Abstract
Climate change effects on coastal ecosystems vary on large spatial scales, but can also be highly site dependent at the regional level. The Wadden Sea in the south-eastern North Sea is warming faster than many other temperate coastal areas, with surface seawater temperature increasing by almost 2 °C over the last 60 years, nearly double the global ocean mean increase. Climate warming is accompanied by rising sea levels, which have increased by approximately 2 mm yr−1 over the last 120 years. For this sedimentary coast, the predicted acceleration of sea-level rise will have profound effects on tidal dynamics and bathymetry in the area. This paper synthesises studies of the effects of ocean warming and sea level rise in the northern Wadden Sea, largely based on research conducted at the Wadden Sea Station Sylt of the Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research. An increasing rate of sea level rise above a critical threshold will lead to coastal erosion and changes in sediment composition, and may cause the transition from a tidal to lagoon-like environment as tidal flats submerge. This involves changes to coastal morphology, and the decline of important habitats such as muddy tidal flats, salt marshes and seagrass meadows, as well as their ecological services (e.g. carbon sequestration). Ocean warming affects plankton dynamics and phenology, as well as benthic community structure by hampering cold-adapted but facilitating warm-adapted species. The latter consist mostly of introduced non-native species originating from warmer coasts, with some epibenthic species acting as ecosystem engineers that create novel habitats on the tidal flats. Warming also changes interactions between species by decoupling existing predator–prey dynamics, as well as forming new interactions in which mass mortalities caused by parasites and pathogens can play an understudied but essential role. However, Wadden Sea organisms can adapt to changing abiotic and biotic parameters via genetic adaptation and phenotypic plasticity, which can also be inherited across generations (transgenerational plasticity), enabling faster plastic responses to future conditions. Important research advances have been made using next-generation molecular tools (-omics), mesocosm experiments simulating future climate scenarios, modelling approaches (ecological network analysis), and internet-based technologies for data collection and archiving. By synthesising these climate change impacts on multiple levels of physical and biological organisation in the northern Wadden Sea, we reveal knowledge gaps that need to be addressed by future investigations and comparative studies in other regions in order to implement management, mitigation and restoration strategies to preserve the uniqueness of this ecosystem of global importance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since its formation after the last ice age about 8000 years ago, the Wadden Sea has been subject to strong abiotic fluctuations in temperature, hydrography, sediment delivery and storm frequency on a seasonal, inter-annual and decadal basis. To this day, the tidal flats of the Wadden Sea continue to be shaped by these dynamics, which pose a challenge to species inhabiting them. Species colonised the Wadden Sea from surrounding areas (no endemic species occur), and due to its comparatively young age and the prevailing variable abiotic conditions, species diversity in the Wadden Sea is comparatively low (Buschbaum and Horn 2024). With the onset of the Anthropocene, the coast of the Wadden Sea was increasingly exposed to human impacts. Initial impacts resulting from agriculture, land reclamation and the construction of dikes and coastal defences have been magnified by industrialised fishing, eutrophication, pollution and impacts of tourism. These regional pressures have affected the Wadden Sea for centuries and continue to do so today. At present, they are being exacerbated by climate change as an additional and globally acting pressure, with rapid warming of seawater (Amorim et al. 2023) and sea level rise being of particular importance.
The current complexity of regional and global human impacts on the Wadden Sea necessitates research foci that assess the role of these factors in ecological functioning and ecosystem services in order to develop and adapt management strategies and mitigation measures. That climate change would become an important additional issue for these shallow coasts was recognised relatively early by the scientific community, as ocean warming and sea level rise were already mentioned in the 1993 Wadden Sea Quality Status Report (QSR 1993). However, it took another 15 years before there was a marked increase in scientific publications about climate change effects in the Wadden Sea. Today, impacts of climate change are considered one of the greatest threats to the outstanding universal values of this UNESCO World Heritage Site (Reise and van Beusekom 2008; Oost et al. 2017; Philippart et al. 2017a, b).
In the northern Wadden Sea, ecological research on regional pressures has a long history starting at the end of the nineteenth century when Karl August Möbius investigated the effects of local fisheries on the declining European oyster population (Ostrea edulis) near the island of Sylt (Möbius 1877). In 1924, the first permanent research station was established on Sylt, which still exists today as the Wadden Sea Station Sylt as part of the large-scale research infrastructure ‘Marine Stations Helgoland and Sylt’ operated by the Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research (AWI; see Alfred-Wegener-Institut Helmholtz-Zentrum für Polar-und Meeresforschung 2023). Since its founding 100 years ago, the Wadden Sea Station Sylt has conducted ecological research using local organisms. Long-term data series on temperature, nutrient concentrations and population dynamics of species have been collected for decades (e.g. Reise et al. 1989; Reise 2005; van Beusekom et al. 2008; Armonies 2017; Armonies et al. 2018, 2023; Amorim et al. 2023; Rick et al. 2023). Thus, there is comprehensive knowledge about long-term changes in the area, which provides a solid baseline for assessing the impacts of additional stressors on the ecosystem.
In this synthesis paper, we provide an overview of the current state of knowledge of the effects of ocean warming and sea level rise in the northern Wadden Sea. We focus mainly on the regional scale, but use information about specific global-scale effects for this area to add a broader context. In addition, we are aware that human impacts and the resulting ecosystem responses (such as changes to nutrient concentrations) are not necessarily uniform across the Wadden Sea, but can show considerable spatial variation (e.g. van Beusekom et al. 2012, 2019; Singer et al. 2023). For this reason, we limit the spatial scope, allowing us to compile information from the level of the ecosystem down to genes to obtain a highly interdisciplinary overall picture covering multiple levels of organisation for this area. The paper is structured to reflect climate change impacts from large- to small-scale processes, describing physical aspects (coastal geomorphology, hydrodynamics, seawater temperature) and impacts at the habitat level focusing on tidal flats, salt marshes, seagrass meadows and bivalve reefs (Fig. 1). Within these habitats, climate change effects at the species level with consequences for the occurrence, abundance and distribution of species as well as interactions between species are presented. Case studies of organism responses via genetic adaptation and phenotypic plasticity are presented. These outline adaptive mechanisms of key species to changing environmental conditions, including molecular mechanisms underlying responses. The characterisation of these effects combines up-to-date methodological approaches such as next-generation molecular tools (-omics), ecological network analysis, mesocosm experiments and internet-based data collection technologies that can facilitate a more holistic approach to ecological research on climate change effects.
Direct (sea level rise, warming, hydrodynamics) and indirect (non-native species) effects of climate change can be of different importance (shown as proportion of outer circles) for selected habitats of the Wadden Sea ecosystem by altering specific processes in the respective areas (inner circles). Note: proportions were estimated based on published studies, personal observations and unpublished data
While the effects of climate change on the Wadden Sea presented here do not represent an exhaustive list of all impacts, a regionally focussed overview can serve as a stimulus for comparative studies on larger spatial scales to identify research gaps for the entire Wadden Sea. Knowledge of the potential impacts of climate change at multiple spatial, temporal and biological scales ranging from genes to ecosystem is essential in order to assess how rapidly changing environmental conditions will affect this unique natural landscape.
Physical climate change impacts on the Northern Wadden Sea
Landscape evolution and coastal geomorphology
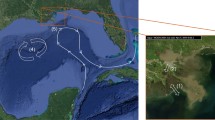
Changes in relative sea level (RSL) affect sedimentary processes that have been the main drivers of landscape evolution and habitat distribution in the Wadden Sea over the last several thousand years (Behre 2002; Flemming and Davis Jr 1994; Lindhorst et al. 2010; Reise 2005). The two barrier islands, Sylt and Rømø, formed by sediment accumulation and longshore drift over the last ca. 6000 years following regional stabilisation of RSL. Tidal flats developed in the back barrier environments (Fruergaard et al. 2021), and today, the List tidal basin (Sylt-Rømø Bight) is one of the largest tidal catchments of the Wadden Sea (~ 400 km2). Due to the construction of two impermeable causeways (Sylt in 1927 and Rømø in 1948) connecting the islands with the mainland, the back barrier environments are today only connected to the open North Sea through the Lister Deep tidal inlet (Fig. 2). Natural dynamics and exchange processes in the tidal basin have also been affected by the construction of dikes and reclamation of salt marshes, which have been effectively disconnected from the tidal basin in a stepwise process over the last five centuries (Reise 2005).
One of the crucial aspects when studying rising RSL in the Wadden Sea is the potential geomorphological response, for which the prognosis is highly uncertain. An elevated RSL is expected to increase the tidal prism (volume of water leaving the Wadden Sea during ebb tide) more than the cross-sectional area of the tidal inlets (Wachler et al. 2020). This leads to increased tidal current velocities in the main tidal inlets and channels (Wachler et al. 2020), and subsequently, to enhanced erosion of the ebb-tidal delta and a deepening of the channels. Sediment will be internally redistributed onto the tidal flats resulting in an increased height difference between channels and the tidal flats (Hofstede 2015; Dissanayake et al. 2009, 2012). Predicted changes in hydrodynamics and in particular tidal asymmetry (higher velocities during either ebb or flood at certain locations) will, in turn, modify sediment transport patterns and sediment budgets for each subarea of the Wadden Sea (Chernetsky et al. 2010; Jiang et al. 2020; Wachler et al. 2020; Jordan et al. 2021; Hagen et al. 2022).
Over longer time scales, tidal flats depend on a sufficient supply and deposition of sediments to be able to keep up with RSL. When RSL rises faster than sedimentation rates, tidal flats are at risk of constant submersion, turning tidal flats into shallow subtidal areas, particularly in the lower intertidal zone. Sediment budget analyses based on both observations (Benninghoff and Winter 2019; Hagen et al. 2022) and modelling (Becherer et al. 2018; Dissanayake et al. 2009, 2012; Hofstede et al. 2018) expect an increasing sediment accumulation in the Wadden Sea basins in response to rising RSL and larger tidal ranges. In intertidal areas of the List tidal basin, sedimentation has matched the rate of RSL rise for at least the last 2000 years (Madsen et al. 2010). Simulations for the area show that this will continue up to a RSL rise of 6–7 mm yr−1 (Hofstede et al. 2019), indicating that the tidal flats could compensate for a threefold increase in the current RSL. At this rate, the channels and outer coasts will act as sediment sources (Hofstede et al. 2019). However, when RSL rises much faster, more water will be transported directly over the tidal flats, reducing flow velocities in channels, leading to sediment deposition rather than erosion (Becherer et al. 2018; Hofstede et al. 2018). Thus, the system remains stable under lower RSL scenarios, but if the rate of RSL increase exceeds a critical limit, tidal flats and coastal habitats may become increasingly inundated (Hofstede 2015; Hofstede et al. 2018; Lodder et al. 2019; Saintilan et al. 2023). Although hydro- and morphodynamic responses of individual basins within the Wadden Sea differ depending on a number of factors (e.g. geometry, availability of sediment sources, coastal protection measures), many tidal areas of the Wadden Sea may transition from a tidal to lagoon-like environment under higher RSL scenarios (Becherer et al. 2018; Dissanayake et al. 2012; Huismans et al. 2022), posing significant challenges to coastal stability and the ecological functioning of the system.
