type species, but this was not an originally included species; British Isles, Jurassic.
Remarks. Purpuroidea? minioi Leonardi, 1935 from the Early Triassic of the Italian Alps certainly does not represent Purpuroidea. This species could rather belong to Angularia Koken, 1892 (Angularia minioi comb. nov.). Purpuroidea carpathica Jekelius, 1935 and P.? lovisatii Tommasi, 1896 (both Ladinian) likewise do not represent this genus. The first species really belonging to Purpuroidea have a Carnian age (e.g., Kittl 1894; Kutassy 1927). Purpuroidea dilophosignata Fallahi et al., 1983 is another latest Triassic representative of this genus from the Norian/Rhaetian Nayband Formation of Iran (Nützel and Senowbari-Daryan 1999; Nützel et al. 2003, 2010).
Range. Carnian (e.g., Kittl 1894; Kutassy 1927)–Turonian (Wenz 1938).
Purpuroidea moosleitneri sp. nov.
Figure 18
Purpuroidea moosleitneri sp. nov. a Paratype, SNSB-BSPG 2009 IV 42a (Rötelwand). b Paratype, SNSB-BSPG 2009 IV 43c (Rötelwand). c Holotype SNSB-BSPG 2021 XVI 55 (Rötelwand). d Paratype NHMW 2021/0122/0016 (Rötelwand). e Paratype NHMW 2021/0122/0017 (Rötelwand). f Paratype NHMW 2021/0122/0018 (Rötelwand)
urn:lsid:zoobank.org:act:57E171DF-42E2-4CB2-B7B2-FD3C1C701C80.
?1856Turbo subcoronatus Hörn.–Hörnes: 23, pl. 1, fig. 2.
2004Purpuroidea sp.–Moosleitner: 63, pl. 17, fig. 12.
Material. 68 specimens; two specimens from Gosaukamm, 30 specimens from Rötelwand (Gaissau); holotype, Rötelwand (Gaissau), SNSB-BSPG 2021 XVI 55; five paratypes, Rötelwand (Gaissau), SNSB-BSPG 2009 IV 42a, SNSB-BSPG 2009 IV 43c, NHMW 2021/0122/0016–2021/0122/0018; NHMW 2021/0122/0019, 2021/0122/0020, NHMW 2021/0121/0010, 2021/0121/0011, SNSB-BSPG 1980 I 196–198, SNSB-BSPG 2008 I 90b, c, SNSB-BSPG 2009 IV 41 h–k, SNSB-BSPG 2011 XXXVIII 2, SNSB-BSPG 2021 XVI 48b, 49b, 50b, 56–63; 36 additional unnumbered specimens NHMW.
Type locality and stratum. Rötelwand (Gaissau), Austria, Oberrhät Formation, Late Triassic, Rhaetian.
Etymology. In honor of Gero Mossleitner who shared his deep knowledge of the paleontology of the Austrian Alps with us and helped us during field work.
Description. Shell globular, large, largest specimen ca. 90 mm high, 80 mm wide; apical angle 90°–100°; last whorl higher than spire; spire gradate; whorls convex with slightly inclined to almost horizontal ramp in late whorls; ramp more inclined in early whorls; last whorl somewhat deflected downward; whorls ornamented with rows of spirally arranged nodes and spiral cords between them; spiral cords may also cross nodes; most prominent nodes situated at edge of ramp with nodes pointing in adapical abaxial direction; up to 11 nodes on last whorl; row of weaker, spirally elongated nodes in suprasutral position in spire whorls, 2 undulating spiral cords between adapical rows of nodes; base convex, evenly rounded, not demarcated from whorl face; base ornamented with rows of spirally elongated nodes of similar strength as nodes of second row; base with spiral cords between rows of nodes.
Remarks. A species that is known as Purpuroidea subcoronata (Hörnes, 1856) from the Late Triassic of Slovenia (Unterpetzen = Podpeca) closely resembles Purpuroidea moosleitneri sp. nov. Hörnes, (1856) claimed that his species was identical with Pleurotomaria coronata Münster, 1841 from the St. Cassian Formation and that Pleurotomaria coronata represents the genus Turbo. To avoid secondary homonymy with Turbo coronatus (Roemer, 1841), he introduced the name Turbo subcoronatus which therefore is a replacement name. Meanwhile, Pleurotomaria coronata has been transferred to the Pleurotomariida genus Worthenia and recently to the genus Nodocingulum Karapunar and Nützel, 2021. Worthenia coronata is certainly not conspecific with the species described and illustrated by Hörnes (1856). The material identified as Turbo subcoronatus by Hörnes (1856) represents an undescribed species. Purpuroidea moosleitneri sp. nov. has a more acute, elevated spire than that species, its ramp is more inclined; it has more spiral cords on the whorl face; the second row of nodes is covered by succeeding whorls; its whorl face is straight, not convex.
Purpuroidea ferenczii Kutassy, 1927 from the Late Triassic (Norian) Dachsteinkalk of Hungary closely resembles Purpuroidea moosleitneri sp. nov. However, Purpuroidea ferenczii has a narrower spiral angle, the adapical nodes are less pronounced, and it has more spiral cords that are closer to each other (see Kutassy 1927, 1932, 1934, 1937) (Fig. 19). Purpuroidea ferenczii was listed by Zapfe (1962, p. 348) as being present in the Gosaukamm reefs and it is likely that his specimens represent Purpuroidea moosleitneri.
Purpuroidea ferenczii, Kutassy's (1927) type specimen from the Norian of Hungary; photo by courtesy Jànos Szabó
Purpuroidea applanata Kittl, 1894 and P. crassenodosa Kittl, 1894, both from the Carnian St. Cassian Formation, differ considerably in shape and ornament. Purpuroidea excelsior Koken, 1897 has a more inclined ramp, fewer rows of spirally arranged nodules and weaker nodes.
Purpuroidea dilophosignata Fallahi et al., 1983 has only two rows of nodes and lacks ornament on the base. Purpuroidea taramellii (Stoppani) as figured by Tommasi (1903, pl. 3, figs. 25, 26) has smaller nodes and a narrower apical angle. Purpuroidea baconica Kittl, 1900 has only a single row of nodes and a narrower apical angle. Purpuroidea carpathica Jekelius, 1935 is much more high-spired and differs in ornamentation.
Genus Angularia Koken, 1892 non Busk, 1881
Type species. Turbo subpleurotomarius Münster, 1841, by monotypy, Late Triassic, Carnian, St. Cassian Formation, South Tyrol, Italy; Koken (1892) provided a preliminary diagnosis of Angularia. A full description was given by Wöhrmann and Koken (1892).
Reversal of precedence. Angularia Koken, 1892 and Angularia Busk, 1881 under ICZN Art. 23.9.
The genus-group name Angularia Busk, 1881 [Bryozoa] was introduced without any included species but with a short description, and is thus an available name under Art. 12.1. To our knowledge, the only mention of this name after 1899 is by Canu and Bassler (1927: 24) as an entry in a list: “Angularia Busk, 1881. No species indicated. Dropped by author”, which does not qualify as the use of a valid name of a taxon. Conversely, the name Angularia Koken, 1892 has been used in at least 25 works published by at least ten authors in the last 50 years: Bandel (1992: 67, 1993: 10, 26, 1994: 139, 153, 156, 2016: 159), Bandel and Dockery (2016: 51), Baumgarte and Schulz (1986: 86), Brayard et al. (2015: 40, 50, 55), Caruthers and Stanley (2008: 167), Frýda and Blodgett (2001: 216), Fürsich and Wendt (1977: 275), Galácz and Szabó (2001: 20), Gründel (1980: 51; 2010: 9, 20), Guzhov (2004: 9, 12, 13), Harzhauser and Schneider (2014: 370), Hasibuan and Grant-Mackie (2007: 257, 265), Kiel et al. (2000: 24), Kramm (2004: 77), Pan Hua-zhang (1977: 119, 1982: 99), Nützel (2005a, b: 20), Nützel and Erwin (2004: 383, 394, 395), Nützel and Senowbari-Daryan (1999: 110), Ponder et al. (2008: 358, 368), Riedel (2000: 166), Sepkoski (2002: 372), Szabó (2011a: 9; 2011b: 227), and Tracey et al. (1993: 146).
Apart from these recent references, Angularia Koken was also included in the widely used handbooks of Cossmann (1906) and Wenz (1938–1944) including descriptions and illustrations.
Based on this evidence, under Art. 23.9.2, we here declare Angularia Busk, 1881 to be a nomen oblitum, and Angularia Koken, 1892 a nomen protectum.
Range. Early Triassic (Olenekian)–Hettangian. Brayard et al. (2015) reported Angularia sp. from the Early Triassic (Olenekian) of the U.S.A. Purpuroidea? minioi Leonardi, 1935 from the Early Triassic of Italy obviously represents Angularia (Angularia minioi comb. nov.). The genus Angularia has been reported from the Hettangian by Gründel (2010) and by Meier and Meiers (1988) as Pleurotomaria obliqua Terquem.
Angularia corallina sp. nov.
Figure 20a, b
Angularia corallina sp. nov. a Paratype, SNSB-BSPG 2009 IV 43a (Rötelwand). b Holotype SNSB-BSPG 2021 XVI 19b (Rötelwand). c Pictavia sp., NHMW 2021/0121/0012 (Gosaukamm). d Protorcula sp., specimen from Krispler collection. e Coelostylina? sp. 1, Rötelwand (Gaissau), SNSB-BSPG 2009 IV 47. f Coelostylina? sp. 2, Rötelwand (Gaissau), specimen from Krispler collection. g Coelostylina? sp. 3, NHMW 2021/0122/0021 (Rötelwand). h Cryptaulax sp. auf großer Stufe, Rötelwand (Gaissau), SNSB-BSPG 2009 IV 41b
urn:lsid:zoobank.org:act:21CBF3B8-F5A5-4CA1-92AF-A8B83F80B289.
Type locality and stratum. Gaissau, Austria, Oberrhät Formation, Late Triassic, Rhaetian.
Etymology. According to its occurrence in coral-rich limestones.
Material. Three specimens from Gaissau; holotype SNSB-BSPG 2021 XVI 19b; paratype SNSB-BSPG 2009 IV 43a and 2009 IV 41b.
Description. Shell fusiform, moderately high-spired; spire gradate with angulation at mid-whorl of last whorl, somewhat above suture in spire whorls; high, steep ramp, with straight-to-slightly convex outline, concave above angulation; whorl face straight below angulation; axially elongated nodes on angulation, ca. 15 nodes per whorls; whorls ornamented with strengthened growth lines, prosocline on ramp, orthocline prosocyrt below ramp and on base; growth lines forming sinus at angulation; whorls adpressed in narrow zone at suture with growth lines strongly curving in adapertural direction; base strongly convex with evenly rounded transition to whorl face.
Remarks. Several similar Late Triassic species were placed in the genus Angularia for instance A. multinodosa Kutassy, 1937 and A. plicata Kutassy, 1937. None of these species have the angulation as low as Angularia corallina sp. nov. on the spire whorls. A similar species was illustrated as Pleurotomaria obliqua Terquem from the Hettangian of Luxembourg by Meier and Meiers (1988, fig. 13a, b). This specimen differs from Angularia corallina in lacking nodes on the angulation. It certainly does not represent Pleurotomaria obliqua which is much broader nor does it represent the genus Pleurotomaria. Instead, this Hettangian specimen can be placed in Angularia.
Superfamily Campaniloidea Douvillé, 1904
Family Ampullinidae Cossmann, 1919 (in Cossmann and Peyrot).
Genus Pictavia Cossmann, 1925
Type species. Natica pictaviensis d'Orbigny, 1850; Bathonian (Middle Jurassic), Noyer, France; original designation.
Remarks. Pictavia unites many Mesozoic littoriniform to bulbous, smooth-shelled species that are difficult to differentiate from each other. Some of them are close to Coelostylindiae. Gründel and Kaim (2006) reported Late Jurassic (Oxfordian) Pictavia species having smooth larval shells that are close to the Late Triassic type species of Coelostylina, C. conica, from the Late Triassic St. Cassian Formation, as recently reported by Hausmann et al. (2021).
Range. Anisian (Ahlburg 1906; Cossmann 1925)–Cenomanian (Smettan 1997).
Pictavia sp.
Figure 20c
Material. One specimen, Gosaukamm, NHMW 2021/0121/0012.
Description. Shell turbiniform, bulbous, with acute spire; relatively large, 85 mm high, 72 wide; whorls evenly convex, rapidly expanding, smooth; whorls embrace at rounded periphery; body whorl much higher than spire; transition from whorl face to base evenly rounded; base rounded convex; aperture and umbilical region not observed.