Coastal hydrodynamics
Hydrodynamics in the Wadden Sea are driven by tidal, atmospheric, wind and river forcing (Plüß 2003; Gräwe et al. 2014; Androsov et al. 2019; Fofonova et al. 2019). RSL-related changes in water depth will modify the propagation and dissipation of tidal waves due to changes in bottom friction and wave speed, resulting in a complex non-linear response of the coastal circulation pattern (Pickering et al. 2012; Holleman and Stacey 2014; Idier et al. 2017; Vermeersen et al. 2018; Devlin and Pan 2020). Rising RSL can affect tidal dynamics on a basin-wide scale, modifying tidal maps and shifting the position of amphidromic points (sites where the tidal range is zero) in the North Sea, with consequences for tidal patterns in the adjacent Wadden Sea area (Pugh 1987, Arbic and Garrett 2010; Pickering et al. 2012; Jänicke et al. 2021). In addition to rising RSL, warming also affects the water column by stabilising it against turbulent dissipation and allowing for higher tidal elevations at the coast (Jänicke et al. 2021; Gräwe et al. 2014). Consequently, the combination of rising RSL and warming is expected to lead to spatially heterogeneous modifications of tidal-induced mixing, transport and elevation, driven by additive effects of local and large-scale processes within the tidal basins. Such changes have already been observed, resulting in contrasting trends in tidal range for 70 North Sea tide gauges between the UK (− 1.0 mm yr−1) and the German Bight (3.3 mm yr−1) since 1958 (Jänicke et al. 2021).
Winds also significantly determine water transport and mixing processes within the Wadden Sea, and thus, the spread of plumes from rivers (Schrum 1997; Duran-Matute et al. 2016; Gräwe et al. 2016; Gerkema and Duran-Matute 2017; Reef et al. 2018; Donatelli et al. 2022). Waters from the Elbe and Weser rivers usually reach the northern Wadden Sea as a buoyancy-driven current, which is mediated by the Earth's rotation. Westerly and south-westerly winds accelerate this process and confine river water close to the coast. Westerly, north-westerly and south-westerly winds predominate in this area throughout the year. However, winds are characterised by significant changes on interannual to decadal time scales, mostly due to variability in large-scale atmospheric pressure systems such as the North Atlantic Oscillation (Sigismund and Schrum 2001; Rubinetti et al. 2023). Importantly, wind-driven tidal levels can exceed normal tide heights, and intense winds can cause severe flooding in coastal areas (storm surges). According to future climate projections, the frequency of such flooding events is expected to increase because of strengthening westerly winds (Ganske et al. 2016). In the Wadden Sea, Danish and North Frisian coasts are expected to be most exposed to storm surges, while East and West Frisian regions should not experience a significant change in storm surge frequency (Feser et al. 2015; Lang and Mikolajewicz 2020; Mayer et al. 2022). In contrast, easterly and north-easterly winds do not show a significantly increasing trend either in magnitude or frequency of occurrence (Ganske et al. 2016; Rubinetti et al. 2023). Importantly, the effects of storm surges are expected to be amplified by increasing RSL (Arns et al. 2015; Oppenheimer et al. 2019), with potential consequences for current coastal protection strategies and infrastructures (Arns et al. 2017; Hermans et al. 2023).
Tracking sea surface temperature using long-term time series
The Sylt Roads time series (SR) represents one of the longest shallow coastal data time series in the world. Since 1973, surface water samples have been collected twice a week from SR (55.03°N, 8.46°E; Fig. 2) and analysed for temperature as well as various physical, chemical and biological parameters (Rick et al. 2023). In order to extend the SR sea surface temperature (SST) time series to cover the same time period as the Helgoland Roads time series (1962–present), SR data were merged with SST data from a nearby sampling station located in List harbour (55.017°N, 8.44°E; method described in Amorim et al. 2023). One of the clearest trends of the time series is a strong increase in mean SST by 0.31 °C per decade over the past six decades Fig. 3. This amounts to an overall increase in annual mean SST of 1.8 °C, almost double the global ocean mean increase (Amorim et al. 2023).
Sylt Roads sea surface temperature (°C) monthly anomalies time series (black line; relative to 1962–2019 monthly climatological means) and linear trend (red dashed line). Adapted from Amorim et al. (2023)
Sea surface temperatures in the German Wadden Sea and North Sea are, in general, governed by large continental land masses rather than North Atlantic temperatures (Amorim et al. 2023). As a consequence, these shallow seas are highly variable on a seasonal basis and subject to temperature extremes, especially in winter. For instance, the winter minimum temperature in the northern Wadden Sea shows much stronger variability than the summer maximum (Amorim et al. 2023). This is particularly obvious for years before 1991 (1962–1990), whereas after 1991 (1991–2019), variability in winter decreased but increased in autumn. By analysing the densities/frequencies of temperature within the time series, two broad peaks emerged (i.e. longer durations of SSTs). One modal peak of cold temperatures occurred in the winter season, and another peak of warm temperatures occurred in summer. Since 1991, a clear shift towards higher temperatures is observed, with more warmer temperatures occurring in winter (higher density) and more maximum temperatures being reached in summer (Fig. 4a).
Distribution of monthly mean sea surface temperatures (SST) at Sylt Roads (SR) in terms of; (a) probability density function for the first (blue) and second (orange) half of the time series and; (b) running mean SST anomalies (red: positive anomalies and blue: negative anomalies). SST monthly anomalies are relative to the 1962–2019 period. Running mean is computed using a 12-month window (adapted from Amorim et al. 2023). Note: negative temperatures in; (a) are a smoothing artefact of the probability density function method
The number of extreme cold and warm temperature days at SR were also analysed to investigate the relevance of heatwaves and sudden cold periods due to blocking systems in winter. There has been a significant shift towards more warm months at SR since 1991 (see also Gimenez et al. 2024), whereas extremely cold months (mean values below < 2–3 °C) became less frequent (Amorim et al. 2023). For instance, the percentage of months with mean temperatures below 2 °C decreased from 16.6% between 1962 and 1990 to 7.8% between 1991 and 2019, whereas the percentage of months with mean temperatures above 17 °C went up by over 6% across the whole time series. Running mean SST anomalies (1962–2019 climatological mean reference) reflect the sharp increase around the end of the 1980s (Fig. 4b), a feature that is not unique to the shallow Wadden Sea, but is observable also at larger spatial scales in the North Sea and North Atlantic (Rodionov and Krovnin 1992; Reid et al. 2016; Amorim et al. 2023). This change marks the beginning of decades of positive temperature anomalies which characterise the ‘new normal’ in SST. Still, in comparison to more offshore sites, surface waters of the Wadden Sea heat up faster and cool down more. Therefore, in the long run, it is expected that differential effects of cooling versus warming will become even more pronounced in the Wadden Sea.
Changing climate effects on Wadden Sea habitats
Climate change impacts at the land–water interface
Tidal flats exposed during low tide are the most conspicuous feature of the Wadden Sea. They serve as nursery grounds for crab, shrimp and fish, and provide habitat for a rich benthic community which is an important food source, especially for migrating and resident shorebirds (Reise 1985). Tidal flats are also a hotspot for carbon and nutrient exchange across the land–water interface, and play a key role in coastal protection because they reduce the kinetic energy from the open sea. Nevertheless, coastal erosion is a major threat to tidal flats and substantial erosion has already been observed in higher tidal zones of many tidal basins (Flemming and Nyandwi 1994). For example, through the reduction of tidal catchment areas due to the construction of embankments, hydrodynamic energy levels have increased, resulting in higher erosion rates (Flemming and Nyandwi 1994). Accelerated sea level rise is projected to cause further increases in hydrodynamic energy (see above), which will exacerbate erosion problems in the future.
Variation in hydrodynamics is also reflected in the tidal flat sediment composition. Tidal flats in exposed areas, where waves and currents have a stronger impact, are predominantly composed of sandy sediments. Tidal flats of fine sediments (mixed or muddy sediments) establish only in sheltered areas where calm hydrodynamic conditions allow small particles to settle. Increasing hydrodynamic energy causes resuspension of fine-grained sediments and a selective erosion of mud. A long-term analysis of the List tidal basin revealed a severe depletion of mud (Dolch and Hass 2008). This was facilitated by reduced seagrass cover, as seagrass cannot tolerate excessive hydrodynamic energy. Mud depletion and a coarsening trend in the grain size composition can be observed in the entire Wadden Sea (Flemming and Nyandwi 1994, Flemming and Bartholomä 1997, Mai and Bartholomä 2000). It is assumed that this coarsening has an impact on the benthos (Flemming and Nyandwi 1994), as well as the biogeochemical functioning of the sediment. For example, sulphate reduction is often the main mineralisation pathway in muddy sediments, whereas aerobic respiration dominates in sandy sediments (de Beer et al. 2005; Jansen et al. 2009). Combined with differences in transport processes such as internal porewater advection (Billerbeck et al. 2006; Røy et al. 2008; Schutte et al. 2019), organic carbon turnover rates and greenhouse gas emissions vary largely between muddy and sandy intertidal sediments, with potential consequences for ‘blue carbon’ mitigation strategies (see Box 1).
Box 1: Blue carbon in the Wadden Sea
Blue carbon refers to atmospheric CO2 trapped as organic material in vegetated coastal sediments, thereby potentially mitigating global climate change (Duarte et al. 2013; Serrano et al. 2019; Kuwae and Hori 2019). The most important vegetated coastal ecosystems, including salt marshes, seagrass meadows and mangrove forests, are recognised for their exceptional capacity to bury carbon in their soils and sediments, demonstrating carbon burial rates more than ten times higher than those of temperate and boreal forests (McLeod et al. 2011; Himes-Cornell et al. 2018; Ouyang and Lee 2014). In the Wadden Sea, seagrass meadows and salt marshes serve as the primary CO2 sinks, accumulating substantial amounts of blue carbon. Despite this significance, relatively few studies have quantified carbon storage in the Wadden Sea. Carbon storage in Wadden Sea salt marshes is mainly minerogenic (composed of mineral particles; Allen 2000), and sediments are mostly only partially waterlogged, allowing for the intrusion of atmospheric oxygen (Keshta et al. 2020). Consequently, high mineralisation rates are facilitated, leading to the degradation of accumulated organic matter (Mueller et al. 2017). As a result, the Wadden Sea's salt marshes generally exhibit lower organic carbon concentrations compared to peat-forming organogenic (composed from organic substances) marshes found in other parts of the world. Still, Wadden Sea salt marshes can have substantial accretion rates (Esselink et al. 2017, Schulze et al. 2021), contributing to their carbon sequestration potential. Carbon sequestration rates have been found in the range of 17–149 g C m−2 yr−1 (Graversen et al. 2022; Mueller et al. 2019; Elschot et al. 2015; Andersen et al. 2011), which fall below the reported world average for salt marshes estimated at 242.2 ± 25.9 g C m−2 yr−1 (Ouyang and Lee 2014) The amount of organic carbon stored below seagrass meadows in the Wadden Sea remains largely unknown, but is the subject of ongoing investigations. Importantly, the different sediment types that seagrasses inhabit might lead to significant variation in carbon storage. For instance, a comparison of carbon storage in Wadden Sea salt marshes and sandy-substrate seagrass meadows showed that salt marshes had higher organic density (0.015 g C cm−3) in the permanently buried soil layers (> 20–30 cm) compared to seagrass meadows, which exhibited a markedly lower organic density (0.004 g C cm−3). Interestingly, salt marshes and seagrass meadows in the Wadden Sea showed considerably lower carbon density than the global averages for both salt marshes (about 0.04 g C cm−3; Ouyang and Lee 2014; Chmura et al. 2003) and seagrass meadows (about 0.02 g C cm−3; Fourqurean et al. 2012). However, salt marshes and seagrass meadows combined cover approximately 63,000 ha (13%) of the Wadden Sea tidal flat area (470,000 ha), highlighting their potential as a substantial CO2 sink. In addition, recent studies have demonstrated that inorganic carbon in the Wadden Sea contributes significantly to the total soil carbon stock, accounting for 29% of the total carbon content (Mueller et al. 2023). In light of ongoing sea level rise and global warming, more studies aiming to quantify organic and inorganic carbon storage in coastal ecosystem habitats are needed to better understand the fate of blue carbon in the unique ecosystem of the Wadden Sea |
The depletion of mud increases the proportion of sandy sediments, which, together with increasing hydrodynamic energy, enables the formation of large sandy sedimentary bedforms on the tidal flats (Fig. 5). Fields of large sandy bedforms have expanded significantly in the northern Wadden Sea over the last decades (Dolch and Reise 2010). As large sandy bedforms move back and forth, they cause unstable sediment conditions which severely affect epibenthic habitats such as intertidal seagrass meadows and mussel beds. Expanding fields of large sandy bedforms appear to have the potential to dis- or replace epibenthic biotic habitats. Also, endobenthic organisms can be inhibited by sediment instability, and sensitive species are expected to decline where new large sandy bedforms develop. Consequently, a decline in benthic biomass production may occur in the Wadden Sea, with implications for its nursery function and foraging ground potential for coastal birds (Dolch and Reise 2010).
Salt marshes and sea level rise
Coastal salt marshes are vegetated areas of the upper intertidal or lower supratidal zones that are exposed to periodic flooding by tides and episodic surges. Salt marshes in the northern Wadden Sea primarily exist in the form of back-barrier and foreland marshes. Foreland marshes mostly form within brushwood groynes (artificial structures to increase sedimentation) and are often heavily managed (e.g. by grazing, artificial drainage). The overall aerial extent of salt marshes in the federal state of Schleswig–Holstein, Germany, amounts to 13,240 ha (out of 40,000 ha of salt marshes in the entire Wadden Sea; Esselink et al. 2017), and thus, presently constitutes a smaller area in comparison to historical distributions within Schleswig–Holstein (Reise 2005). Former marsh losses in the Wadden Sea were mainly caused by human intervention (e.g. land reclamation or coastal protection measures) that started approximately 1000 ad and reached well into the twentieth century (Esselink et al. 2017; Reise 2005). Such intervention has subsequently been reduced since salt marshes are increasingly recognised as valuable habitats for a specific and species-rich community (including fish, invertebrates and birds), resulting in a steady increase in areal extent (up to 30% more area) during the last 30 years (Esselink et al. 2017). Moreover, more ecosystem services of salt marshes have been identified such as their contribution to coastal protection through wave attenuation and long-term carbon sequestration, both highlighting their importance to coastal communities (see Box 1, Möller et al. 2014; Mueller et al. 2019).
Despite their value, future salt marsh persistence may be threatened by climate change effects such as accelerated rates of sea level rise and changes to storm surge frequency and magnitude. Currently, most Wadden Sea salt marshes are keeping pace with present levels of sea level rise due to high accretion rates (Suchrow et al. 2012; Nolte et al. 2013; Esselink et al. 2017). However, further increases in RSL and more frequent inundations may negatively affect plant growth, leading to a negative feedback loop ensuing marsh submergence and, finally, marsh loss (Chmura 2013; Kirwan and Guntenspergen 2012). Importantly, how salt marsh vegetation responds to increased flooding frequencies was shown to vary not only between but also within plant species (Kirwan and Guntenspergen 2012; Reents et al. 2022), with consequences for community-level responses and the resistance of salt marshes to accelerated rates of RSL rise in the future. It is expected that salt marshes will migrate further inland in response to accelerated rates of sea level rise. However, the coastline along the Wadden Sea is largely protected by hard structures such as dikes that restrict landward expansion (‘coastal squeeze’), which could decrease marsh resistance and further increase the risk of salt marsh loss (Schuerch et al. 2018).
The effects of more frequent inundations can have opposing effects on the biogeomorphology of salt marshes. On the one hand, overwash events (flooding during storm surges that carry large amounts of water and sediment onto the marsh platform) during storm tides add sediment for vertical accretion and positively contribute to marsh resistance (Schuerch et al. 2018). A model that incorporated changing storm activity and overwash events in the future predicted that a salt marsh on the barrier island of Sylt could even survive sea level increases of up to 20 mm yr−1 (Schuerch et al. 2013). On the other hand, wind waves can cause severe damage to marshes via erosion of the marsh edge (Leonardi and Fagherazzi 2015; Schwimmer 2001). Erosion along the edges of salt marshes is presently widespread in the List tidal basin, and marsh erosion contributes an estimated 3000 t yr−1 of fine-grained deposits to the local sediment budget of tidal flats (Pejrup et al. 1997). The vegetation of salt marshes plays an important role in marsh resistance under wave exposure, since roots and rhizomes stabilise the sediments, while aboveground parts attenuate waves and currents. Above certain hydrodynamic conditions, however, stem breakage and biomass loss can cause a reduction in wave attenuation (Rupprecht et al. 2017), which highlights the importance to better understand the vegetation’s susceptibility to severe wave-induced damage. Recent studies suggest that plant resistance to wave impact is highly species specific and is determined by plant properties such as vegetation height, aboveground biomass and stem flexibility (Reents et al. 2022; Schoutens et al. 2019). Since plant responses to both increasing flooding frequency as well as higher hydrodynamic forcing are species specific, future salt marsh plant community composition may change, with potential implications for not only salt marsh resistance, but also for ecosystem services such as carbon sequestration (see Box 1).
Seagrass as an indicator of climate change impacts
Seagrass meadows are the dominant and highly productive vegetation of shallow, sedimentary coasts. In the Wadden Sea, they grow on tidal flats, mainly in the mid- to upper intertidal where they fulfil a variety of ecologically important functions such as providing habitat and nursery grounds, a food source and protection from predators for many organisms including gastropods, polychaetes, bivalves, crustaceans and fish. In addition, they promote sedimentation, reduce erosion with their dense root–rhizome system and counteract climate change directly by storing atmospheric CO2 as organic carbon in their underlying sediment (Larkum et al. 2006). By this process, seagrass meadows store carbon beneath them in the sediment and can build up large carbon deposits over decades and centuries (Fourqurean et al. 2012), and thus provide similar ecosystem services as salt marshes (see Box 1).
At the same time, seagrasses are very sensitive to a variety of environmental parameters such as pollution and eutrophication. Since the late 1990s, a reduction in nutrient discharges into the Wadden Sea has resulted in the recovery of seagrass meadows in the northern Wadden Sea: since 2011, a fivefold increase in seagrass meadow area has been recorded (Dolch et al. 2017). A further increase in seagrass meadow area has occurred since 2019, likely due to high seed availability. Indeed, an analysis of historical aerial photographs revealed that since 2010, seagrass meadow area in the northern Wadden Sea is larger than it was in the 1930s (Dolch et al. 2013, 2017). However, this positive trend will likely be reversed by increasing impacts of climate change effects. For instance, seagrass meadows only form in sheltered locations and depend on calm hydrodynamic conditions (Dolch et al. 2013). More extreme storm events as well as increased hydrodynamic energy as a result of an increasing tidal range in combination with increasing wave wash may result in a loss of seagrass meadow area and of suitable habitats for seagrass (Schanz et al. 2002; Schanz and Asmus 2003). In addition, seagrasses are sensitive to large temperature changes. Especially during heatwaves, seagrasses are exposed to high air temperatures during ebb tide, which culminate in severe heat stress and desiccation. Critically, if seagrass meadows are damaged or destroyed, they will transform from valuable carbon reservoirs (see Box 1) into CO2 emitters that will further increase impacts of climate change. Consequently, protecting and promoting the natural expansion of seagrass meadows are important contributions not only to mitigating climate change, but also to preserving the many other important ecological functions of these habitats.