Remarks. Eight Triassic nominate species have been assigned to Pictavia. Pictavia sp. resembles P. alsatica (Koken, 1898) from the Anisian Muschelkalk. Pictavia comes (Ammon, 1878), and P. limnaeoides (Ammon, 1878) from the Norian Hauptdolomit of the Alps are similar but much smaller. Pictavia silicea (Quenstedt, 1858) from the Late Jurassic of Germany is similar but much smaller (Gründel et al. 2019, pl. 2, figs. 17, 18). The Late Jurassic species Globularia hebertana (d'Orbigny, 1851) from France is similar and also rather large (Fischer and Weber 1997, pl. 16, fig. 10). However, the Eocene type species of Globularia Swainson, 1840 has a lower spire and is more globular. Without more information about growth-line pattern, aperture and early ontogenetic shell, an identification is hardly possible. Given the exceptional large size of the present specimen, we assume that it represents a yet undescribed species.
Family Coelostylinidae Cossmann, 1908
Genus Coelostylina Kittl, 1894
Type species. Melania conica Münster, 1841; St. Cassian Formation, Carnian, Late Triassic, northern Italy; subsequent designation by Cossmann (1895).
Remarks. High-spired caenogastropods with umbilicus or an umbilical chink are diverse and abundant in the Triassic, and are commonly assigned to the genera Coelostylina or Omphaloptychia which could represent synonyms. Coelostylina has a well-documented type species including a well-preserved type specimen from the St. Cassian Formation (Hausmann et al. 2021). By contrast Omphaloptychia nota, the type species of Omphaloptychia is not documented as well and its type material is not as well preserved.
Range. Permian, Wuchiapingian (e.g., Dietz 1911)–Middle Jurassic (Bajocian) (Cox 1965).
Coelostylina? sp. 1
Figure 20e
Material. One specimen, SNSN-BSPG 2009 IV 47, Rötelwand (Gaissau).
Description. Shell of about eight whorls, apex missing, 65 mm high, 26 mm wide; shell high-spired, conical; apical angle ca. 35°; whorl face distinctly convex; periphery low on whorls where whorls embrace; whorls smooth; sutures distinct; transition from whorl face to base rounded; base distinctly convex.
Remarks. This specimen resembles Coelostylina? sp. 2, but has a more convex, extended base. Coelostylina? sp. 3 has a less convex whorl face.
Coelostylina? sp. 2
Figure 20f
Material. One specimen, Rötelwand (Gaissau), Krispler collection.
Description. Shell of about six whorls, apex missing, 41 mm high, 21 mm wide; shell high-spired, conical; apical angle ca. 30°; whorl face convex; periphery low on whorls where whorls embrace; whorls smooth; sutures distinct; transition from whorl face to base rounded; base flatly convex.
Remarks. This specimen resembles Coelostylina? sp. 1, but has a somewhat more convex whorl face, more distinct sutures, and a flatter base. Coelostylina? sp. 3 has a flatter whorl face and indistinct sutures.
Coelostylina? sp. 3
Figure 20g
Material. One specimen, Rötelwand (Gaissau) NHMW 2021/0122/0021.
Description. Teleoconch fragment of about 5–6 whorls, apex missing, 33 mm high, 19 mm wide; shell high-spired, conical; apical angle ca. 40°; whorl face slightly convex-to-almost straight; periphery low on whorls where whorls embrace; whorls smooth; sutures shallow, indistinct; transition from whorl face to base rounded; base rounded, convex, conical.
Remarks. “Chemnitzia” sp. as reported by Stoppani (1860, pl. 2, fig. 23) from the Rhaetian of the South Alps has deeper sutures and more convex whorls. Another “Chemnitzia” sp. as reported by Stoppani (1860, pl. 28, fig. 3) from the Rhaetian of the South Alps could be conspecific, but has an angular transition from whorl face to base. Several Triassic species assigned to the genera Omphaloptychia (e.g., O. muensteri Böhm, 1895), Toxoconcha, and Coelostylina (for instance C.? baconica Kittl, 1900) are similar.
Family Protorculidae Bandel, 1991
Genus Protorcula Kittl, 1894
Type species. Turritella subpunctata Münster, 1841; St. Cassian Formation, Carnian, Late Triassic, northern Italy; subsequent designation by Cossmann (1895).
Range. Capitanian (Nützel et al. 2012)–Pliensbachian (Cossmann 1913; Gründel 2007a). Wenz (1938) noted that Protorcula ranges to the Bajocian, but we do not know the reference for this occurrence. Protorcula? quadricostata Gründel, 2007b from the Sinemurian differs very much from the type species of Protorcula in teleoconch ornament and is likely not a member of the genus. Nützel and Senowbari-Daryan (1999) and Nützel et al. (2012) reported Protorcula from the Norian/Rhaetian Nayband Formation, Iran.
Protorcula sp.
Figure 20d
Material. One specimen from Rötelwand (Gaissau), Krispler collection.
Description. Shell high-spired, turreted, comprising ca. 15 whorls (apical whorls missing), 5 cm high, 1 cm wide; whorls low; whorl face distinctly concave, sutures situated on sharp crests; whorls apparently lacking ornament but are insufficiently preserved; aperture not preserved.
Remarks. This high-spired shell with concave whorl face resembles some Jurassic/Cretaceous Nerineidae. However, knowledge of the aperture and internal folds would be needed to place it in that group. To date, Nerineidae have no certain fossil record in the Triassic.
Family Cryptaulacidae Gründel, 1976
Genus Cryptaulax Tate, 1869
Type species. Cerithium tortile Hébert and Eudes-Deslongchamps, 1860 (= Procerithium protortile Cox, 1965); Jurassic, France; original designation.
Remarks. Several latest Triassic (Norian/Rhaetian) Cryptaulax species have been reported (Haas 1953; Nützel and Senowbari-Daryan 1999; Nützel et al. 2003, 2010).
Range. Norian (Haas 1953; Nützel and Erwin 2004)–Early Cretaceous (Kaim 2004).
Cryptaulax sp.
Figure 20h
Material. One specimen on large block, Rötelwand (Gaissau), SNSB-BSPG 2009 IV 41 l.
Description. Shell high-spired, slender, acutely conical; apical angle 30°; ca. 8 whorls preserved, 46 mm high, 17 wide; earlier preserved whorls with ca. ten orthocline axial ribs, separated by wide interspaces, and two spiral cords; adapical spiral cord situated above mid-whorl; abapical spiral cord above suture; intersections of axial ribs and spiral cords nodular; last whorl with two additional spiral cords intercalated between primary spiral cords; last preserved whorl with distinctly more axial ribs as earlier whorls (ca. 15 ribs); sutures shallow but distinct; base convex, with at least three nodular spiral cords.
Remarks. Despite the rather poor preservation, this shell is characteristic by its increase in axial and spiral ornamentation just on the last preserved whorls. The present specimen is large for the genus Cryptaulax. It probably represents an undescribed species.
Class Bivalvia Linnaeus, 1758
Remarks. The classification of bivalves follows Hautmann (2001), with modifications as indicated.
Infraclass Autolamellibranchiata Grobben, 1894
Subclass Pteriomorphia Beurlen, 1944
Order Arcoida Stoliczka, 1871
Superfamily Arcoidea Lamarck, 1809
Family Parallelodontidae Dall, 1898
Subfamily Grammatodontinae Branson, 1942
Genus Grammatodon Meek and Hayden, 1860
Subgenus Cosmetodon Branson, 1942
Type species. Arca keyserlingii d´Orbigny, 1850 (original designation).
Grammatodon (Cosmetodon) buckmanni (Richardson, 1843)
Figure 21a
Bivalvia. a Grammatodon (Cosmetodon) buckmanni (Richardson, 1843), left valve from Rötelwand (Gaissau), NHMW 2021/0122/0022. b Rhaetavicula contorta (Portlock, 1843), left valve from Gosaukamm, NHMW 2021/0121/0016. c Cassianella sp., left valve from Gosaukamm, NHMW 2021/0121/0022. d Gen. et sp. indet., internal mold of right valve from Gosaukamm, NHMW 2021/121/0023. e, f Oxytoma inaequivalve (Sowerby, 1821); e left valve from Gosaukamm, NHMW 2021/0121/0025; f right valve from Gosaukamm, NHMW 2021/0121/0026. g, h Chlamys tatrica (Goetel, 1917); g right valve from Rötelwand, NHMW 2021/122/0023; h left valve from Rötelwand, NHMW 2021/122/0024. i Plagiostoma subvaloniense Krumbeck, 1923, right valve from Gosaukamm, NHMW 2021/121/0038. j Schafhaeutlia sphaerioides (Boettger, 1880), left valve from Gosaukamm, NHMW 2021/121/0043
*1843Arca buckmanni sp. nov.—Richardson p. 504, fig. 243.
1855Cucullaea hettangiensis sp. nov.—Terquem, p. 90, pl. XXI, fig. 3.
1963Parallelodon hettangiensis (Terquem)—Zapfe, p. 214.
2001Grammatodon (Cosmetodon) sp. A.—Hautmann, p. 36. pl. 3, figs. 1, 3.
2001Grammatodon (Cosmetodon) sp. B.—Hautmann, p. 36. pl. 3, fig. 2a–b.
2020Grammatodon (Cosmetodon) buckmanni (Richardson, 1843)—Knight and Morris, p. 1189, figs. 10a–h.
Material. One left valve from Rötelwand (Gaissau) NHMW 2021/0122/0022, one left and one right valve from Gosaukamm NHMW 2021/0121/0013, 2021/0121/0014; one complete internal mold from Gosaukamm NHMW 2021/0121/0015.
Description. Relatively large (up to 4 cm in length) Cosmetodon with shallow central sulcus running from the prosogyrous umbones to the ventral margin of the valves. Outline of valves rectangular, beaks located c. 25% of overall length behind anterior margin. Ornamentation with fine radial ribs superimposed by commarginal growth ridges varying in strength.
Remarks. Knight and Morris (2020) indicated a close similarity between Arca buckmanni Richardson, 1843 and Cucullaea hettangiensis Terquem, 1855, but they kept both species separately, based on a supposedly greater elongation of the former. Because the type material of Cucullaea hettangiensis seems to be lost and the description and figures in Terquem (1855) do not show a difference to Arca buckmanni in the elongation or in any other feature, we regard both taxa tentatively as synonyms. The presence of this Jurassic species (as “Parallelodon hettangiensis”) in the Upper Rhaetian reef limestone was already recognized by Zapfe (1963). We include the two specimens of Cosmetodon from the Rhaetian of east-central Iran described in open nomenclature by Hautmann (2001) in G. (C.) buckmanni. In the Rhaetian, Grammatodon (Cosmetodon) buckmanni was thus a trans-Tethyan species, which survived the end-Triassic mass extinction.
Order Pterioida Newell, 1965
Suborder Pteriina Newell, 1965
Superfamily Pterioidea Gray, 1847
Family Pteriidae Gray, 1847
Genus Rhaetavicula Cox, 1962
Type species. Avicula contorta Portlock, 1843 (original designation).
Rhaetavicula contorta (Portlock, 1843)
Figure 21b
*1843Avicula contorta sp. nov.—Portlock: 126, pl. 25, fig. 16.
1963Pteria (Rhaetavicula) contorta (Portlock)—Zapfe: 223.
2001Rhaetavicula contorta (Portlock 1843)—Hautmann, p. 46, pl. 5, fig. 7 (cum synonymis).
Material. Six left valves from Gosaukamm NHMW 2021/0121/0016–2021/0121/0021.
Description. The studied specimens show the characteristic twisted appearance of the left valve, which is ornamented by up to 20 radial ribs intercalated in two ranks.
Remarks. This species was widespread in the Rhaetian of Europe, North America and the Tethys realm (Hautmann 2001).
Family Cassianellidae Ichikawa, 1958
Genus Cassianella Beyrich, 1862
Type species. Avicula gryphaeata Münster (in Goldfuss), 1838 (original designation).
Cassianella sp.
Figure 21c
Material. One left valve from Gosaukamm NHMW 2021/0121/0022.
Description. Internal mold of a left valve, showing the characteristic strongly convex and obliquely curved main body of the shell, the markedly convex anterior auricle, and the short but well-developed posterior auricle.
Remarks. Because the ornamentation of the shell exterior is not preserved, a species assignment is not possible.
Incertae familiae
gen. et sp. indet.
Figure 21d
Material. Two internal molds of right valves from Gosaukamm NHMW 2021/0121/0023, 2021/0121/0024.
Description. Shell medium-sized, obliquely oval in outline. Dorsal margin straight, without differentiated auricles or wings. Beak not terminal, umbo slightly protruding above hinge margin. Ornamentation of broad concentric folds. Ligament not observed.
Remarks. This taxon is represented by two incomplete internal molds of right valves. Neither its ligament area nor the anterior part of the shell is preserved, which makes a full determination impossible. Shape and ornamentation of the shell resemble Retroceramus Koshelkina, 1957, a genus that is most common in the Jurassic of high palaeo-latitudes (Damborenea 1990; Zakharov et al. 2020), but has also been questionable reported from the Late Triassic (Cox et al. 1969).