Climate-induced changes to bivalve reefs
Aggregations of epibenthic bivalves are vital coastal structures that fulfil key ecological functions (Goss-Custard et al. 1982; Gutierrez et al. 2003), and at the same time are economically important as an aquaculture food source (Seaman and Ruth 1997). Historically, bivalve beds in the northern Wadden Sea were formed by blue mussel (Mytilus edulis) in the intertidal and European oyster (Ostrea edulis) in subtidal areas (Möbius 1877; Hagmeier and Kändler 1927). Another important habitat structure was formed by Sabbelaria spinulosa, a reef-forming tube worm, in the deep subtidal (Fig. 6). During the early twentieth century, oyster beds and Sabellaria reefs including their associated fauna (e.g. sponges and cnidaria) disappeared completely from the northern Wadden Sea due to overfishing and have not returned (Reise 1982). Subsequently, blue mussel (M. edulis) became the only reef-forming bivalve species in the Wadden Sea and also spread into subtidal areas (Reise 1982; Reise and Schubert 1987; Reise et al. 1989). Beds of blue mussels can persist over long time periods if losses (e.g. by predation, storms, fisheries) can be compensated for by regular recruitment events (Nehls and Thiel 1993; Büttger et al. 2014; van der Meer et al. 2019). In general, mussel recruitment is favoured by severe winters that minimise the abundance of predators (shrimps and crabs) on tidal flats the following spring, causing a temporal mismatch between predator and prey (Strasser and Günther 2001; Beukema and Dekker 2014; Beukema et al. 2015). However, due to climate change, cold winters are becoming extremely rare events (Büttger et al. 2014, Amorim et al. 2023), resulting in recruitment failures and even shortages of local mussel populations in the Wadden Sea (Nehls et al. 2006; Beukema et al. 2015).
Schematic cross-section through a tidal area including common habitat-forming species in the past (left) and present Wadden Sea (right). H and L indicate high and low tide level, respectively. Mussels: Mytilus edulis; European oysters: Ostrea edulis; reef-forming tube worms: Sabellaria spinulosa; Pacific oysters: Magallana gigas
In contrast to the native mussel M. edulis, Pacific oysters (Magallana gigas) profit from warmer water conditions, resulting in increased proliferation (Diederich et al. 2005; Nehls et al. 2006). Pacific oysters originating from East Asia were introduced to the northern Wadden Sea for aquaculture purposes in the late 1980s and quickly spread throughout the area (Reise 1998). Periods with high summer temperatures during the late 1990s and early 2000s resulted in an increase in oyster abundance, making M. gigas a common habitat-forming organism throughout the entire Wadden Sea (Wehrmann et al. 2000; Troost 2010; Reise et al. 2017). Since Pacific oyster settle on hard substrates like shell fragments or mussel beds (Reise 1998; Diederich 2005; Troost 2010), oyster settlement led to a transformation of most intertidal mussel beds into mixed reefs of mussels and oysters in the Wadden Sea (Reise et al. 2017). Pure mussel beds are primarily confined to the high intertidal zone (Fig. 6), where environmental conditions are less favourable for Pacific oysters (Waser et al. 2016; Reise et al. 2017). Mixed reefs of mussels and oysters are generally more stable against abiotic and biotic pressures than pure mussel beds. For instance, oysters offer mussels shelter from predation (Eschweiler and Christensen 2011; Waser et al. 2015, 2016; Buschbaum et al. 2016), and the permanent attachment of oysters, even when dead, forms mixed reefs of rigid and persistent structures (Markert et al. 2010; Reise et al. 2017; van der Meer et al. 2019). It is expected that mixed reef structures will increase in area in the future (van der Meer et al. 2019), and Pacific oysters will further extend to subtidal areas (Fig. 6; Diederich et al. 2005; Ricklefs et al. 2020). The spread of Pacific oysters into subtidal areas also offers new settling substrates for species that formerly were associated to beds of European oysters. For example, many sedentary-living species like hydrozoans that became rare in the absence of European oysters (Reise 1982) are currently abundant owing to the occurrence of Pacific oysters (Waser et al. unpublished data).
Wadden Sea species diversity under climate change
Nutrient and plankton dynamics in a changing Wadden Sea
The impact of climate change on nutrient and plankton dynamics interacts with other ecological changes like eutrophication and invasive species (Reise and van Beusekom 2008). Eutrophication has exerted a major impact on the Wadden Sea ecosystem in conjunction with climate change. For example, extreme wet years in the 1980s with high freshwater discharge coincided with maximum nutrient concentrations, resulting in maximum nutrient loads, phytoplankton abundance and organic matter turnover throughout the Wadden Sea (van Beusekom et al. 2019). Political decisions in the 1980s initiated measures to reduce riverine nutrient loads (de Jong 2007), leading to a reduced eutrophication status in the entire Wadden Sea. Since the 1980s, freshwater discharge has shown a slight downward trend (van Beusekom et al. 2019). However, extreme droughts, which can be expected to increase due to climate change (Sieck et al. 2021; Ossó et al. 2022), also play a role since they will disproportionally reduce nitrogen loads in particular. Specifically, drought will increase residence times of nutrients in the river basin and increase the proportion of nitrogen from agriculture and wastewater lost by denitrification (Schulz et al. 2023). Conversely, extreme rain events are also predicted to occur more frequently in the future. A record flood in 2013 transported large amounts of freshwater and nitrogen into the inner German Bight, but mainly impacted the southern Wadden Sea (Kerimoglu et al. 2020). To what extent such floods may impact the northern Wadden Sea will strongly depend on contingent hydrodynamic conditions.
Both riverine nutrient loads and changing temperatures have strong effects on plankton dynamics in the northern Wadden Sea. Whereas decreasing nitrogen loads lead to lower summer phytoplankton biomass (van Beusekom et al. 2019), low winter temperatures enhance the spring bloom (van Beusekom et al. 2009). This is likely due to suppression of both benthic and pelagic grazing pressure (Martens and van Beusekom 2008; van Beusekom et al. 2009). Recent data support the impact of winter temperature on the spring phytoplankton bloom (Fig. 7a). Like with phytoplankton, temperature conditions during the first part of the year also have a strong impact on zooplankton dynamics. There is a significant correlation between mean annual copepod abundance (excluding Oithona spp.) and mean winter/spring temperatures (January–May) in the northern Wadden Sea (Martens and van Beusekom 2008), suggesting that climate warming will lead to an earlier start of copepod seasonal development, and the comparatively strong temperature increase in autumn months (e.g. 0.13 °C yr−1 in September between 1984 and 2005) might also lead to a longer season with higher mean annual abundances. However, data from 2005 to 2011 show a more complicated picture (Fig. 7b). Despite high winter temperatures, copepod biomass was clearly below levels observed during the 1990s. Whereas the correlation is still significant, the explained variability decreased from 0.40 to 0.15, likely due to low annual copepod abundance since 2000 despite warm winter temperatures.
a Spring phytoplankton bloom dynamics depending on mean winter temperature, and; (b) time series of mean winter–spring temperature (January–May) and mean annual copepod abundance. Copepod abundance includes Acartia, Centropages, Paracalanus, Pseudocalanus and Temora, but does not include the ambush-feeding copepod Oithona
How ongoing climate change will alter phytoplankton and zooplankton dynamics in the Wadden Sea will also depend on other ecological developments. For instance, herbivorous zooplankton will benefit from higher temperatures over a longer season, and will, thus, exert more grazing pressure on phytoplankton, as will higher benthic filtration rates at higher temperatures, with both factors suppressing phytoplankton blooms. On the other hand, the observed shift to a larger proportion of carnivorous zooplankton (Martens and van Beusekom 2008) accompanied by the invasion of new carnivorous zooplankton (e.g. Mnemiopsis leydi; Boersma et al. 2007) could also increase grazing pressure on herbivores. Furthermore, higher temperatures will also increase remineralisation rates, leading to faster recycling of nutrients that could alleviate nutrient limitation, especially during summer (Reise and van Beusekom 2008). In general, it is predicted that global change factors like increased temperatures and reduced river discharge in conjunction with EU policies to further reduce eutrophication will reduce phytoplankton blooms. However, we note that other, yet unknown ecosystem changes like increased feeding pressure on copepods from, e.g., fish species extending their ranges northward may have opposite effects on phytoplankton bloom dynamics (Reise and van Beusekom 2008). How these top-down and bottom-up effects on the plankton community will interact is an important future research area for the Wadden Sea.
Increasing SST has a significant influence not only on overall plankton biomass in the Wadden Sea, but also on the phenology of individual species and the seasonal development of the plankton community as a whole. A comparison of the timing of occurrence for phytoplankton taxa between two time periods (1962–1988 and 1989–2015) at a nearby monitoring station (Helgoland Roads) showed significant temporal shifts in many key diatom and dinoflagellate taxa (Scharfe and Wiltshire 2019). The direction of these shifts was dependent on species-specific preferences, with some taxa occurring earlier in the year whilst others showed delayed peak abundances, and shifts in timing varied from several days to a couple of weeks. Taxa with spring blooms tended to shift to earlier peak abundances (e.g. diatoms Asterionellopsis glacialis, Thalassiosira spp, Ditylum brightwellii, Thalassionema nitzschioides, Skeletonema costatum), reflecting the warmer SST earlier in the season. Examples of phytoplankton taxa with delayed blooms in the summer–early autumn seasons include D. brightwelliii, T. nitzschioides and S. costatum, whereas Thalassiosira spp. tended to occur earlier in the summer. The dinoflagellates Prorocentrum micans and Tripos fusus showed earlier summer occurrences and also lower abundances. Water temperature and light availability are the dominant abiotic factors driving the phenology of phytoplankton blooms, together with zooplankton dynamics (Wiltshire et al. 2015). Phenological changes of phytoplankton taxa in response to increasing SST may impact not only interspecific interactions, but also the phytoplankton–zooplankton energy transfer link, with the potential for cascading effects on food web dynamics.
Changes to fish diversity
The Wadden Sea is of large ecological importance for many fish species that use this area for at least one stage in their life cycle (Tulp et al. 2017; van der Veer et al. 2015). It acts as a nursery and feeding ground, and offers protection from predators for post-larval and juvenile fishes. Moreover, it offers a refuge for seasonal migrants en route to marine or freshwater spawning areas (Elliott et al. 2007; Tulp et al. 2017). Climate warming influences fish species by causing shifts in phenology (van Walraven et al. 2017), poleward shifts in species distributions (Montero-Serra et al. 2015), changes to predator–prey interactions (Durant et al. 2007), dispersal to deeper water of bottom-dwelling species (Dulvy et al. 2008) and changes to community assemblages (Fossheim et al. 2015; Rutterford et al. 2023).
A high-resolution monthly juvenile fish monitoring program that has been running since 2007 in the northern Wadden Sea near Sylt identified 55 fish species belonging to three biogeographic guilds (Boreal, Lusitanian and Atlantic) and three habitats (benthic, benthopelagic and pelagic). This time series shows that the local community exhibits seasonal assemblages based on SST preferences (Fig. 8). The winter season is dominated by smelt (Osmerus eperlanus), dab (Limanda limanda), bull-rout (Myoxocephalus scorpius), sand goby (Pomatoschistus minutus) and flounder (Platichthys flesus). Rock gunnel (Pholis gunnellus), sprat (Sprattus sprattus), sea snail (Liparis liparis) and great sand eel (Hyperoplus lanceolatus) are dominant in spring. Summer is dominated by herring (Clupea harengus), small sand eel (Ammodytes tobianus), whiting (Merlangius merlangus) and Nilsson’s pipefish (Syngnathus rostellatus), while Atlantic cod (Gadus morhua) and hooknose (Agonus cataphractus) dominate in autumn. However, there are seasonal overlaps with some species present year-round, and others such as horse mackerel (Trachurus trachurus) that are season specific. Therefore, the seasonal assemblage structure shown in Fig. 8 represents their peak abundances.
Redundancy analysis (RDA) triplot of seasonal assemblages of dominant fish species in the List tidal basin. Seasons (explanatory variable) are marked by small green squares and fish species with blue numbered lines (see legend for species names). Fish images were modified from Fishbase, Scandinavian Fishing Yearbook and WoRMS
Over the long term, fish species composition showed three major abundance patterns between 2007 and 2019. Herring and small sand eel were the most dominant species, and together with great sand eel and pipefish had peak abundances after severe winters, whereas all other species declined (but showed recovery) after typical winters (Odongo et al. 2024). Severe winters (more than 30 days of ice coverage on the coast; Strasser et al. 2003) cause a delay in copepod development and a decline in benthic organisms (Armonies et al. 2001), resulting in a mismatch between fish predators and their prey, which likely explains the observed differences in trends found.
In comparison to previous investigations covering the periods from 1989 to 1995 (Herrmann et al. 1998; Vorberg and Breckling 1999), four fish species were lost, while eight additional species were found. This indicates that long-term effects of climate warming on fish communities have been observed in the northern Wadden Sea, but new species are still rare with low abundances. Warmer autumn SSTs (Rick et al. 2023) have led to a shift in phenology for cod and whiting, with longer residence times in the List tidal basin. Similarly, two species of goby (P. minutus and P. microps) are staying longer in the shallow intertidal areas before winter migration to more stable temperatures in deeper areas. Changing global climate patterns such as warming winters (Clark et al. 2020) and autumns (Rick et al. 2023) will likely lead to competitive disadvantages for northern species and advantages for southern species (Elliott and Hemingway 2002), which may lead to changes in fish community structure (Meyer et al. 2016; Clark et al. 2020), with consequences for ecosystem functions as well as fisheries.
Neobiota in a warming Wadden Sea
Species richness of an ecosystem is not a stable state, but is subject to constant natural change. During its development, the Wadden Sea was colonised by species from surrounding ecosystems, which is likely why no endemic species occur (Buschbaum and Horn 2024). Exceptional climatic variability in the North Atlantic region since the formation of the Wadden Sea around 8000 years ago coupled with highly fluctuating abiotic conditions kept species diversity at a low level, and favoured the occurrence of a few opportunistic and well-adapted species, with some now reaching densities up to several thousands of individuals per square metre (Reise et al. 2023). Low species diversity coupled with high three-dimensional habitat availability on and below the seafloor results in comparatively low interspecific competition, making the Wadden Sea an immigration-friendly ecosystem with many empty niches and a high capacity to accommodate new species from foreign coasts (Buschbaum and Horn 2024).
As SST rises, species originating from more southern coasts may extend their distribution limits to the Wadden Sea. The first arrivals were and will mainly be small pelagic fish species that respond quickly to rising temperatures (see above), but in the long term, benthic invertebrates will also immigrate (Hiscock et al. 2004; Reise and van Beusekom 2008). However, the number of new macroinvertebrate species expanding their distribution northwards will be limited because most coastal benthic species of the East Atlantic already have wide latitudinal distribution ranges (Reise and van Beusekom 2008; Weinert et al. 2016). These species can still be considered ‘natural’ immigrants from adjacent regions because they are not actively introduced by human activities. However, the number and importance of human-introduced organisms is much higher than natural immigration. In the past 150 years, biodiversity in the Wadden Sea has increased by more than 100 introduced species (detailed in Lackschewitz et al. 2022; Reise et al. 2023). The rate of species introductions was low at the beginning of the twentieth century, but has increased exponentially since the 1990s (Büttger et al. 2022), in large part due to increased cargo shipping between the continents and the use of non-native marine species for aquaculture (Ojaveer et al. 2018).
Most of the already established introduced species in the Wadden Sea originate from warmer areas. As a consequence, rising temperatures facilitate ecological ‘sleepers’ (Spear et al. 2021), which are non-native species that have already established but respond with increased abundance after temperatures increase. A prominent example is the Australian barnacle Austrominius modestus. This species was introduced to the Wadden Sea in the 1950s but remained rare until the 1990s, when it showed an exponential increase in abundance as a result of a series of mild winters and warm summers (Witte et al. 2010). A large proportion of the new species are suspension feeders such as bivalves, tunicates and bryozoans, with hitherto largely unknown effects on Wadden Sea food webs. However, continually increasing densities indicate that suspension feeders are currently not food limited in the Wadden Sea (Reise and van Beusekom 2008).
Interestingly, many of the introduced species are habitat engineers. The Pacific oyster, macroalgal species and epibenthic organisms such as the American slipper limpet Crepidula fornicata have successfully established (Reise et al. 2023), whereas only a few new endobenthic soft-bottom species managed to do so (less than 30% of all known non-native species; Büttger et al. 2022). Perhaps, these species are introduced less frequently because their transport is mostly restricted to ballast water, whereas hard-bottom species can be introduced by additional vectors such as ship hulls and aquaculture organisms (Buschbaum and Horn 2024). Non-native hard-bottom species benefit from an increase in artificial structures made from stone and concrete used for coastal protection, as these provide not only protection from storms but also settlement substrate. This also applies to the foundations of offshore wind turbines, which represent stepping stones that facilitate dispersal (Reise and Lackschewitz 2023; Reise et al. 2023). Thus, artificial and human-altered coastal areas offer non-native species entrance doors for successful establishment within the Wadden Sea ecosystem.
The combined effects of global trade, artificial hard substrates and climate change lead to more introductions and successful establishment of non-native species in the Wadden Sea, with a current rate of about two new species per year (Reise et al. 2023). Management of non-native species is the subject of intense debate with opposing opinions ranging from acceptance to eradication (Buschbaum and Horn 2024). Irrespective of this sometimes-emotional discussion, the introduction of new species should be prevented, as it represents a man-made change to the ecosystem, does not correspond to the guiding principles of a World Heritage site and does not take into account that every additional new species represents an ecological risk (Reise et al. 2023).
Direct and indirect climate change effects on macroalgae
The unstable sediments of the Wadden Sea are not suitable habitat for macroalgae, which require hard substrate for attachment. Only extensive epibenthic biogenic structures such as mussel beds have naturally occurring macroalgae, but with comparatively low diversity. The only abundant native colonising macroalga on Wadden Sea intertidal mussel beds is a special form of rockweed, Fucus vesiculosus forma mytili. This brown alga reproduces purely vegetatively and lacks a holdfast (Albrecht 1998), but anchors to mussel beds using the dense byssus thread-mesh produced by the mussels. Mussel beds covered with F. vesiculosus forma mytili represent a specific habitat type. The alga enhances the accumulation of mud by decreasing currents and hydrodynamics above the mussel beds, resulting in lower densities of mussel bed–associated species such as barnacles and crabs below the algal canopy. At the same time, it attracts grazing herbivores such as snails, isopods and amphipods, therefore increasing overall benthic and habitat diversity (Albrecht and Reise 1994). Climate change is predicted to influence the ecological functioning of this community since warming temperatures may alter the phenology of F. vesiculosus in spring and summer, and decrease survival of F. vesiculosus germlings in late summer (Al-Janabi et al. 2016; Graiff et al. 2017).
Besides direct consequences of climate warming on habitat-forming native macroalgae, indirect effects are also predicted to increase in the Wadden Sea. For example, higher temperatures associated with mild winters and warm summers have strongly facilitated the development of mixed bivalve reefs (see above), with Pacific oysters dominating the top layer of the reef and mussels mainly occurring near the bottom between the oysters (Reise et al. 2017). This spatial distribution pattern hampers the fixation of F. vesiculosus forma mytili on bivalve aggregations (Mayr 2009), which has resulted in its reduced abundance on mixed reefs in comparison to pure mussel beds before the introduction of the Pacific oyster. Like Pacific oysters, some species of non-native habitat-forming macroalgae also profit from milder temperatures in the Wadden Sea. One prominent example is the Japanese seaweed Sargassum muticum, which is now very abundant in the lower intertidal and shallow subtidal zone (Fig. 9), where it provides habitat for a rich associated community, but also competes for light and space with native algae (Buschbaum et al. 2006; Lang and Buschbaum 2010). Recently, the non-native kelp Undaria pinnatifida, native to East Asian shores, was found on mixed bivalve reefs near Sylt, but its function as a habitat-building organism is still unknown for the Wadden Sea area (Schiller et al. 2018). This species also occurs attached to artificial hard substrates such as coastal protection structures, which are increasingly being built as a result of sea level rise (Buschbaum et al. 2012; Reise et al. 2023), suggesting a corresponding increase in U. pinnatifida in the future.
The introduced macroalgae Japanese seaweed Sargassum muticum (left) and Vaucheria sp. (right) represent the most common macroalgae on tidal flats in the List tidal basin. Sargassum muticum requires hard substrate such as bivalve shells on the sediment surface for attachment, while Vaucheria sp. is rooted in the sedimentary bottom
A new phenomenon emerged with the establishment of two non-native grass-like Vaucheria species (Vaucheria longicaulis and V. velutina; Xanthophyceae), which have spread rapidly around the island of Sylt and already covered an area of 180 ha in 2020 (Fig. 9). Unlike most other introduced macroalgae, these species do not require hard substrates for attachment, but are rooted in the sediment at and below the low tide level, where a dense algal turf accumulates and stabilises mud up to a height of 20 cm. By enriching sediments, the introduction of these algal species may improve the capacity of tidal flats to compensate for accelerated sea level rise, but also strongly affects the composition of the endobenthic species community (Reise et al. 2022a, b; Rybalka et al. 2022).