Order Pectinoida Gray, 1854
Suborder Monotidina Waterhouse, 2001 emend. Wasmer et al. 2012.
Family Oxytomidae Ichikawa, 1958
Genus Oxytoma Meek, 1864
Type species. Avicula muensteri Bronn, 1830 = Avicula inaequivalvis Sowerby, 1821 (original designation).
Oxytoma inaequivalve (Sowerby, 1819)
Figures 21e, f
*1819Avicula inaequivalvis sp. nov.―Sowerby: 78, pl. 244.
1901Avicula (Oxytoma) inaequivalvis Sowerby―Waagen: 24, pl. 1, figs. 1–17 (cum synonymis).
1963Oxytoma inaequivalve intermedium (Emmrich)―Zapfe: 223.
1963Oxytoma inaequivalve muensteri (Bronn)―Zapfe: 223.
Material. Ten partly fragmented left valves from Gosaukamm NHMW 2021/0121/0027–2021/0121/0036; one left valve from Gosaukamm NHMW 2021/0121/0025; one right valve from Gosaukamm NHMW 2021/0121/0026.
Description. Shell pteriiform, bialate, inequivalve. Left valve strongly convex, with small anterior auricle and elongated posterior wing. Ornamentation with 10–11 first-order radial ribs. Second-order ribs intercalated at about 30–50% of shell height, most prominent on posterior part of the valves, faint or absent anteriorly. Third-order ribs occasionally present but faint. Posterior wing smooth except for weak radial ridges parallel to hinge margin, which are invisible in internal molds. Right valve slightly convex, with small anterior auricle forming a deep embayment toward disc. Posterior wing large and elongated. Disc of right valve retrocrescent, covered with faint radial ribs intercalated in two ranks.
Remarks. This species is very variable in terms of its ornamentation, which is reflected by considerable taxonomic splitting, as reviewed by Waagen (1901). The impression from our limited material is that there is much variation with regard to the ornamentation within populations.
Oxytoma inaequivalve (Sowerby, 1821) is among the species of the Oberrhät Limestone that survived into the Jurassic.
Suborder Pectinina Waller, 1978
Superfamily Pectinoidea Rafinesque, 1815
Family Pectinidae Rafinesque, 1815
Subfamily Chlamydinae von Teppner, 1922
Genus Chlamys Röding, 1798
Subgenus Chlamys Röding, 1798
Type species. Pecten islandicus Müller, 1776 (subsequent designation Herrmannsen 1846).
Chlamys tatrica (Goetel, 1917)
Figure 21g–h
*1917Pecten favrii Stoppani var. nov. tatrica—Goetel: 148, pl. 8, fig. 11.
1963Pecten (Chlamys) favrii tatricus Goetel—Zapfe: 225, pl. 2, fig. 8.
1998Praechlamys sp.—Chen Chuzhen: pl. 1, fig. 8.
Material. One left valve from Rötelwand NHMW 2021/0122/0024; one external mold of a right valve from Gosaukamm NHMW 2021/0121/0037; two right valves from Rötelwand (Gaissau) NHMW 2021/0122/0023, 2021/0122/0025.
Description. Disc of right valve up to 4 cm high, covered with 20–24 radial plicae arranged in pairs. Apical angle of disc c. 80°. Posterior disc margin with numerous transverse ridges. Anterior auricle of right valve well developed, with deep byssal notch; posterior auricle of right valve large, with three radial ridges. Disc of left valve with ribs intercalated in two ranks: second-order ribs notably weaker than first-order ribs.
Remarks. This species was introduced by Goetel (1917) as a subspecies of Pecten favrii Stoppani, 1863. Hautmann (2001) noted that Goetel’s (1917) taxon can be separated consistently from Stoppani’s species based on the much lower number of plicae and the smaller apical angle. The material from the Oberrhät Limestone confirms this conclusion.
Order Limoida Waller, 1978
Superfamily Limoidea Rafinesque, 1815
Family Limidae Rafinesque, 1815
Genus Plagiostoma J. Sowerby, 1814
Type species. Plagiostoma giganteum J. Sowerby, 1814 (subsequent designation Stoliczka 1871).
Plagiostoma subvaloniense Krumbeck, 1923
Figure 21i
*1923Plagiostoma subvaloniense sp. nov.―Krumbeck: 202, pl. 12, figs. 17–19.
?1963Lima (Plagiostoma) punctata Sowerby―Zapfe: 230.
2001Plagiostoma subvaloniense Krumbeck, 1923―Hautmann: 94, pl. 21, figs. 15–19; pl. 22, fig. 1 (cum synonymis)
Material. One left valve (internal mold with small fragments of shell) from Gosaukamm NHMW 2021/0121/0041; internal molds of one right and one left valve and one complete specimen from Gosaukamm NHMW 2021/0121/0038–2021/0121/0040; one fragment of an internal mold with conjoined valves from Gosaukamm NHMW 2021/0121/0042.
Description. Medium-sized Plagiostoma (height c. 4 cm) with an apical angle of c. 90°. Ornamentation, where preserved, with densely spaced radial ribs.
Remarks. Although this taxon is not completely preserved, the size, overall morphology, apical angle and rudimentary preserved ornamentation is suggestive for Plagiostoma subvaloniense Krumbeck, 1923.
Subclass Heteroconchia Hertwig, 1895
Superorder Heterodonta Neumayr, 1884.
Order Veneroida Adams and Adams, 1856.
Incertae familiae
Genus Schafhaeutlia Cossmann, 1897
Type species. Gonodon schafhaeutli Salomon, 1895 [= Gonodon ovatum Schafhäutl, 1863] (monotypy).
Remarks. Although the hinge of this genus can be interpreted as lucinid, the massive, arched hinge teeth, thick spheroidal shell and strongly incoiled umbones are unlike most other lucinoid genera. The systematic position of this taxon must therefore currently be regarded as uncertain.
Schafhaeutlia sphaerioides (Boettger, 1880)
Figure 21j
*1880Lucina (Loripes) sphaerioides sp. nov.―Boettger (in: Ver beek et al.): 37, pl. 1, figs. 16, 17.
1963Schafhaeutlia cf. manzavinii (Bittner)―Zapfe: 216, pl. 3, fig. 9.
2001Schafhaeutlia sphaerioides (Boettger 1880)―Hautmann: 128, text-fig. 25, pl. 30. figs. 1–5 (cum synonymis).
Material. Three left valves from Gosaukamm NHMW 2021/0121/0043–2021/0121/0045.
Description. Thick-shelled, strongly convex Schafhaeutlia. Height of valves approximately equal to length, umbones centrally placed, strongly prosogyrate. Ornamentation with commarginal ribs that are asymmetrical in cross section.
Remarks. Following Hautmann (2001, p. 128), this species is interpreted as an epifaunal recliner that rested at the flattened anterior part of the shell, supported by the strongly incurved umbones.
Discussion
The demise of the widespread reefs and mountain-building carbonate platforms in the western Tethys is one of the hallmarks of the end-Triassic mass extinction event. At the Rötelwand, the guild of reef-building corals is only present with three genera and species (admittedly more species exist in Retiophyllia; see above) in the studied samples, only one of which Pinacophyllum survived the extinction with certainty. Cycliphyllia and possibly also Retiopyhllia did not survive the perturbation at the end of the Rhaetian. Globally, corals were hit severely, with recent estimations of generic loss between 51 and 53% (Kiessling et al. 2009), based on an updated compilation of stratigraphic ranges by Lathuilière and Marchal (2009) as well as data from the PBDB.
However, the present study is not focused on the reefs themselves but on their associated fauna. Our documentation confirms that the diversity of such “accessory” faunas may be much larger than that of the reef builders that provide their ecological context.
Kiessling et al. (2007) and Kiessling and Aberhan (2007) suggested that reef-associated taxa and taxa that preferred carbonate substrata have had a significantly higher extinction risk than level-bottom dwellers during the end-Triassic mass extinction, which seems plausible: if reefs disappear, reef dwellers lose their habitats and might also become extinct. Our new data on reef dwellers or reef-associated invertebrates and the facies of the fossiliferous rocks from Gaissau (Rötelwand Reef, Salzburg area) and Gosaukamm (Dachstein Mountains) provide an opportunity to test this hypothesis.
Among the 11 foraminiferid genera found in the studied thin sections, eight have had their last appearance in the Rhaetian. Thus, Foraminifera at the Rötelwand locality suffered 73% extinction at the genus level, which is well above the average marine genus extinction (46.8%; Bambach et al. 2004) during this event. This high extinction of Foraminifera is contrasted by a surprisingly low extinction proportion of reef-associated mollusc taxa (bivalves and gastropods), which are present with 47 identified genera from the studied latest Triassic reefal carbonates (bivalve data also include Zapfe's 1963 study). Among these, only 12 genera became extinct (25.5%) at the end of the Triassic. However, most of the species are known only from the Late Triassic, except for two bivalve species (Grammatodon (C.) buckmanni and Oxytoma inaequivalve) from our material (Table 2) and one bivalve species described by Zapfe (“Ostrea anomala” = Liostrea hisingeri; Zapfe 1963), which are also known from the Jurassic.
Gastropods are present with 17 genera and 19 species. Six of these species are described as new, including two relatively large and conspicuous ones (Microschiza rhaetica and Purpuroidea moosleitneri) that are also abundant. It is remarkable that such species have not been described earlier from this well-researched area. This is probably due to their rather spotty or not easily accessible occurrence. None of the present gastropod species is known to cross the Triassic–Jurassic boundary, but this is common in gastropods, because Triassic species are usually restricted to a single stage, and many are known from a single or few occurrences (Nützel 2005b). Among the 17 gastropod genera, three do not cross the boundary and became extinct during the end-Triassic crisis. However, most genera (82%) survived and are also known from Jurassic and Cretaceous deposits. Most of the present genera originated in the Triassic (eight in the Late Triassic) and only two in the Permian. Taken together, the gastropod fauna has a Triassic character, although most genera survived into the Jurassic. Nützel (2005b, fig. 1) showed that the Rhaetian gastropod standing diversity is very low (ca. 200 species including those in open nomenclature), and the drop from the Norian is pronounced. However, the Rhaetian is much shorter than the Norian (Rhaetian: 4 Ma, Norian: 13 Ma; Gradstein et al. 2004), and according to Kiessling et al. (2007), the number of Rhaetian fossil occurrences is generally very low, so that sample standardization would be needed to correct for these differences. The gastropod species diversity peaks in the Carnian (Nützel 2005b), which is largely due to the extremely diverse fauna of the St. Cassian Formation. This Liberation Lagerstätte (Roden et al. 2020) is characterized by low lithification, so that small, fragile gastropods can be easily extracted. By contrast, the Upper Rhaetian Reef Limestone and the Gosaukamm carbonates are strongly lithified. Most of the studied gastropod species are medium-sized or large, and must be extracted from the rock by laborious preparation, whereas tiny gastropod species are lacking. The study of the thin sections revealed that small gastropods were present, but they cannot be extracted. This taphonomic bias must be taken into account when the St. Cassian fauna is compared with the present one. Pieroni et al. (2021) emphasized that the study of gastropods from latest Triassic carbonate platforms is necessary to understand the evolution of their diversity dynamics during that critical time interval despite their commonly poor preservation.
It is remarkable that the present gastropod fauna resembles the Hettangian gastropod fauna from Luxembourg (Brouch), as reported by Terquem (1855), Meier and Meiers (1988), Monari et al. (2011), and Gründel (2011). Both faunas are dominated by similar species representing the genus Microschiza. Moreover, similar species representing the genera Pleurotomaria, Trochotoma, Angulomphalus, and Angularia, among others, are present at both sites. It seems that this Hettangian fauna consists largely of descendants or close relatives of the latest Triassic Tethyan gastropod faunas. This similarity is also remarkable, because Brouch represents a non-reefal, siliciclastic (sandstone)-dominated environment (Faber and Weiss 2005). Differences between both faunas may be explained with this facies difference, notably the absence of Purpuroidea at Brouch.
The here described bivalve fauna is essentially a subset of the 41 bivalve taxa described by Zapfe (1963; Table 3). Although Zapfe (1963) did not figure the majority of his material, an assignment of the new material to his identifications is possible in most cases, although taxonomic names were partly revised herein.
All bivalve species identified in this study were epifaunal, either byssally attached or reclining (Table 2), which is expected for a reefal environment that is dominated by hard substratum. The vast majority of species described by Zapfe (1963) was also epifaunal, but the presence of few burrowing heterodonts and anomalodesmats in his collection indicates the occasional presence of soft substratum, such as pockets filled with mud within or between the reefs. Palaeogeographically, all identified bivalve species occurred Tethys-wide in the Triassic, although some are known in the eastern Tethys only from isolated occurrences (Table 2).