Species interactions change with rising temperatures
Changes to predator–prey and competitive interactions
Ecological research on species in the northern Wadden Sea dates back to the second half of the nineteenth century. However, it took another 100 years until the mid-1970s before experimental investigations of species interactions came into focus and the first extensive manipulative field studies were conducted (Reise 1985). Experimental changes to natural densities of crabs, fish and birds revealed the important role of predators for population dynamics and the spatial occurrence of endobenthic prey species such as bivalves and polychaete worms. These represent the dominant taxonomic groups in the soft bottom of the Wadden Sea and are the main food source for millions of breeding and migratory birds (Reise 1977, 1978). In recent years, this key predator–prey interaction has been indirectly affected by climate change, shown by a decline of about 55% in breeding bird populations in the Wadden Sea (Koffijberg et al. 2022). Flooding of breeding sites during storm surges and increased frequency and magnitude of storms during the breeding period are responsible factors (van de Pol et al. 2010).
Rising temperatures can also decouple existing predator–prey relationships. Severe winters are an important regulating variable for population dynamics of many benthic species in the Wadden Sea, but have become rare in the last two decades. At the same time, the frequency of hot and dry summers has increased (see above). It is a well-known phenomenon that bivalve recruitment is enhanced after severe winters in the Wadden Sea (Ziegelmeier 1964; Strasser and Günther 2001; Strasser 2002; Strasser et al. 2003). This results less from a higher supply of bivalve larvae, but rather, from lower predation pressure from main predators such as the shore crab Carcinus maeanas. Crab larval development is delayed after severe winters, and therefore, bivalves have outgrown the prey size spectrum of the crabs when they appear on the tidal flats, which can be up to 8 weeks later than the bivalve larvae (Strasser and Günther 2001; Strasser et al. 2003). Thus, cold winter temperatures lead to a temporal mismatch between predatory crabs and their prey species, with positive effects for several bivalve populations (e.g. Cerastoderma edule). However, the trend of increasingly mild winters may result in low recruitment success of many bivalve species.
A number of species interactions are indirectly affected by climate change through the introduction of non-native species originating from warmer coasts that benefit from rising temperatures in the Wadden Sea. In particular, successfully established benthic suspension feeders such as bivalves and ascidians have enhanced the trophic functional group of primary consumers in terms of abundance and biomass, consuming planktonic organisms and affecting the overall energy flow and food web. However, these species also provide a new food source for secondary consumers such as bivalve-eating birds (Buschbaum and Horn 2024). At the same time, the number of introduced secondary consumers is relatively low in the Wadden Sea (Reise et al. 2023), although they can considerably change existing species interactions as well as develop new ones. For example, two introduced Pacific shore crabs, Hemigrapsus sanguineus and Hemigrapsus takanoi, which are adapted to warm temperatures, have recently established in mixed oyster–mussel reefs, where crabs can reach densities of about 350 individuals m−2 (Fig. 10; Cornelius et al. 2021). Both introduced crab species show a similar food spectrum as the native shore crab C. maenas and, therefore, increase predation pressure on many species associated with oyster reefs, such as barnacles, mussels and amphipods (Bleile and Thieltges 2021; Cornelius et al. 2021). Interestingly, a fundamental habitat change caused by an introduced species will not necessarily lead to changes in existing species interactions. For example, within newly developed oyster reefs, the grazing snail Littorina littorea maintains the same level of ecological function as in former pure blue mussel beds by keeping mussels and oysters free from excessive overgrowth with algae and barnacles (Cornelius and Buschbaum 2020).
Competition is another key species interaction shaping coastal communities. With rising temperatures, it is often predicted that warm-adapted introduced species will have a competitive advantage over native species. However, clear evidence for this is still lacking in the Wadden Sea area. Introduced oysters have not displaced native mussels (Reise et al. 2017), nor have non-native crab species caused a decline in the density of native shore crabs (Fig. 10; Cornelius et al. 2021), despite a strong overlap in food preference and a competitive advantage of the introduced crab species over the native C. maenas (Geburzi et al. 2018). Thus, coexistence seems to be the rule rather than the exception (Reise et al. 2023). The pattern of low competition between epibenthic species is also reflected in endobenthic species. In contrast to rocky shores, the sedimentary bottom of the Wadden Sea provides a three-dimensional living space, which presumably results in comparatively low competition for space and food, with competition only occurring when there is an extremely high abundance of individuals (Jensen 1992a). Therefore, low competition enables incredibly high densities, for example, of endobenthic bivalves and worms with several thousands of individuals per square metre (Jensen 1992a, b; Reise et al. 1994). A species-habitat analysis in the northern Wadden Sea revealed that macrobenthic species occur on average in less than half of the suitable sites available (Armonies and Reise 2003). Therefore, many areas that could be used are not occupied by endobenthic resident organisms, thereby providing habitat for introduced species without having to compete with native species for space. Whether continued increases in SST in the Wadden Sea will lead to higher individual species densities and, thus, more competition for space and food is a key area of future studies.
More parasites and disease-causing pathogens with climate warming
Interspecific interactions also include the interaction between parasites (including pathogens that cause disease) with their hosts. Parasites form a major, though often ignored, part of biodiversity (Windsor 1998; Wood and Johnson 2015) that can reach substantial biomass in ecosystems (Kuris et al. 2008) and contribute largely to within-ecosystem connectivity (Dunne et al. 2013). In the Wadden Sea, macroparasite diversity can far exceed host diversity. For example, 26 parasitic species were recovered from 10 mollusc hosts (Thieltges et al. 2006) and 16 parasite species from 4 fish hosts (Schade et al. 2015). Successful parasite transmission can be lower with rising temperatures due to transmission interference by non-suitable hosts (Goedknegt et al. 2015), indicating that also parasite diversity can be influenced by climate change.
Climate change increases the likelihood of species introductions, and this also applies to parasites introduced directly or indirectly with other non-native species (Goedknegt et al. 2016a) from which they can spill over to native organisms (Goedknegt et al. 2016b). In this context, parasitic copepods from the genus Mytilicola that infect the guts of bivalves represent an excellent case study. While early observations associated the invasion of M. intestinalis with mass mortalities of its newly acquired host, the blue mussel Mytilus edulis (Korringa 1950), later studies questioned negative effects (Dethlefsen 1975). Now, this parasite is widespread in the Wadden Sea and can reach prevalences of up to 100% of mussel hosts being infected (Feis et al. 2022). Recent experimental studies using controlled infections could repeatedly demonstrate reduced body condition of infected mussels, suggesting that either the parasite itself or the immune response diverts energetic resources away from mussel growth (Feis et al. 2016, 2018). Furthermore, when mussels were additionally challenged with a disease-causing bacterial pathogen (Vibrio orientalis), they showed increased mortalities as a consequence of not being able to clear the bacterial infection from their haemolymph (Demann and Wegner 2019). This effect was amplified at elevated temperatures (Demann and Wegner 2019), indicating that mussels weakened by a non-lethal parasite infection and stressful environmental conditions suffer increasingly from secondary infections of opportunistic pathogens, potentially leading to bigger disease outbreaks that may also affect other host species besides mussels.
In general, disease outbreaks caused by microbial pathogens (bacteria, protists, viruses) are predicted to increase with climate change (Harvell et al. 1999, 2002) and mass mortalities associated with disease and parasites have been reported for several Wadden Sea species (Jensen and Mouritsen 1992; Watermann et al. 2008). However, the occurrence of diseases is only systematically monitored for mammals and birds, while disease-mediated mortality of invertebrates remains elusive (Ward and Lafferty 2004). To better understand the role of disease within the Wadden Sea ecosystem and its possible future trajectories with increasing temperatures, invertebrate mortalities need to be monitored and causative agents need to be identified.
Identification of disease agents is a time-consuming ‘needle in the haystack’ process since only few microbial pathogens are amenable to cultivation for experimental use. An exception to this are bacteria of the genus Vibrio sp., which contain many cultivatable strains that are pathogenic to marine organisms. Mass mortalities of Pacific oysters caused by virulent Vibrio strains (Le Roux et al. 2016) are also sensitive to water temperature. For one, the occurrence of virulent Vibrio strains across seasons correlates positively with temperature (Wendling et al. 2014; Fig. 11), suggesting that warmer water temperatures in the wake of climate change will also lead to higher Vibrio-induced mortality. Experimental studies suggest that virulence of single Vibrio strains is increased with higher temperatures (Wendling and Wegner 2015). However, infection of oysters with virulent Vibrio can also lead to the increase of other opportunistic bacterial genera such as Arcobacter and Mycoplasma (Lokmer and Wegner 2015), indicating that the microbial community inhabiting a host, i.e. its microbiome, is sensitive to perturbation. Mortality may, thus, not be a direct effect of the initial infection but rather a side effect of microbiome disturbance giving rise to other opportunistic pathogens. Indeed, oyster microbiomes are particularly sensitive to temperature and temperature stress (Wegner et al. 2013; Lokmer and Wegner 2015). If the resulting community shifts result in higher vulnerability of many host organisms to disease, cascading ecosystem effects resulting from mortalities caused by the interaction of parasites and disease-causing pathogens with climate change can be expected.
Seasonal correlation between sea surface temperature (red) and the occurrence of virulent Vibrio isolates (blue). The likelihood of encountering a virulent Vibrio isolate is particularly high after peak summer temperatures, indicating that more virulent strains will be present with climate warming. Modified from Buschbaum et al. (2016)
Furthermore, genetic material that turns harmless bacterial symbionts into pathogens (i.e. virulence genes) can propagate between organisms by horizontal gene transfer. This has been shown for antimicrobial resistance genes, where transfer of mobile genetic elements constitutes an important pathway in the prevalence and proliferation of antibiotic resistance, and its transmission to human pathogens (Séveno et al. 2002). Increasing temperatures can accelerate the acquisition of antimicrobial resistance genes (MacFadden et al. 2018). In addition, climate change modifies the bacterial communities associated to species in different compartments of the ecosystem (Lokmer and Wegner 2015; Scanes et al. 2021), potentially impacting the transmission of mobile virulence and antimicrobial resistance genes within the food web (Ferguson et al. 2021; Zhang et al. 2022).
Adaptation to climate change in the Wadden Sea
Adaptive mechanisms of organisms in a warming ocean
Adaptive responses of organisms to the rapidly warming conditions in the Wadden Sea include shifting distribution ranges (e.g. northward shifts by fish species), genetic adaptation in situ (e.g. rapid adaptation to Vibrio pathogens in pacific oyster; Wendling and Wegner 2015) and/or phenotypic plasticity. With plasticity, individuals (genotypes) adjust their phenotype in response to direct environmental cues. Plasticity is thus a fast response mechanism to cope with changing climate conditions (Munday et al. 2013). Such within-generation plasticity can lead to altered phenologies (e.g. earlier spring phytoplankton blooms), changes in reproductive strategies (e.g. increased reproductive output to compensate for potential warming-induced losses) and thermal tolerance mechanisms (e.g. differential expression of genes involved in thermal regulation; Munday et al. 2013). Importantly, plasticity can also occur across generations (transgenerational plasticity). For example, parents exposed to ocean warming can prime their offspring to perform better in warmer environments (Donelson et al. 2018). Information about past environmental conditions is passed on to the next generation via nutrients, hormones and/or epigenetic modifications within gametes, potentially resulting in faster, adaptive plastic responses to future conditions (Adrian-Kalchhauser et al. 2020). In this way, transgenerational plasticity can buy time for slower genetic adaptation to catch up over the longer term.
Plasticity in response to projected ocean warming scenarios has been investigated using marine threespine stickleback (Gasterosteus aculeatus) as a model system and case study for generalisation to other Wadden Sea species. Controlled laboratory breeding experiments revealed several mechanisms underlying plastic responses to ocean warming. A common finding was that reproductive output of females (egg size, clutch size) and offspring growth (body length) were highly plastic both within and across generations (Fig. 12). In nearly all studies, females acclimated to a + 4 °C warming scenario during gametogenesis produced smaller eggs (compared to 17 °C as the ambient summer mean temperature in the List tidal basin; Shama and Wegner 2014, Shama 2015, Shama 2017), and offspring fish reared at + 4 °C had lower growth rates and smaller body size (Ramler et al. 2014; Schade et al. 2014; Shama et al. 2014). That is, negative within-generation plasticity effects occurred at + 4 °C. However, when mothers were acclimated to + 4 °C during gametogenesis and their offspring also developed at + 4 °C, transgenerational plasticity led to (relatively) larger offspring in the matching parent–offspring + 4 °C environments (Fig. 12a; Shama et al. 2014). One mechanism underlying better offspring growth at + 4 °C was more efficient mitochondrial metabolism inherited from + 4 °C mothers. By breeding a grand-offspring generation, it was shown that metabolism responses were underlain by changes to gene expression depending on the maternal and grandmaternal environment (Shama et al. 2016), indicating an epigenetic basis for transgenerational plasticity (Fellous et al. 2022). In addition to phenotypic effects on individual organisms, such non-genetic inheritance mechanisms can also influence the adaptive potential of populations (e.g. invasion potential, migration propensity, and susceptibility to parasites and pathogens; Adrian-Kalchhauser et al. 2020), making their study a key tool to predict potential impacts of climate change for Wadden Sea populations.
Stickleback offspring growth plasticity at ambient (17 °C) and + 4 °C ocean warming scenario (21 °C) depending on; (a) paternal and maternal acclimation temperature combination and; (b) maternal temperature predictability (constant or variable). In; (a) offspring of 21 °C mothers are relatively larger when grown at 21 °C, reflecting transgenerational plasticity. In; (b) mothers acclimated to unpredictable (variable) environments produce more variably sized offspring, suggesting bet hedging. Redrawn from; (a) Shama et al. 2014 and; (b) Shama 2015
In addition to increasing mean seawater temperature, the Wadden Sea is also experiencing an increase in temperature variability, anomalies and heatwaves (see above; Amorim et al. 2023). How organisms respond to environmental variability can differ markedly from responses to directional change. Whereas transgenerational plasticity is predicted to occur when parent environment is predictive of offspring environment, bet hedging by parents (offspring phenotype diversification) is expected in unpredictable environments. Physiological mechanisms underlying thermal hardening—where acute exposure to temperature extremes increases thermal tolerance—are potential adaptive responses (Munday et al. 2013). In stickleback, bet hedging by mothers acclimated to temperature variation (weekly switches between two temperatures) led to higher variance in offspring size (Fig. 12b; Shama 2015), whereas no bet hedging occurred when parents experienced stochastically fluctuating environments (Shama 2017). By simulating the natural temperature variation experienced by this population in the Wadden Sea, it was shown that a 2.5-fold increase in temperature variability is stressful, and almost always leads to smaller fish, but that parental acclimation to temperature variation could offset some of the offspring size losses (Spence-Jones et al. in press). Smaller size is a common finding for studies of fish species responses to ocean warming (Daufresne et al. 2009), and continued warming as well as increasing temperature variability in the Wadden Sea will likely result in progressively smaller adult fish, with potential implications for population dynamics, species interactions and ecosystem functions.
Omics approaches to identify species responses to climate change
Molecular tools are gaining increasing importance to understand the mechanistic basis of adaptive responses to climate change (Hansen et al. 2012). An explicit link between an organism’s immediate phenotypic response to environmental change and its genetic make-up can be established by the differential activity of genes (RNA sequencing). The cumulative activity of all genes, i.e. the transcriptome, can give insights into how different molecular pathways are utilised to respond to climate change throughout development (Fellous et al. 2022) and across generations (Shama et al. 2016), as well as to investigate regulation of specific genes involved in key organism responses such as heat or stress tolerance and immunity (Wegner et al. 2020).
On the genomic level, hardwired information in the DNA can be coupled to phenotypic data to identify adaptive processes such as heterozygote advantage or hybrid vigour (Wendling and Wegner 2015), and how these interact with changing environmental conditions (Wendling et al. 2016). Coupling different -omics approaches like gene activity (transcriptomics) and genetic differentiation (genomics) can link adaptation processes across different time scales. Also, genetic markers with high resolution can be used to trace sources of species invasions, as have been used to track species introductions into the northern Wadden Sea (Moehler et al. 2011; Feis et al. 2019). More specifically, using Pacific oysters from different invasion sources, this approach revealed that mechanisms generating phenotypic diversity on the molecular level (alternative splicing, transposon activity) are activated in novel environments, and genetic differences in these functions can be selected for over longer periods (Wegner et al. 2020). This suggests that colonisation of novel habitats by introduced species can act as a driver of evolutionary change.
Omics tools can also help in the identification of novel introduced species (DNA barcoding), which is particularly important when colonising species belong to cryptic species complexes and show little morphological differentiation (Goedknegt et al. 2018; Waser et al. 2020; Rybalka et al. 2022). Environmental DNA (eDNA) and metabarcoding, the combination of DNA taxonomy and high-throughput sequencing, are other useful tools that allow biodiversity monitoring and subsequent ecological assessment and planning in a changing environment (Deiner et al. 2017; Song and Liang 2023). Metabarcoding has successfully been applied to groups of organisms across the tree of life as well as in terrestrial, freshwater and marine environments (Beng et al. 2016). This method further allows monitoring of target species (i.e. introduced or elusive) at low densities (Ruppert et al. 2019). Even though eDNA metabarcoding still has its limitations, it can nicely complement and expand on traditional methods, and it is an efficient, non-invasive and sensitive tool that can be used for species or overall biodiversity monitoring on large spatial and temporal scales. Thus, -omics approaches are essential for a deep mechanistic insight into organismal responses to climate change and species interactions (Feis et al. 2016). In some applications, they can go as far as confirming functional roles of uncharacterised target genes using gene knock-outs and knock-ins (Wegner et al. 2019). The combination of -omics approaches can help to identify molecular targets responding to multiple climate change related pressures, and directional changes in these gene targets in terms of gene activity or DNA sequence can provide meaningful markers for the underlying molecular pathways to monitor whether species and communities can keep up with the pace of environmental change.
A holistic ecosystem view using novel approaches
Modelling the Wadden Sea food web
Food webs are complex ecological entities, but can be represented as ecological models that capture the balance of the ecosystem as a network of trophic relationships. Such models can be used to understand how specific changes to the network can alter system structure (Baird and Ulanowicz 1989). Food web models involve simplified simulations of the ecological network functionality, and analyse interactions, behaviour and dynamics (Dunne 2009). However, climate change effects at the food web scale are challenging to capture due to the diversity of different effects occurring simultaneously (Fig. 13). For instance, effects of climate change can alter species composition within an area, physiological processes of individual species and also their phenology, all of which can significantly affect the functioning of the food web. The food web of the Wadden Sea was described in a number of studies focusing on trophic interactions of different tidal basins (Asmus and Asmus 1985; Baird et al. 2004; Schückel et al. 2015; de la Vega et al. 2018a; Horn et al. 2019; Jung et al. 2020) and estuaries (de Jonge and Schückel 2019). All studies confirmed that the Wadden Sea is a highly productive ecosystem, with the base of the food web mainly formed by primary producers (phytoplankton and microphytobenthos) and dead organic material. Whether herbivorous or detrivorous pathways dominate the food web is dependent on the habitat and species composition of a specific geographical region (de la Vega et al. 2018b). Benthos is a key link in the food web, transporting energy from the base of the web to higher trophic levels, such as to fish, birds and seals.
Schematic representation of climate change impact on the different trophic levels of the Wadden Sea food web. Different climate change effects alter the species composition, physiology and phenology of organisms with unknown implication for the entire food web. Effects can potentially cascade from one trophic level to the preceding one. The food web illustration was modified after Horn et al. (2021a). Potential bottom-up cascading effects due to rising temperature were included after Barneche et al. (2021)
Although climate change will heavily impact Wadden Sea organisms, the effects have rarely been investigated at the level of the entire food web. Recently, the influence of rising temperature on the physiology of individual system components was studied using Ecological Network Analyses (ENA). ENA provides a structural understanding of network properties, but also specific functional indices (Kay et al. 1989; Fath and Patten 1999; Ulanowicz 2004; Fath et al. 2007). With rising temperatures, ENA indicates an increase in activity at lower trophic levels and reduced energy transfer to higher trophic levels (Baird et al. 2019). ENA also identified effects of changing species compositions, a key consideration, since climate change will facilitate the introduction of new species (see above). Introduced species will inevitably establish new trophic links and, therefore, alter the recipient food web. Effects of non-native species differ along the Wadden Sea coastline, as shown for Pacific oysters (M. gigas; Baird et al. 2012; Jung et al. 2020) and razor clams (Ensis leei; Horn et al. 2017; Jung et al. 2020), with M. gigas causing more severe effects on the food web’s structure and organisation in the northern Wadden Sea (Baird et al. 2012), while E. leei had larger effects in the Dutch intertidal area (Jung et al. 2020).