The bivalve fauna represents an assemblage that is typical of latest Triassic reef and carbonate platform environments. As noted above, the end-Triassic mass extinction affected these settings heavily, probably because of an ocean acidification event (Hautmann 2004; Hautmann et al. 2008a). This event was followed by a short-term interruption of carbonate deposition (Hautmann 2004; Hautmann et al. 2008a; Greene et al. 2012; McRoberts et al. 2012) and a long-lasting reef eclipse (Kiessling et al. 2009; Lathuilière and Marchal 2009). Consequently, it could be expected that reef and carbonate platform associated taxa also suffered above average, but surprisingly, this seems not to be the case. Among the herein recorded bivalve genera, three (Rhaetavicula, Cassianella, Schafhaeutlia) out of seven (42.8%) went extinct, which is comparable to the average extinction of marine bivalve genera at the end of the Triassic (40.5%; Hautmann et al. 2008a). The small size of this dataset limits the significance of this comparison, but the result is also in accordance with Zapfe’s (1963) larger dataset. Accepting his determinations at the genus level with slight modifications (Table 3), only 9 out of 30 reported genera (30%) went extinct. It should be noted that some determinations in Zapfe (1963) require revision, but the general pattern (no elevated extinction in reef-associated taxa) is likely robust. It is also notable that a bivalve-dominated level-bottom fauna in a siliciclastic setting of the latest Triassic of South Tibet (Hautmann et al. 2008b) suffered much higher extinction at the genus level (53.8%) than the reef-related bivalves reported in this study (30%).
We note that the genus level might be inappropriate for the study of extinction patterns, because differences in the ecology among species of the same genus cannot be ruled out. However, the above-cited analyses of Kiessling and Aberhan (2007) and Kiessling et al. (2007) were also calculated at the genus level; thus, the difference between the results is not an artifact of the taxonomic level. Moreover, the genus is still a low taxonomic category that reflects close phylogenetic relationships of the included species, which usually share similar niches (niche conservatism; e.g., Pyron et al. 2015). Therefore, it is unlikely that a genus comprises species that belong to entirely different guilds.
In summary, the expectation that the end-Triassic reef extinction should have been matched by an elevated extinction of reef-associated taxa is confirmed for foraminifers, but not for gastropods and bivalves. Given the low number of Rhaetian marine invertebrate occurrences (Kiessling et al. 2007), this unexpected observation is relevant for the interpretation of the end-Triassic mass extinction and requires an explanation. The present case study on dwellers of one of the most important latest Triassic reef complexes adds significantly to this discussion, regardless of whether it is a local exception or not. We suggest that the studied Rhaetian gastropod and bivalve genera were not obligate reef dwellers, but had a broader ecological capacity or that they shifted their habitats following the extinction of reefs. This is indicated by the presence of many of the studied gastropod genera in siliciclastic level-bottom settings in the Hettangian of Luxembourg (see above), and likewise, most of the surviving bivalve genera are known from level-bottom settings in the Early Jurassic (e.g., Aberhan 1992). We conclude that most of the Late Triassic reef-dwelling molluscs of the latest Triassic reefs of Austria had sufficient ecological plasticity to survive the global downfall of reef ecosystems in other habitats. Batten's (1973) proposal that a major gastropod faunal turnover occurred after the Rhaetian needs to be tested.
Abbreviations
- FAD:
-
First appearance date
- LAD:
-
Last appearance date
- NHMW:
-
Naturhistorisches Museum Wien
- PBDB:
-
Paleobiology database
- SNSB-BSPG:
-
Staatliche Naturwissenschaftliche Sammlungen Bayerns-Bayerische Staatssammlung für Paläontologie und Geologie
References
Aberhan, M. 1992. Palökologie und zeitliche Verbreitung benthischer Faunengemeinschaften im Unterjura von Chile. Beringeria 5: 1–174.
Adams, H., and A. Adams. 1856. The genera of Recent Mollusca, arranged according to their organisation. Volume 2 (1854–1858). London: John van Vorst.
Ahlburg, J. 1906. Die Trias im südlichen Oberschlesien. Abhandlungen der Königlich Preussischen Geologischen Landesanstalt und Bergakademie. Neue Folge 50: 1–163.
Allasinaz, A. 1972. Revisione dei Pettinidi. Rivista Italiana Di Palaeontologia e Stratigrafia 78: 189–428.
Allison, E.C. 1955. Middle Cretaceous Gastropoda from Punta China, Baja California, Mexico. Journal of Paleontology 29: 400–432.
Ammon, L. 1878. Die Gastropoden des Hauptdolomites und des Plattenkalkes der Alpen. Abhandlungen des Zoologisch-Mineralogischen Vereines in Regensburg 11: 1–72.
Ammon, L. 1893. Die Gastropodenfauna des Hochfellen-Kalkes und über Gastropoden-Reste aus Ablagerungen von Adnet, vom Monte Nota und den Raibler Schichten. Geognostische Jahreshefte 5: 161–219.
Bambach, R.K., A.H. Knoll, and S.C. Wang. 2004. Origination, extinction, and mass depletion of marine diversity. Paleobiology 30: 522–542.
Bandel, K. 1991. Über triassische “Loxonematoidea” und ihre Beziehungen zu rezenten und paläozoischen Schnecken. Paläontologische Zeitschrift 65: 239–268.
Bandel, K. 1992. Über Caenogastropoden der Cassianer Schichten (Obertrias) der Dolomiten (Italien) und ihre taxonomische Bewertung. Mitteilungen aus dem Geologisch-Paläontologischen Institut der Universität Hamburg 73: 37–97.
Bandel, K. 1993. Caenogastropoda during Mesozoic times. Scripta Geologica, Special Issue 2: 7–56.
Bandel, K. 1994. Comparison of Upper Triassic and Lower Jurassic Gastropods from the Peruvian Andes (Pucará Group) and the Alps (Cassian Formation). Palaeontographica Abteilung A 233: 127–160.
Bandel, K. 2007. Description and classification of Late Triassic Neritimorpha (Gastropoda, Mollusca) from the St Cassian Formation, Italian Alps. Bulletin of Geosciences 83: 215–274.
Bandel, K. 2009. The slit bearing nacreous Archaeogastropoda of the Triassic tropical reefs in the St. Cassian Formation with evaluation of the taxonomic value of the selenizone. Berliner Paläobiologische Abhandlungen 10: 5–47.
Bandel, K. 2016. A glimpse into the Jurassic gastropods of the shallow sea with description of Mid-Jurassic species of Madagascar (Sakaraha) and their relation to species of similar age in Europe and elsewhere. Freiberger Forschungshefte C 550: 137–203.
Bandel, K., and D.T. Dockery III. 2016. Mollusca of the Coon Creek Formation in Tennessee and Mississippi with a systematic discussion of the Gastropoda. Bulletin of the Alabama Museum of Natural History 33: 34–96.
Batten, R.L. 1973. The vicissitudes of the gastropods during the interval of the Guadalupian–Ladinian time. Canadian Society of Petroleum Geologists Memoir 2: 596–607.
Baumgarte, D., and M. Schulz. 1986. Stratigraphie und Fauna des Unteren und Mittleren Wellenkalkes (Unteranis/ Pelson) von Müs (Bl. 5423 Großenlüder). Geologisches Jahrbuch Hessen 114: 69–94.
Begg, J.G., and J.A. Grant-Mackie. 2003. New Zealand and New Caledonian Triassic Pleurotomariidae (Gastropoda, Mollusca). Journal of the Royal Society of New Zealand 33: 223–268.
Bernecker, M. 2005. Late Triassic reefs from the Northwest and South Tethys: Distribution, setting, and biotic composition. Facies 51: 442–453.
Beurlen, K. 1944. Beiträge zur stammesgeschichte der Muscheln. Sitzungsberichte der Bayerischen Akademie der Wissenschaften 1944 (1–2): 133–145.
Beyrich, E. 1862. Zwei aus dem deutschen Muschelkalk noch nicht bekannte, Avicula-artige Muscheln. Zeitschrift der Deutschen Geologischen Gesellschaft 14: 9–10.
Blodgett, R.B., J. Frýda, and G.D.J. Stanley. 2001. Delphinulopsidae, a new neritopsoidean gastropod family from the Upper Triassic (upper Carnian or lower Norian) of the Wallowa terrane, northeastern Oregon. Journal of the Czech Geological Society 46: 305–318.
Boettger, O. 1880. Die Tertiärformation von Sumatra und ihre Tierreste. III. Die Conchylien der unteren Tertiärschichten. Palaeontographica, Supplementband III: 29–120.
Böhm, J. 1895. Die Gastropoden des Marmolatakalkes. Palaeontographica 42: 211–308.
Bouchet, P., J. Frýda, B. Hausdorf, W.F. Ponder, Á. Valdés, and A. Warén. 2005. Working classification of the Gastropoda. In Classification and nomenclator of gastropod families, eds. P. Bouchet, and J.-P. Rocroi. Malacologia 47: 1–397.
Bouchet, P., J.-P. Rocroi, B. Hausdorf, A. Kaim, Y. Kano, A. Nützel, P. Parkhaev, M. Schrödl, and E.E. Strong. 2017. Revised classification, nomenclator and typification of gastropod and monoplacophoran families. Malacologia 61: 1–526.
Branson, C.C. 1942. Parallelodon, Grammatodon, and Beushausenia (=Cosmetodon, new name). Journal of Paleontology 16: 247–249.
Brayard, A., M. Meier, G. Escarguel, E. Fara, A. Nützel, N. Olivier, K.G. Bylund, J.F. Jenks, D.A. Stephen, M. Hautmann, E. Vennin, and H. Bucher. 2015. Early Triassic Gulliver gastropods: Spatio-temporal distribution and significance for the biotic recovery after the end-Permian mass extinction. Earth-Science Reviews 164: 31–64.
Bronn, H. 1830. Über die Muschelkalkversteinerungen des Süddeutschen Steinsalzgebirges, welche bisher unter dem Namen Pectinites salinarius zusammengefasst wurden. Leonard und Bronns Jahrbuch für Mineralogie 1: 282.
Brönnimann, P., L. Zaninetti, F. Bozorgnia, G.R. Dashti, and A. Moshtaghian. 1971. Lithostratigraphy and foraminifera of the Upper Triassic Naiband Formation Iran. Revue de Micropaléontologie 14: 7–16.
Busk, G. 1881. Notes on a peculiar form of Polyzoa closely allied to Bugula (Kinetoskias, Kor. and Dan.). Quarterly Journal of Microscopical Science 21: 1–14.
Buvignier, A. 1843. Mémoire sur quelques fossiles nouveaux des départements de la Meuse et des Ardennes. Mémoires de la Société Philomatique de Verdun (Meuse) 2: 225–252.
Canu, F., and R.S. Bassler. 1927. Classification of the cheilostomatous Bryozoa. Proceedings of the United States National Museum 69: 1–43.
Caruthers, A.H., and G.D.J. Stanley. 2008. Late Triassic silicified shallow-water corals and other marine fossils from Wrangellia and the Alexander terrane, Alaska, and Vancouver Island, British Columbia. The Geological Society of America, Special Paper 442: 151–179.
Cherchi, A., and R. Schroeder. 2010. Boring sponges (Entobia) on Mesozoic Lithocodium calcimicrobial crusts. Rivista Italiana di Paleontologia e Stratigrafia 116 (3): 351–356.
Chen Chuzhen. 1998. Some Triassic bivalves from Pamir and Karakorum Region. In Palaeontology of the Karakorum-Kunlun Mountains, ed. Wen Shixuan, 299–308, 1–2. Beijing: Science Press.
Ciriacks, K.W. 1963. Permian and Eotriassic bivalves of the middle Rockies. Bulletin of the American Museum of Natural History 125: 1–100.
Conti, M.A., and S. Monari. 2001. Middle Jurassic gastropods from Central High Atlas, Morocco. Geobios 34: 183–214.
Cossmann, M. 1895. [Review of] Die Gastropoden der Schichten von St-Cassian der Südalpinen Trias. von (Les Gasteropodes des couches de St-Cassian, dans le Trias sud-alpin. Par) Ernst Kittl (1). Journal de Conchyliologie 43: 61–68.
Cossmann, M. 1897. Revue Critique de Paléozoologie.
Cossmann, M. 1903. Essais de Paléoconchologie Comparée 5, 215 pp. Paris: M. Cossmann, F.R. de Rudeval.
Cossmann, M. 1906. Essais de Paléoconchologie Comparée 7, 261 pp. Paris: M. Cossmann, F.R. de Rudeval.
Cossmann, M. 1908. [Review of] Système silurien du centre de la Bohème, 1ère partie. Recherches paléontologiques. Vol. IV: Gastropodes, T. II, par J. Perner. Revue Critique de Paléozoologie 12(2): 91–95.
Cossmann, M. 1909. Essais de Paléoconchologie Comparée 8, 248 pp. Paris: M. Cossmann, F.R. de Rudeval.
Cossmann, M. 1913. Contribution a la Paléontologie Française des terrains Jurassiques. III. Cerithiacea et Loxonematacea. Mémoires de la Société Géologique de France. Paléontologie 46: 89–264.
Cossmann, M. 1918. Essais de Paléoconchologie Comparée 11. 388 pp. Paris: M. Cossmann.
Cossmann, M. 1925. Essais de Paléoconchologie Comparée 13. 345 pp. Paris: Les Presses Universitaires de France.
Cossmann, M., and A. Peyrot. 1919. Conchologie néogénique de l’Aquitaine. Tome 3, Gastropodes, Scaphopodes et Amphineures. Bordeaux. 695 pp., 17 pls. Published in parallel in Actes de la Société Linnéenne de Bordeaux.
Cox, L.R. 1955. Proposed use of the plenary powers (1) to validate the generic name “Pleurotomaria” Sowerby (J.), 1821 (class Gastropoda) if it is judged that that name is invalid, (2) to validate the name “anglicus” Sowerby (J.), 1818, as published in the combination “Trochus anglicus” and (3) to designate the species so named to be the type species of the genus “Pleurotomaria” Sowerby, 1821. Bulletin of Zoololgical Nomenclature 11: 21–27.
Cox, L.R. 1962. New genera and subgenera of Mesozoic Bivalvia. Palaeontology 4 (4): 592–598.
Cox, L.R. 1965. Gastéropodes Jurassiques du sud-est Tunisien. Annales de Paléontologie (invertébrés) 40: 243–268.
Cox, L.R., N.D. Newell, D.W. Boyd, C.C. Branson, R. Casey, A. Chavan, A.H. Coogan, C. Dechaseux, C.A. Fleming, F. Haas, L.G. Hertlein, E.G. Kauffman, A. Myra Keen, A. La Rocque, A.L. Mc Alester, R.C. Moore, C.P. Nuttall, B.F. Perkins, H.S. Puri, L.A. Smith, T. Soot-Ryen, H.B. Stenzel, E.R. Trueman, R.D. Turner, and J. Weir. 1969. Bivalvia. In Treatise on invertebrate paleontology. Part N. Mollusca 6, ed. R.C. Moore, 1224 pp. Boulder, Colorado and Lawrence, Kansas: Geological Society of America and University of Kansas Press.
Cuvier, G. 1795. Second mémoire sur l’organisation et les rapports des animaux à sang blanc, dans lequel on traite de la structure des Mollusques et de leur division en ordres, lu à la Société d’histoire naturelle de Paris, le 11 Prairial, an III. Magazin Encyclopédique, ou Journal des Sciences, des Lettres et des Arts 2: 433–449.
Dall, W.H. 1898. Contribution to the Tertiary fauna of Florida with special reference to the Silex beds of Tampa and the Pliocene beds of the Caloosahatchie River. Transactions of the Wagner Free Institute of Science of Philadelphia 3: 571–947.
Damborenea, S.E. 1990. Middle Jurassic inoceramids from Argentina. Journal of Paleontology 64: 736–759.
Davies, G.R. 1977. Former magnesian calcite and aragonite submarine cements in Upper Palaeozoic reefs in the Canadian Arctic: A summary. Geology 5: 11–15.
Davies, G.R., and W.W. Nassischuk. 1990. Submarine cements and fabrics in Carboniferous to Lower Permian, reefal, shelf margin and slope carbonates, northwestern Ellesmere Island, Canadian Arctic Archipelago. Geological Survey of Canada, Bulletin 399: 77 pp.
de Lamarck, J.B. 1809. Philosophie zoologique, ou, Exposition des considérations relative à l’histoire naturelle des animaux. Paris: Dentu.
Defrance, M.J.L. 1826. Genus Pleurotomaria. In Dictionnaire des Sciences Naturelles, dans Lequel on Traite Méthodiquement des pifferens Êtres de la Nature, Considérés Soit en Eux-mêmes d'Après l'État Actuel de nos Connoissances, soit Relativement à l'Utilité qu'en Peuvent Retirer la Medicine, l'Agriculture, le Commerce et les Artes: Suivi d'une Biographie des plus Célèbres Naturalists, par Plusieurs Professeurs du Muséum National d'Histoire Naturelle et des Autres Principales de Écoles de Paris, Volume 41, ed. F. Cuvier, p. 381. Paris and Strasbourg.
Dietz, E. 1911. Ein Beitrag zur Kenntnis der deutschen Zechsteinschnecken. Jahrbuch der Preußischen Geologischen Landesanstalt zu Berlin für das Jahr 1909 (30): 444–506.
Dittmar, A. 1864. Die Contorta-Zone (Zone der Avicula Contorta Portl.), ihre Verbreitung und organischen Einschlüsse. München: Herrmann Manz.
Douvillé, H. 1904. Mollusques fossiles. In Mission scientifique en Perse par J. de Morgan, tome 3, partie IV, 192–380, pls. 25–50. Paris: Leroux.
Elliott, G.F. 1956. Further records of fossil calcareous algae from the Middle East. Micropaleontology 2: 327–334.
Eudes-Deslongchamps, E.M. 1842. Mémoire sur les Cérites fossiles des terrains secondaires du Calvados. Mémoires de la Société Linnéenne de Normandie 7: 189–214.
Eudes-Deslongchamps, J.A. 1849. Mémoire sur les Pleurotomaires des terrains secondaires du Calvados. Mémoires de la Société Linnéenne de Normandie 8: 1–157.
Faber, A., and R. Weiss. 2005. Le Grès de Luxembourg: Intérêt scientifique et patrimonial de ses sites fossilifères. Ferrantia 44: 161–164.
Fallahi, M., B. Gruber, and G. Tichy. 1983. Gastropoden und Bivalven aus dem oberen Teil der Nayband-Formation (Obertrias) von Baqirabad (Isfahan, Iran). Schriftenreihe der Erdwissenschaftlichen Kommissionen 5: 57–82.
Ferrari, S.M., S.E. Damborenea, M.O. Manceñido, and M. Griffin. 2015. Early Jurassic Trochotomidae (Vetigastropoda, Pleurotomarioidea) from the Neuquén Basin, Argentina. Journal of Paleontology 89: 331–345.
Fischer, P. 1880–1887. Manuel de Conchyliologie et de Paléontologie Conchyliologique. Paris: Savy.
Fischer, J.-C., and C. Weber. 1997. Révision critique de la Paléontologie Française d’Alcide d’Orbigny (incluant la réédition de l'original). Volume II Gasteropodes Jurassiques. Paris: Masson.
Flügel, E. 1963. Zur Geologie der Sauwand bei Gußwerk (Steiermark). Mitteilungen Naturwissenschaftlicher Verein für Steiermark 93: 64–105.
Flügel, E. 1981. Palaeoecology and facies of Upper Triassic reefs in the Northern Calcareous Alps. SEPM Special Publication 30: 291–359.
Flügel, E. 2002. Triassic reef pattens. In Phanerozoic reef patterns, eds. W. Kiessling, E. Flügel, and J. Golonka. SEPM Special Publication 72: 391–461.
Flügel, E. 2004. Microfacies of carbonate rocks. Berlin, Heidelberg: Springer.
Flügel, E., and E. Flügel-Kahler. 1963. Mikrofazielle und geochemische Gliederung eines obertriadischen Riffes der Nördlichen Kalkalpen (Sauwand bei Gußwerk, Steiermark, Österreich). Mitteilungen des Museums für Bergbau, Geologie und Technik am Landesmuseum Joanneum 24 (1962): 1–129.
Frentzen, K. 1941. Die Foraminiferenfaunen des Lias, Doggers und unteren Malms der umgebung von Blumberg (Oberes Wutachgebiet). Beiträge zur Naturkundlichen Forschung im Oberrheingebiet 6: 125–402.
Frýda, J., and R.B. Blodgett. 2001. Chulitnacula, a new paleobiogeographically distinctive gastropod genus from Upper Triassic strata in accreted terranes of southern Alaska. Journal of the Czech Geological Society 46: 299–306.
Fürsich, F.T., and J. Wendt. 1977. Biostratinomy and Palaeoecology of the Cassian Formation (Triassic) of the Southern Alps. Palaeogeography, Palaeoclimatology, Palaeoecology 22: 257–323.
Galácz, A., and J. Szabó. 2001. Toarcian gastropods from the Gerecse Mts (Hungary). Fragmenta Palaeontologica Hungarica 19: 15–24.
Gale, L., R. Rettori, R. Martini, A. Šmuc, T. Kolar-JurkovŠek, and B. Rozic. 2011. Duostominidae (Foraminifera, Robertinida) from the Upper Triassic beds of the Slovenian Basin (Southern Alps, Slovenia). Rivista Italiana di Paleontologia e Stratigrafia 117 (3): 375–397.
Gale, L., T. Kolar-JurkovŠek, A. Šmuc, and B. Rozic. 2012. Integrated Rhaetian foraminiferal and conodont biostratigraphy from the Slovenian Basin, eastern Southern Alps. Swiss Journal of Geosciences 105: 435–462.
Gemmellaro, G.G. 1872–1882. Alcune Faune Giuresi e Liasiche della Sicilia. Milano: The author, 434 pp.
Goetel, M.W. 1917. Die rhätische Stufe und der untere Lias der subtatrischen Zone in der Tatra. Bulletin international de l´academie des sciences de Cracovie - Classe des sciences mathématiques et naturelles, série A: sciences mathématiques, Nov.–Dez. 1916: 1–222.
Goldfuss, A. 1833–1840. Petrefacta Germaniae. Abbildungen und Beschreibungen der Petrefacten Deutschlands und der angränzenden Länder, unter Mitwirkung des Herrn Grafen Georg zu Münster, herausgegeben von August Goldfuss. Zweiter Teil. Düsseldorf: Arnz and Co.
Gradstein, F., J. Ogg, and A. Smith. 2004. A geologic timescale. Cambrige: Cambridge University Press.
Gray, J.E. 1847. A list of genera of Recent Mollusca, their synonyms and types. Proceedings of the Zoological Society of London 15: 129–219.
Gray, J.E. 1854. A revision of the arrangement of the families of bivalve shells (Conchifera). Annals and Magazine of Natural History, Series 2 (13): 408–418.
Greene, S.E., R.C. Martindale, K.A. Ritterbush, D.J. Bottjer, F.A. Corsetti, and F.A. Berelson. 2012. Recognising ocean acidification in deep time: An evaluation of the evidence for acidification across the Triassic–Jurassic boundary. Earth Science Reviews 113: 72–93.
Grobben, K. 1894. Zur Kenntnis der Morphologie, der Verwandtschaftsverhältnisse und des systems der mollusken. Sitzungsberichte der Akademie der Wissenschaften, Mathematisch-Naturwissenschaftliche Klasse Wien 103: 61–86.
Gründel, J. 1976. Zur Taxonomie und Phylogenie der Bittium-gruppe. Malakologische Abhandlungen, Staatliches Museum für Tierkunde in Dresden 3: 33–59.
Gründel, J. 1980. Zur Gastropodenfauna des Unteren Muschelkalkes im Raum Halle-Unstruttal (DDR). Freiberger Forschungshefte C 34: 41–59.
Gründel, J. 1998. Archaeo- und Caenogastropoden aus dem Dogger Deutschlands und Nordpolens. Stuttgarter Beiträge Zur Naturkunde, Serie B 260: 1–39.
Gründel, J. 2000. Archaeogastropoda aus dem Dogger Norddeutschlands und des nordwestlichen Polens. Berliner Geowissenschaftliche Abhandlungen Reihe E 34: 205–253.
Gründel, J. 2003. Gastropoden aus dem Unteren Lias (Ober-Hettangium bis Unter-Sinemurium) Südwestdeutschlands. Stuttgarter Beiträge zur Naturkunde. Serie B (Geologie und Paläontologie) 340: 1–55.
Gründel, J. 2007a. Gastropoden aus dem unteren Pliensbachium von Feuguerolles (Normandie, Frankreich). Freiberger Forschungshefte C 524: 1–34.
Gründel, J. 2007b. Jurassische Gastropoden aus der Betakalkbank (oberes Sinemurium, obere Obtusum-Zone) Südwestdeutschlands. Stuttgarter Beiträge Zur Naturkunde, Serie B 370: 1–29.
Gründel, J. 2010. Neubeschreibung der Gastropodenfauna aus dem Hettangium (unterster Jura) des Kanonenberges bei Halberstadt (Deutschland). Beringeria 41: 3–24.
Gründel, J. 2011. Die Ptychomphalidae Wenz, 1938, (Ptychomphaloidea, Gastropoda) im Jura. Freiberger Forschungshefte C 539: 59–69.
Gründel, J., and A. Kaim. 2006. Shallow-water gastropods from Late Oxfordian sands on Kleby (Pomeriana, Poland). Acta Geologica Polonica 56: 121–157.
Gründel, J., H. Keupp, and F. Lang. 2015. Die Arten der Unterklasse Neritimorpha Koken, 1896 (Gastropoda) aus der Korallenfazies des oberen Kimmeridgiums (oberer Jura) von Saal bei Kelheim und dem Gebiet Nattheim (Süddeutschland). Zitteliana A 55: 77–106.
Gründel, J., H. Keupp, and F. Lang. 2019. Arten der Unterklasse Caenogastropoda aus der Korallenfazies des oberen Kimmeridgiums (Ober-Jura) von Saal bei Kelheim und dem Gebiet Nattheim (Süddeutschland). Zitteliana 93: 97–142.
Gugenberger, O. 1933. Die Cardita-Schichten von Launsdorf in Mittelkärnten und ihre Fauna II. Gastropoden. Sitzungsberichte der Akademie der Wissenschaften in Wien, mathematisch-naturwissenschaftliche Klasse, Abteilung 1 142: 157–184.
Gürich, G. 1934. Die Leitfossilien. Ein Hilfsbuch zum Bestimmen von Versteinerungen bei geologischen Arbeiten in der Sammlung und im Felde. Siebente Lieferung: Wirbellose des Jura von E. Dacqué. Berlin: Borntraeger.
Guzhov, A.V. 2004. Jurassic gastropods of European Russia (orders Cerithiiformes, Bucciniformes, and Epitoniiformes). Paleontological Journal 36 Supplement 5: S457–S592.
Haas, O. 1953. Mesozoic invertebrate faunas of Peru. Bulletin of the American Museum of Natural History 101: 1–328.
Haber, G. 1932. Fossilium Catalogus, Pars 53, Gastropoda, Amphineura et Scaphopoda jurassica. Berlin: I.W. Junk.
Hallam, A., and P.B. Wignall. 1997. Mass extinctions and their aftermath. Oxford: Oxford University Press.
Harasewych, M.G. 2002. Pleurotomarioidean gastropods. Advances in Marine Biology 42: 237–294.
Harasewych, M.G., and S. Kiel. 2007. Upper Jurassic Pleurotomariidae (Gastropoda) from southwestern Madagascar. The Nautilus 121: 76–89.
Harzhauser, M., and S. Schneider. 2014. A new family of giant Jurassic–Cretaceous littorinoid gastropods from the northern Tethys shelf. Acta Palaeontologica Polonica 59: 367–378. https://doi.org/10.4202/app.2011.0196.
Hasibuan, F., and J.A. Grant-Mackie. 2007. Triassic and Jurassic Gastropods from the Misool Archipelago, Eastern Indonesia. Jurnal Sumber Daya Geologi XVII (4): 257–272.
Hausmann, I.M., A. Nützel, V.J. Roden, and M. Reich. 2021. Diversity and palaeoecology of Late Triassic invertebrate assemblages from the tropical marine basins near Lake Misurina (Dolomites, Italy). Acta Palaeontologica Polonica 66: 143–192.
Hautmann, M. 2001. Die Muschelfauna der Nayband-Formation (Obertrias, Nor-Rhät) des östlichen Zentraliran. Beringeria 29: 1–181.
Hautmann, M. 2004. Effect of end-Triassic CO2 maximum on carbonate sedimentation and marine mass extinction. Facies 50: 257–261.
Hautmann, M. 2021. Extinction: end-Triassic mass extinction. eLS 2: 1–15. https://doi.org/10.1002/9780470015902.a0029378
Hautmann, M., M. Benton, and A. Tomasových. 2008a. Catastrophic ocean acidification at the Triassic–Jurassic boundary. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 249: 119–127.
Hautmann, M., F. Stiller, Cai Huawei, and Sha Jingeng. 2008b. Extinction-recovery pattern of level-bottom faunas across the Triassic-Jurassic boundary in Tibet: Implications for potential killing mechanisms. Palaios 23: 711–718.
Hébert, E., and E. Eudes-Deslongchamps. 1860. Mémoire sur les fossiles de Montreuil-Bellay (Maine-et-Loire). 1re. partie. Céphalopodes et Gastéropodes. Bulletin de la Société Linnéenne de Normandie 5: 153–240.
Herrmannsen, A.N. 1846–1852. Indicis Generum Malacozoorum primordia. Cassel: Fischer.
Hertwig, C.W.T.R. 1895. Lehrbuch der Zoologie, 3rd ed. Jena: Fischer.
Hong-fu, Yin, and E.L. Yochelson. 1983. Middle Triassic Gastropoda from Qingyan, Guizhou Province, China: 2—Trochacea and Neritacea. Journal of Paleontology 57: 515–538.
Hörnes, M. 1855. Über die Gastropoden und Acephalen der Hallstätter Schichten. Denkschriften der Kaiserlichen Akademie der Wissenschaften, Mathematisch Naturwissenschaftliche Classe 9: 35–54.
Hörnes, M. 1856. Gastropoden aus der Trias der Alpen. Denkschriften der Kaiserlichen Akademie der Wissenschaften, Mathematisch Naturwissenschaftliche Classe 12: 21–34.
Hua-zhang, Pan. 1977. Mesozoic and Cenozoic Fossil Gastropoda from Yunnan. Mesozoic Fossils from Yunnan, China 2: 83–188.
Hua-zhang, Pan. 1982. Triassic marine gastropods from SW China. Bulletin of the Nanjing Institute of Geology and Paleontology 4: 153–188.
Ichikawa, K. 1958. Zur Taxonomie und Phylogenie der triadischen “Pteriidae” (Lamellibranch.). Palaeontographica, Abteilung A 111: 131–212.
Jekelius, E. 1935. Der weiße Triaskalk von Brasov und seine Fauna. Anuarul Institutului Geologic al Romdniei 17: 1–109.
Kaim, A. 2004. The evolution of conch ontogeny in Mesozoic open sea gastropods. Palaeontologia Polonica 62: 1–182.
Karapunar, B., and A. Nützel. 2021. Slit-band gastropods (Pleurotomariida) from the Late Triassic Cassian Formation and their diversity dynamics. Zootaxa 5042 (1): 1–165.
Karapunar, B., A. Nützel, B. Seuss, and R.H. Mapes. 2022. Taxonomy and diversity of slit-band gastropods (order Pleurotomariida) from the Pennsylvanian of the USA. Papers in Palaeontology. https://doi.org/10.1002/spp2.1417.
Kendall, A.C. 1985. Radiaxial fibrous calcite: a reappraisal. In Carbonate Cements, eds. N. Schneiderman, and P.M. Harris. Society for Economic Paleontologists and Mineralogists, Special Publication 36: 59–77.
Kiel, S., and K. Bandel. 2000. New slit-bearing Archaeogastropoda from the Late Cretaceous of Spain. Berliner Geowissenschaftliche Abhandlungen, Reihe E 34: 269–277.
Kiel, S., K. Bandel, N. Banjac, and M.D.C. Perilliat. 2000. On Cretaceous Campanilidae (Caenogastropoda, Mollusca). Freiberger Forschungshefte C 490: 15–26.
Kiessling, W. 2005. Long-term relationship between ecological stability and biodiversity in Phanerozoic reefs. Nature 433: 410–413.
Kiessling, W., and M. Aberhan. 2007. Environmental determinants of marine benthic biodiversity dynamics through Triassic–Jurassic time. Paleobiology 33: 414–434.
Kiessling, W., M. Aberhan, B. Brenneis, and P.J. Wagner. 2007. Extinction trajectories of benthic organisms across the Triassic–Jurassic boundary. Palaeogeography, Palaeoclimatology, Palaeoecology 244: 201–222.
Kiessling, W., E. Roniewicz, L. Villier, P. Léonide, and U. Struck. 2009. An early Hettangian coral reef in southern France: Implications for the end-Triassic reef crisis. Palaios 24: 657–671.
Kittl, E. 1891. Die Gastropoden der Schichten von St. Cassian der südalpinen Trias. I. Theil. Annalen des Kaiserlich-Königlichen Naturhistorischen Hofmuseums 6: 166–262.
Kittl, E. 1892. Die Gastropoden der Schichten von St. Cassian der südalpinen Trias. II. Theil. Annalen des Kaiserlich-Königlichen Naturhistorischen Hofmuseums 7: 35–97.
Kittl, E. 1894. Die Gastropoden der Schichten von St. Cassian der südalpinen Trias. III. Theil. Annalen des Kaiserlich-Königlichen Naturhistorischen Hofmuseums 9: 143–277.
Kittl, E. 1899. Die Gastropoden der Esinokalke, nebst einer Revision der Gastropoden der Marmolatakalke. Annalen des Kaiserlich-Königlichen Naturhistorischen Hofmuseums 14: 1–237.
Kittl, E. 1900. Trias-Gastropoden des Bakonyer Waldes. Resultate der wissenschaftlichen Erforschung des Balatonsees: 1–57.
Kittl, E. 1908. Beiträge zur Kenntnis der Triasbildungen der nordöstlichen Dobrudscha. Denkschriften der mathematisch-naturwissenschaftlichen Klasse der kaiserlichen Akademie der Wissenschaften 81: 447–532.
Knight, R.I., and N.J. Morris. 2020. Revision of the British Lower Jurassic Arcoidea (Bivalvia): Palaeobiological, palaeoecological and evolutionary implications. Historical Biology 32 (9): 1176–1205.
Knight, J.B., L.R. Cox, A.M. Keen, R.L. Batten, E.L. Yochelson, and R. Robertson. 1960. Systematic descriptions. In Treatise of Invertebrate Palaeontology, Part I, Mollusca 1, ed. R.C. Moore, I169–I310. Lawrence: Geological Society of America University Kansas Press.
Koken, E. 1892. Ueber die Gastropoden der rothen Schlernschichten nebst Bemerkungen über Verbreitung und Herkunft einiger triassischer Gattungen. Neues Jahrbuch für Mineralogie, Geologie und Paläontologie 2: 25–36.
Koken, E. 1896. Die Gastropoden der Trias um Hallstatt. Jahrbuch der Kaiserlich-Königlichen Geologischen Reichsanstalt 46: 37–126.
Koken, E. 1897. Die Gastropoden der Trias um Hallstatt. Abhandlungen der Kaiserlich-Königlichen Geologischen Reichsanstalt 17: 1–112.
Koken, E. 1898. Beiträge zur Kenntnis der Gastropoden des süddeutschen Muschelkalkes. Abhandlungen zur Geologischen Specialkarte von Elsass-Lothringen, Neue Folge 2: 1–49.
Kollarova-Andrusova, V., and M. Kochanova. 1973. Die Molluskenfauna des Bleskovy Pramen bei Drnava (Nor, Westkarpaten). Slovenskej Akademie vied, Bratislava: 1–215.
Koshelkina, Z.V. 1957. Paleontological substantiation of the stage subdivision of Jurassic marine deposits of the Vilyii Depression and Cis-Verkhoyansk Foredeep, in Tr. Mezhved. soveshch. po razrabotke unifitsirovannykh stratigr. skhem Sibiri 1956 g. Doklady po stratigrafii mezozoiskikh i kainozoiskikh otlozhenii (Resolutions of Interdisciplinary Meeting on Development of Unified Stratigraphic Schemes of Siberia, 1956. Reports on Stratigraphy of Mesozoic and Cenozoic Deposits), Leningrad: Gostoptekhizdat, 1957: 38–45.
Kramm, E. 2004. Fauna der unteren Schaumkalkbank (Trias, Anis) von Rodges (Hessen). Beiträge zur Naturkunde in Osthessen 39: 73–82.
Kristan, E. 1957. Ophthalmidiidae und Tetraxinae (Foraminifera) aus dem Rhät der Hohen Wand in Nieder-Österreich. Jahrbuch der Geologischen Bundesanstalt, Sonderabdruck 100 Heft 2: 269–298.
Kristan-Tollmann, E. 1960. Rotaliidea (Foraminifera) aus der Trias der Ostalpen. Jahrbuch Geologische Bundesanstalt, Sonderband 5: 47–78.
Kristan-Tollmann, E. 1964. Die Foraminiferen aus den rhätischen Zlambachmergeln der Fischerwiese bei Aussee im Salzkammergut. Jahrbuch Geologische Bundesanstalt, Sonderband 10: 1–189.
Krumbeck, L. 1923. Geologische Ergebnisse der Reisen K. Deningers in den Molluken. III. Brachiopoden, Lamellibranchiaten und Gastropoden aus der Oberen Trias der Insel Seran. Palaeontographica, Supplement IV III: 186–246.
Krumbeck, L. 1924. XXII. Die Brachiopoden, Lamellibranchiaten und Gastropoden der Trias von Timor II. In Paläontologie von Timor, XIII. Lieferung, ed. J. Wanner, 38–90. Stuttgart: Schweizerbart.
Krystyn, L., G.W. Mandl, and M. Schauer. 2009. Growth and termination of the Upper Triassic platform margin of the Dachstein area (Northern Calcareous Alps, Austria). Austrian Journal of Earth Sciences 101: 23–33.
Kühn, O. 1942. Zur Kenntnis des Rhät von Vorarlberg. Mitteilungen des Alpenländischen Geologischen Vereines (Mitteilungen der Geologischen Gesellschaft in Wien) 33: 111–147.
Kutassy, A. 1927. Beiträge zur Stratigraphie und Paläontologie der alpinen Triasschichten in der Umgebung von Budapest. Magyar kir. Földtani Intézet Évkönyve 27: 1–71 [107–175].
Kutassy, A. 1932. Újabb adatok a Budapest-Környéki Dachsteini Mészkö Faunájának ismeretéhez [Weitere Beiträge zur Kenntnis der Fauna des Dachsteinkalkes in der Umgebung von Budapest]. Magyar Tudományos Akadémia Matematikai És Termézettudományi Értesítöje [Mathematischer und Naturwisssenschaftlicher Anzeiger der Ungarischen Akademie der Wissenschaften] 49: 222–250.
Kutassy, A. 1934. Die Fauna des Norischen Dachsteinkalkes von St. Anna bei Neumarktl (Oberkrain). Földtani Közlöny 64: 65–80.
Kutassy, A. 1937. Triadische Faunen aus dem Bihar-Gebirge. Geologica Hungarica, Series Palaeontologica 13: 1–80.
Lathuilière, B., and D. Marchal. 2009. Extinction, survival and recovery of corals from the Triassic to Middle Jurassic time. Terra Nova 21 (1): 57–66.
Leischner, W. 1961. Zur Kenntnis der Mikrofauna und -flora der Salzburger Kalkalpen. Neues Jahrbuch Für Geologie Und Paläontologie, Abhandlungen 112: 1–47.
Leonardi, P. 1935. Il Trias inferiore delle Venezie. Memoirie degli Istituti de Geologia e Mineralogia dell´ Università di Padova 11: 1–136.
Leonardi, P., and F. Fiscon. 1959. La Fauna Cassiana di Cortina D’Ampezzo. Memorie degli Istituti di Geologia e Mineralogia Dell’università di Padova 21: 1–103.
Lindström, A., and J.S. Peel. 2010. Shell repair and shell form in Jurassic pleurotomarioid gastropodes from England. Bulletin of Geosciences 85 (4): 541–550.
Linnaeus, C. 1758. Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis. Tomus I. Editio Decima. Stockholm: Laurentius Salvius.
Lycett, J. 1848. Notes on the distribution of the fossil conchology of the Oolitic formations in the vicinity of Minchinhampton, Gloucestershire. Annals and Magazine of Natural History 2: 248–259.
Mandl, G.W. 2000. The Alpine sector of the Tethys shelf—Examples of Triassic to Jurassic sedimentation and deformation from the Northern Calcareous Alps. Mitteilungen der Österreichischen Geologischen Gesellschaft 92: 61–77.
McLean, J.H., and M.G. Harasewych. 1995. Review of western Atlantic species of cocculinid and pseudococculinid limpets, with descriptions of new species (Gastropoda: Cocculiniformia). Contributions in Science, Natural History Museum of Los Angeles County 453: 1–33.
McRoberts, C.A., L. Krystyn, and M. Hautmann. 2012. Macrofaunal response to the end-Triassic mass extinction in the West-Tethyan Kössen Basin Austria. Palaios 27 (9): 607–616.
Meek, F.B. 1864. Check list of the invertebrate fossils of North America. Cretaceous and Jurassic. Smithsonian Miscellaneous Collection 177: 1–40.
Meek, F.B., and F.V. Hayden. 1860. Systematic catalogue of Jurassic, Cretaceous and Tertiary fossils collected in Nebraska. Proceedings of the Academy of Natural Sciences of Philadelphia 12: 417–433.
Meier, H., and K. Meiers. 1988. Die Gastropodenfauna der Angulata-Zone des Steinbruchs Reckingerwald bei Brouch. Musée National d'Histoire Naturelle, Luxembourg, Travaux scientifiques du Mnhn Tome XIII: 1–88.
Monari, S., M. Valentini, and M.A. Conti. 2011. Earliest Jurassic patellogastropod, vetigastropod, and neritimorph gastropods from Luxembourg with considerations on the Triassic–Jurassic faunal turnover. Acta Palaeontologica Polonica 56: 349–384.
Monari, S., R. Gatto, and M. Valentini. 2018. Vetigastropoda and Neritimorpha from the Lower Bajocian of Luxembourg and palaeobiogeography of Aalenian-Bajocian (Middle Jurassic) gastropods of western Europe. Journal of Systematic Palaeontology 16: 449–492.
Moosleitner, G. 2004. Fossilien sammeln im Salzburger Land. Wiebelsheim: Edition Goldschneck, Quelle & Meyer.
Müller, O.F. 1776. Zoologiae Danicae Prodromus, seu Animalium Daniae et Norvegiae Indigenarum Characteres, Nomina, et Synonyma Imprimis Popularium. Impenis Auctoris. Hallageriis, Havniae (Copenhagen). xxxii + 282 p.
Münster, G. von. 1841. Beschreibung und Abbildung der in den Kalkmergelschichten von St. Cassian gefundenen Versteinerungen. In Beiträge zur Geognosie und Petrefacten-Kunde des Südöstlichen Tirol’s vorzüglich der Schichten von St. Cassian Heft 4, eds. H.L. Wissmann, G. von Münster, and K.F. Braun. Bayreuth: Buchner.
Neumayr, M. 1884. Zur Morphologie des Bivalvenschlosses. Sitzungsberichte der Akademie der Wissenschaften in Wien, Mathematisch-Naturwissenschaftliche Klasse, Abteilung 1, 88: 385–418.
Neumayr, M. 1891. Beiträge zu einer morphologischen Einteilung der Bivalven. Denkschriften der Mathematisch-Naturwissenschaftliche Classe der Kaiserlichen Akademie der Wissenschaften Wien 58: 701–801.
Newell, N.D. 1965. Classification of the Bivalvia. American Museum Novitates 2206: 1–25.
Nützel, A. 2005a. A new Early Triassic gastropod genus and the recovery of gastropods from the Permian/Triassic extinction. Acta Palaeontologica Polonica 50: 19–24.
Nützel, A. 2005b. Recovery of gastropods in the Early Triassic. Comptes Rendus Palevol 4: 501–515.
Nützel, A., and D.H. Erwin. 2004. Late Triassic (Late Norian) gastropods from the Wallowa Terrane (Idaho, USA). Paläontologische Zeitschrift 78: 361–416.
Nützel, A., and J. Gründel. 2015. Early Jurassic (Pliensbachian) gastropods from Franconia, South Germany. Palaeontographica Abteilung A 305: 1–87.
Nützel, A., and K. Nakazawa. 2012. Permian (Capitanian) gastropods from the Akasaka Limestone (Gifu Prefecture, Japan). Journal of Systematic Palaeontology 10: 103–169.
Nützel, A., and B. Senowbari-Daryan. 1999. Gastropods from the Upper Triassic (Norian-Rhaetian) Nayband Formation of central Iran. Beringeria 23: 93–132.
Nützel, A., A. Hamedani, and B. Senowbari-Daryan. 2003. Some Late Triassic Gastropods from the Nayband Formation in Central Iran. Facies 48: 127–134.
Nützel, A., M. Mannani, B. Senowbari-Daryan, and M. Yazdi. 2010. Gastropods from the Late Triassic Nayband Formation (Iran), their relationships to other Tethyan faunas and remarks on the Triassic gastropod body size problem. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 256 (2): 213–228.
Nützel, A., B. Aghababalou, and B. Senowbari-Daryan. 2012. Gastropods from the Norian (Late Triassic) Nayband Formation near Natanz (Iran). Bulletin of Geosciences 87: 53–65.
Orbigny, A. D’. 1849–1852. Prodrome de paléontologie stratigraphique universelle des animaux mollusques et rayonnés. Paris: V. Masson.
Orbigny, A. D’. 1851–1860. Paléontologie Française, terrains jurassique. Tom II. Gasteropodes. Paris: V. Masson.
Osswald, K. 1930. Über einige Rätfossilien aus dem Risserkogelgebiet (südlich Tegernsee). Jahrbuch der Preußischen Geologischen Landesanstalt zu Berlin für das Jahr 1929 50 Teil 3: 733–750.
Pieroni, V., S. Monari, and J.A. Todd. 2021. A new caenogastropod from the upper Rhaetian of Lombardy: Palaeobiogeographical history and implications for the Early Jurassic gastropod recovery. Acta Palaeontologica Polonica 66: 193–206.
Plöchinger, B. 1973. Erläuterungen zur Geologischen Karte des Wolfgangseegebietes 1:25.000. Wien: Geologische Bundesanstalt.
Plöchinger, B. 1982. Erläuterungen zu Blatt 95 Sankt Wolfgang im Salzkammergut. Wien: Geologische Bundesanstalt.
Ponder, W.F., D.J. Colgan, J.M. Healy, A. Nützel, L.R.L. Simone, and E.E. Strong. 2008. Caenogastropoda. In Phylogeny and evolution of the Mollusca, ed. W.F. Ponder and D.L. Lindberg, 331–383. Berkeley, Los Angeles, London: University of California Press.
Portlock, J. 1843. Reports on the Geology of the County of Londonderry, and of parts of Tyrone and Fermanagh. Dublin: Milikan and Longmans.
Pyron, R.A., G.C. Costa, M.A. Patten, and F.T. Burbrink. 2015. Phylogenetic niche conservatism and the evolutionary basis of ecological speciation. Biological Reviews 90 (4): 1248–1262.
Quenstedt, F.A. 1858. Der Jura. Tübingen: Laupp.
Rafinesque, C.S. 1815. Analyse de la Nature, ou Tableau de l’univers et des corps organisés. Palermo: The author.
Richardson, G.F. 1843. Geology for beginners. London: Longman, Brown, Green and Longmans.
Riedel, F. 2000. Ursprung und Evolution der “höheren” Caenogastropoda. Berliner Geowissenschaftliche Abhandlungen, Reihe E 26: 1–240.
Roden, V., I.M. Hausmann, A. Nützel, B. Seuss, M. Reich, M. Urlichs, H. Hagdorn, and W. Kiessling. 2020. Fossil liberation: A model to explain high biodiversity in the Triassic Cassian Formation. Palaeontology 63: 85–102. https://doi.org/10.1111/pala.124418.
Röding, P.F. 1798. Museum Boltenianum sive Catalogus Cimeliorum e Tribus Regnis Naturae quae olim Collegerat Joa. [Joachim] Fried. Bolten.. Pars Secunda Continens Conchylia sive Testacea Univalvia, Bivalvia, & Multivalvia. Hamburg: Johan. Christi. Trappii.
Roemer, F.A. 1841. Die Versteinerungen des Norddeutschen Kreidegebirges. Hannover: Hahn.
Sachariewa-Kowatschewa, K. 1962. Die Trias von Kotel (Ost-Balkan). II. Teil. Scaphopoden und Gastropoden. Annuaire de l’Université de Sofia, Faculté de Biologie, Géologie et Géographie, Livre 2. Géologie 55: 91–140.
Salomon, W. 1895. Geologische und palaeontologische Studien über die Marmolata. Palaeontographica 42: 1–210.
Schäfer, P. 1979. Fazielle Entwicklung und palökologische Zonierung zweier obertriadischer Riffstrukturen in den Nördlichen Kalkalpen (“Oberrhät”-Riff-Kalke, Salzburg). Facies 1: 3–245.
Schäfer, P. 1984. Development of ecologic coral reefs during the latest Triassic (Rhaetian) of the Northern Limestone Alps. Palaeontographica Americana 54: 210–218.
Schäfer, P., and B. Senowbari-Daryan. 1978a. Neue Korallen (Scleractinia) aus Oberrhät-Riffkalken südlich von Salzburg (Nördliche Kalkalpen, Österreich). Senckenbergiana Lethaea 59: 117–135.
Schäfer, P., and B. Senowbari-Daryan. 1978b. Die Häufigkeitsverteilung der Foraminiferen in drei oberrhätischen Riff-Komplexen der Nördlichen Kalkalpen (Salzburg, Österreich). Verhandlungen Geologische Bundesanstalt 1978 (2): 73–96.
Schäfer, P., and B. Senowbari-Daryan. 1981. Facies development and paleoecological zonation of four Upper Triassic patch-reefs, Northern Calcareous Alps near Salzburg, Austria. SEPM Special Publication 30: 241–259.
Schlagintweit, F. 2011. Taxonomic revision of Late Triassic “Lithocodium aggregatum Elliott” (Northern Calcareous Alps, Austria). Jahrbuch Geologische Bundes-Anstalt 151 (3–4): 375–396.
Schlagintweit, F., T. Bover-Arnal, and S. Salas. 2010. New insights into Lithocodium aggregatum Elliott 1956 and Bacinella irregularis Radoicic 1959 (Late Jurassic-Lower Cretaceous): Two ulvophycean green algae (?Order Ulotrichales) with a heteromorphic life cycle (epilithic/euendolithic). Facies 56: 509–547.
Schmid, D.U. 1995. “Tubiphytes” morronensis - eine fakultativ inkrustierende Foraminifere mit endosymbiotischen Algen. Profil 8: 305–317.
Schmid, D.U., and R.R. Leinfelder. 1996. The Jurassic Lithocodium aggregatum-Troglotella incrustans foraminiferal consortium. Palaeontology 39: 21–52.
Schnetzer, R. 1934. Die Muschelkalkfauna des Öfenbachgrabens bei Saalfelden. Palaeontographica Abteilung A 81: 1–159.
Senowbari-Daryan, B. 1980. Fazielle und paläontologische Untersuchungen in oberrhätischen Riffen (Feichtenstein- und Gruberriff bei Hintersee, Salzburg, Nördliche Kalkalpen). Facies 3: 1–237.
Senowbari-Daryan, B. 2013. Tubiphytes Maslov, 1956 and description of similar organisms from Triassic reefs of the Tethys. Facies 59: 75–112.
Senowbari-Daryan, B., I. Bucur, F. Schlagintweit, E. Sasaran, and J. Matyszkiewicz. 2008. Crescentiella, a new name for “Tubipyhtes” morronensis Crescenti, 1969: An enigmatic Jurassic-Cretaceous microfossil. Geologia Croatica 61: 185–214.
Sepkoski, J.J. 2002. A compendium of fossil marine animal genera. Bulletins of American Paleontology 363: 1–560.
Smettan, K. 1997. Bivalven, Gastropoden und Serpuliden aus den Branderfleckschichten (Cenoman) der Fahrenbergmulde (Nördliche Kalkalpen, Bayern): Taxonomie und Palökologie. Zitteliana 21: 99–158.
Sowerby, J. 1812–1822. The mineral conchology of Great Britain. London: Meredith.
Stanton, R.J., and E. Flügel. 1987. Palecology of Upper Triassic Reefs in the Northern Calcareous Alps: Reef Communities. Facies 16: 157–186.
Stanton, R.J., and E. Flügel. 1995. An accretionary distally steepened ramp at an intrashelf basin margin: An alternative explanation for the Upper Triassic Steinplatte “reef” (Northern Calcareous Alps, Austria). Sedimentary Geology 95: 269–286.
Stoliczka, F. 1870–1871. Cretaceous fauna of southern India. 3. The Pelecypoda, with a review of all known genera of this class, fossil and Recent. Paleontologica Indica, Memoirs 6: 1–537.
Stoppani, A. 1858–1860. Paléontologie Lombarde ou description des fossiles de Lombardie. Les pétrifications d'Esino. Les gasteropodes, les acéphales, les brachiopodes, les céphalopodes, les crinoides, les zoophytes et les amorhozoaires. Milano: Joseph Bernadoni.
Stoppani, A. 1860–1865. Géologie et Paléontologie des Couches à Avicula contorta en Lombardie. Paléontologie Lombarde ou Description des Fossiles de Lombardie III. Milano: Joseph Bernardoni.
Swainson, W. 1840. A treatise on malacology; or shells and shell fish. London: Longman.
Szabó, J. 2011a. Corrections to three gastropod genera, established by Kutassy on Late Triassic type species from Dachstein Limestone localities of Budapest (Hungary). Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 261: 37–47.
Szabó, J. 2011b. A budapesti (Budai-hegység) felső-triász Dachsteini Mészkő legendás gastropoda-faunájának revíziója, ésgondolatok a típusgyűjtemény hányatott sorsa okán (A revision of the nomenclature of well-known gastropod fauna from the Upper Triassic Dachstein Limestone of Budapest (Buda Hills) and thoughts on fate of the type collection). Földtani Közlöni 141 (3): 217–232.
Szabó, J., M.A. Conti, S. Monari, and J. Wendt. 2021. Gastropods from the Jurassic neptunian sills of Rocca Busambra (north-western Sicily, Italy): Patellogastropoda, Pleurotomarioidea, Scissurelloidea, Fissurelloidea and Eucycloidea. Papers in Palaeontology 7: 27–110.
Tate, R. 1869. Contributions to Jurassic Palaeontology. 1. Cryptaulax, a new Genus of Cerithiadae. The Annals and Magazine of Natural History. Series 4, No. 24: 417–419.
Teppner, W. von. 1922. Lamellibranchia Tertiaria, Anisomyaria II. In Fossilium Catalogus, I. Animalia Pars 15, ed. C. Diener, 67–296. Berlin: W. Junk.
Terquem, O. 1855. Paléontologie de l´ étage inférieur de la formation Liasique de la Pronince de Luxembourg, Grand-Duché (Holland) et de Hettange du Departement de la Moselle. Mémoires de la Société géologique de France, 2(5): 29: 245–279.
Terquem, O., and E. Piette. 1865. Le Lias inférieur de l’est de la France comprenant la Meurthe, la Moselle, le Grand-Duché de France, la France et la Meuse. Mémoires de la Société géologique de France, 2ème séries 8. Mémoire 1: 1–175.
Tichy, G. 1979. Gastropoden aus den triassischen Hallstätterkalk-Blöcken von West-Timor (Indonesien). Beiträge Zur Paläontologie von Österreich 6: 119–133.
Tichy, G. 1980. Gastropoden aus dem Prezzokalk (Anis) von Lenna im Val Brembana(Südalpen, Italien). Verhandlungen der Geologischen Bundesanstalt Jahrgang 1979. Heft 3: 423–441.
Tollmann, A. 1985. Geologie von Österreich, vol. II. Wien: Deuticke.
Tommasi, A. 1896. Nuovi fossili triassici in Sardegna. Bollettino Della Societa Geologica Italiana 15: 497–503.
Tommasi, A. 1903. Revisione della Fauna a Molluschi di dolomia principale di Lombardia. Palaeontograhica Italica 9: 95–112.
Tracey, S., J.A. Todd, and D.H. Erwin. 1993. Mollusca: Gastropoda. In The Fossil Record 2, ed. M.J. Benton, 131–167 (chapter 8). London: Chapman & Hall.
Ulrich, E.O., and W.H. Scofield. 1897. The lower Silurian Gastropoda of Minnesota. The Geology of Minnesota: Paleontology 3: 813–1081.
Salvini-Plawen, L. von. 1980. A reconsideration of systematics in the Mollusca (phylogeny and higher classification). Malacologia 19: 249–278.
Schafhäutl, K.F.E. von. 1863. Südbayerns Lethea geognostica. Leipzig: Leopold Voss.
Wöhrmann, S. von, and E. Koken. 1892. Die Fauna der Raibler Schichten vom Schlernplateau. Zeitschrift Der Deutschen Geologischen Gesellschaft 44: 167–223.
Waagen, L. 1901. Der Formenkreis des Oxytoma inaequivalve Sowerby. Jahrbuch der Kaiserlich Königlichen Geologischen Reichsanstalt 51: 1–24.
Wagreich, M., F. Böhm, and H. Lobitzer. 1996. Sedimentologie des kalkalpinen Mesozoikums in Salzburg und Oberösterreich (Jura, Kreide). Exkursionsführer Sediment 96, 11. Sedimentologentreffen Wien 1996.
Waller, T.R. 1978. Morphology, morphoclines and a new classification of the Pteriomorphia (Mollusca: Bivalvia). Philosophical Transactions of the Royal Society of London Ser. B 284: 345–365.
Wasmer, M., M. Hautmann, E. Hermann, D. Ware, G. Roohi, K. Ur-Rehman, A. Yaseen, and H. Bucher. 2012. Olenekian (Early Triassic) bivalves from the Salt Range and Surghar Range Pakistan. Palaeontology 55 (5): 1043–1073.
Waterhouse, J.B. 2001. Late Paleozoic Brachiopoda and Mollusca chiefly from Wairaki Downs, New Zealand, with notes on Scyphozoa and Triassic ammonoids and new classifications of Linoproductoidea (Brachiopoda) and Pectinida (Bivalvia). Earthwise 3: 1–196.
Wenz, W. 1938–1944. Gastropoda, Teil I. In Handbuch der Paläozoologie 6, ed. O.H. Schindewolf. Berlin: Borntraeger.
Wilson, J.L. 1975. Carbonate facies in geologic history. Berlin, Heidelberg, New York: Springer.
Wolff, H. 1967. Zur Rät-fazies des östlichen Wendelstein-Gebietes. Mitteilungen der Bayerischen Staatssammlung für Paläontologie und Historische Geologie 7: 227–243.
Woodward, S.P. 1851–1856. A manual of the Mollusca; or, rudimentary treatise of Recent and fossil shells. London: Weale.
Yu, W., Pan, H.-Z., and Wang, H. 1974. Triassic gastropods. In The Stratigraphical and Paleontological Handbook of Southwestern China, ed. Nanjing Institute of Geology and Paleontology, Academia Sinica, 320–326. Beijing: Science Press.
Zakharov, V.A., A.P. Ippolitov, N.G. Zverkov, P.A. Beznosov, and D.N. Kiselev. 2020. Middle jurassic retroceramids and unionids from the Izhma River Basin, North of European Russia. Stratigraphy and Geological Correlation 28 (4): 381–401.
Zaninetti, L., D. Altiner, Z. Dager, and B. Ducret. 1982. Les Milioliporidae (Foraminiferes) dans le Trias superieur a facies recifal du Taurus, Turquie. I: Proposition pour une nouvelle subdivision. Revue de Paléobiologie 1: 93–100.
Zapfe, H. 1962. Beiträge zur Paläontologie der nordalpinen Riffe. Ein Massenvorkommen von Gastropoden im Dachsteinkalk des Tennengebirges. Salzburg. Annalen des Naturhistorischen Museums Wien 65: 57–69.
Zapfe, H. 1963. Beiträge zur Paläontologie der nordalpinen Riffe. Zur Kenntnis der Fauna der oberrhätischen Riffkalkes von Adnet, Salzburg (exkl. Riffbildner). Annalen des Naturhistorischen Museums Wien 66: 207–259.
Zapfe, H. 1967a. Untersuchungen im obertriadischen Riff des Gosaukammes (Dachsteingebiet, Oberösterreich) VIII. Verhandlungen der Geologischen Bundesanstalt 1967: 13–27.
Zapfe, H. 1967b. Beiträge zur Paläontologie der nordalpinen Riffe. Die Fauna der Zlambach-Mergel der Fischerwiese bei Aussee. Steiermark. Annalen des Naturhistorischen Museums Wien 71: 413–480.
Zittel, K.A. 1895. Grundzüge der Palaeozoologie. München: Oldenbourg.
Acknowledgements
We would like to thank the following persons: Gero Moosleitner helped during field work and provided access to private collections. Susana Ester Damborena and Stefano Monari provided helpful reviews. Simon Schneider and Mike Reich for helpful remarks and editorial corrections. We thank Philippe Bouchet for helping with the reversal of precedence for Angularia. Manuela Schellenberger assisted with photography. Elisabeth Lange prepared some of the studied specimens. Stefan Sónyi provided thin sections for facies and reef builder analysis. Alexander Lukeneder and Thomas Nichterl gave access to the collections at the NHMW. Walter Krispler gave us access to his private collection. Baran Karapunar provided valuable advice on the taxonomy of the pleurotomariidan species. János Szabó provided a photograph of the type specimen of Purpuroidea ferenczii. Vittorio Pieroni helped to clarify the identity of Trochotoma praecursor. The Deutsche Forschungsgemeinschaft (DFG) is acknowledged for the financial support (projects NU 96/13-1, NU 96/14-1). This research received support from the SYNTHESYS Project (http://www.synthesys.info/), which is financed by a European Community Research Infrastructure Action under the FP7 “Capacities” Program (AT TAF-1797) and provided a grant to AN for a collection visit to NHMW.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Simon Schneider.
This article is registered in Zoobank under https://zoobank.org/References/918a7d0d-cc94-451e-92a6-fd0af11e8316.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nützel, A., Nose, M., Hautmann, M. et al. Latest Triassic (Sevatian–Rhaetian) reef carbonates from the Northern Calcareous Alps (Austria), their mollusc dwellers, and their fate at the end-Triassic extinction event. PalZ 97, 265–309 (2023). https://doi.org/10.1007/s12542-022-00631-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12542-022-00631-9