Modelling approaches such as ENA are useful to provide insights into the functioning of the system and changes due to selected climate change related pressures. In response to changing climate effects such as warming, many biotic components of the food web and abiotic influences are shifting in space and time, leading to alterations to a number of direct and indirect interactions within the network. Food web models analysed with ENA are, however, static in space and time, and results should be interpreted as snapshots of the system. Investigations of continuously resolved influences of environmental changes on food webs and ecosystem responses are not readily feasible with this method (Horn et al. 2021a, b). An extended model with a modified ENA is required to allow investigations of dynamic shifts in and on the system (Horn et al. 2021a, b). The framework Ecopath with Ecosim (EwE; Christensen and Pauly 1992) is also a promising approach to capture food web dynamics and combines general trophic mass-balanced food web modelling with differential equations to describe dynamic energy flows within a food web. Recently, an EwE model determined bottom-up impacts of climate change on a food web using simulations considering a medium and high emissions climate scenario (Whitehouse et al. 2021). Thus, EwE could provide a suitable tool to investigate climate change effects, also in combination with other stressors. In the northern Wadden Sea, EwE was used to investigate potential effects of seagrass recovery, and revealed changes in the associated benthic fauna and its predators due to higher food availability and shelter provision caused by increased seagrass biomass (Horn et al. 2021a), highlighting the importance to include mediating effects into modelling approaches.
Model simulations using EwE could also be valuable for exploring potential ecosystem-based management decisions. Since the early 2000s, the European Commission has brought forward several directives which explicitly promote the implementation of ecosystem-based management decisions within the European Union (Safi et al. 2019; de Jonge and Schückel 2021). For example, assessing the quality of the structure and the functioning of ecosystems are major concerns of the Water Framework Directive and the Marine Strategy Framework Directive (Safi et al. 2019; Oprandi et al. 2023). Accordingly, both directives call for the use of suitable indicators (such as ENA indicators) in the assessment of ‘Good Environmental Status’. However, in marine conservation and policy, the application of status indicators is still developing because links between indicators and the pressures affecting them can be obscured by food web dynamics, data limitations or cumulative effects of multiple pressures (McQuatters-Gollop et al. 2019). A first approach was recently published in Schückel et al. (2023). Holistic, function-based criteria and indices provided by models that feed into ecosystem-based management decisions and inform decision-making processes can foster marine biodiversity conservation (Safi et al. 2019).
Mesocosms as a unique infrastructure to simulate climate change at the ecosystem level
Experiments are helpful to elucidate causal relationships and mechanisms underlying interactions between abiotic changes and responses of organisms, but they are often too simplistic (e.g. single-species laboratory experiments) or too complex with many variables that cannot be controlled (e.g. field studies). Mesocosms are a unique approach to address important questions relevant for coastal ecology, and have become an indispensable tool for climate-change related research to bridge the gap between field and laboratory experiments. Importantly, they allow the manipulation of environmental factors under near-natural conditions at the community level.
As part of its research infrastructure, the Wadden Sea Station Sylt has a unique mesocosm facility (AWISOM; Alfred-Wegener-Institut Helmholtz-Zentrum für Polar-und Meeresforschung 2023) that was specifically designed to investigate effects of future climate change scenarios on marine intertidal and subtidal biota in a near-natural way. The facility consists of 24 large outdoor mesocosms (each containing 1800 L of seawater), plus an additional 6 mesocosms housed within a greenhouse that are used for experiments during winter (Fig. 14), making it one of the largest and most technically advanced mesocosm facilities in Europe. The system is equipped with software for simulating tidal cycles and water currents, and for precisely regulating water temperatures and pCO2 (Pansch et al. 2016), for example, in accordance with IPCC climate scenarios (IPCC 2022). Integrated multi-parameter probes ensure continuous recording of abiotic factors such as dissolved oxygen, pH, water temperature and conductivity, facilitating digital data collection and future internet-based observation to archive technologies (see Box 2).
Box 2: Observation to archive—the future of data collection
With the rise of digital transformation, data handling will be revolutionised during the next few years, especially with regard to field work. Digital tools will gradually replace traditional methods due to increased efficiency and accuracy of data collection, and improved accessibility and exchange. Despite the fact that digitalisation has affected virtually every aspect of research, many scientists still use handwritten notes that are manually transferred into a digital format that allows further processing (e.g. sharing, analysis, visualisation). Thus, in contrast to the enormous expansion of data processing capabilities, data collection has not evolved along with digitalisation. Importantly, digital transformation plays a significant role in promoting FAIR (Findable, Accessible, Interoperable, Reusable) standards for scientific data, which are now mandatory for many research institutions. Internet of Things (IoT) technologies (networking of internet-enabled devices) offer promising approaches to address data collection issues (e.g. by using smartphone applications). These cloud-based techniques provide an initial quality check of acquired data in the field based on quality-assurance algorithms, and data can be transferred in real time for further processing in other web services. Data formats are compatible with other applications, which is a key component for the functionality of IoT technologies to allow complementary analyses. At the same time, IoT-based measurement systems can be easily adapted to specific requirements, remotely parameterised and combined to create individual measurement networks. Internet-based, bidirectional communication with measuring devices also facilitates citizen science, as data can be integrated directly into measurement campaigns, thereby increasing the number of data points. Such enhanced data exchange and flexibility can facilitate the early detection of trends or potential shifts in the overall system. At the Wadden Sea Station Sylt, usability tests of digital data collection via voice control as well as automated simulation of abiotic parameters in mesocosm experiments showed that data can be provided online without time delay, can be downloaded on local devices in various formats and fed into, e.g., time series databases |
The first experiments conducted in the mesocosms investigated the impacts of warming, ocean acidification and increased nutrient input on benthic Wadden Sea communities. Exposing macroalgae (Fucus vesiculosus), bivalves (Pacific oyster and blue mussel), gastropods (Littorina spp.) and amphipods (Gammarus spp.) for several months to environmental conditions predicted for 2100 showed how different species and trophic levels within the community responded to climate stressors, both alone and in combination (Pansch et al. 2016; Mensch et al. 2016, Pansch 2019). Pelagic community responses to specific IPCC climate scenarios were also investigated by exposing phyto- and zooplankton to increased temperature and CO2. These experiments could show that a tipping point occurred between two IPCC scenarios, and that large shifts in traits and processes within planktonic food webs are to be expected in the future (Moreno et al. 2022). In salt marsh plants, higher temperature and CO2 concentrations led to differential responses of C3 (Elymus athericus) and C4 (Spartina anglica) plant species. While Spartina anglica showed increases in stem density and biomass (Koop-Jakobsen and Dolch 2023), as well as improved biomechanical traits such as stem diameter and flexural rigidity (Paul et al. 2022), Elymus arthericus showed no response to altered climate conditions. These observed alterations of biomass production may have implications for blue carbon sequestration (see Box 1). Responses of organisms at higher trophic levels (e.g. small fish species) were also tested using mesocosms experiments. For instance, two studies of ocean warming impacts on threespine stickleback mate choice showed that smaller males had higher reproductive success at warmer temperatures (Fuxjäger et al. 2019; Wanzenböck et al. 2022), supporting the premise that “bigger is not always better” under climate change. Currently, increasing ocean temperature variation and extreme events such as heatwaves are posing the largest threat to Wadden Sea communities, and mesocosm experiments that simulate different heatwave intensities and durations will play a key role in our ability to predict responses at the community level, and potential consequences for ecosystem processes.
Conclusions
Our synthesis of research investigating consequences of climate change in the northern Wadden Sea reveals that warming and the associated rise in sea level cause effects from the ecosystem to the genetic level. Coastal morphology and hydrodynamics are affected by increasing relative sea level (RSL). Both warming and rising RSL alter tidal flats, salt marshes and seagrass meadows, as well as their ecological services (e.g. carbon sequestration), and warming influences species interactions, genetic and phenotypic adaptation mechanisms of species, and the functioning of food webs (Box 3). Climate change impacts are clearly reflected at all levels of the ecosystem, underlining the overarching consequences of these human-induced pressures on the Wadden Sea.
Box 3: Climate change effects in the northern Wadden Sea
• Rising relative sea level (RSL) leads to erosion, changes to coastal geomorphology and sediment composition, and coupled with stronger winds to an increase in storm surge frequency and magnitude • Overall mean increase in sea surface temperature (SST) of 1.8 °C is almost double the global average, and there is a clear trend of more warmer winters since 1991 • Salt marshes and seagrass meadows important for coastal protection and blue carbon storage are affected by both rising RSL and SST • Increasing SST reduces spring phytoplankton blooms, alters plankton phenology and changes plankton as well as fish community composition • Warming SST facilitates non-native species introductions (e.g. habitat engineers, parasites, pathogens) and leads to changing species interactions (e.g. predator–prey temporal mismatch) and food web dynamics • Organisms adapt to increasing SST via genetic adaptation and phenotypic plasticity, underlain by molecular mechanisms for thermal tolerance |
Despite the research highlighted in this synthesis, there are still many uncertainties about the effects of climate change on the Wadden Sea. Long-term investigations show that climate change has led to a rise in species numbers and biomass of both epibenthic and endobenthic organisms (Beukema et al. 2017; Beukema and Dekker 2020; Lackschewitz et al. 2022). Thus, species diversity does not appear to have suffered from climate change so far. In the long term, however, warming and sea level rise will affect the habitat structure of the Wadden Sea with impacts on associated species communities. For example, potential changes in the upper intertidal (via area loss and prolonged flooding) and lower intertidal (permanently submerged) caused by an increasing rate of sea level rise will have consequences for species community patterns, especially for species that require the entire tidal range for their ontogeny. For example, lugworms, Arenicola marina, and tellinid clams, Macoma balthica, settle in the higher intertidal zone but migrate to lower intertidal zones as adults (Armonies and Hellwig-Armonies 1992; Reise et al. 2001). Therefore, any change in spatial extent and water residence time of the tidal flats in the different intertidal zones will significantly affect the occurrence of some species.
Long-term descriptive studies of species occurrence provide important insights in population dynamics and developments (Reise 2003; Thieltges et al. 2004; Beukema and Dekker 2020), but cannot explain the underlying processes. Experimental approaches in the field and within mesocosms in which future warming and inundation scenarios of tidal flats are simulated can provide first hints about population-level consequences. Moreover, a combination of research approaches from across disciplines could be an important instrument to close existing knowledge gaps and merge disciplines whose separation is still partly visible in this synthesis. For example, changes to sediment composition and budgets are well investigated by geologists, but information about potential consequences for habitats (e.g. bivalve reefs, seagrass meadows) as well as benthic species communities and population dynamics are hardly known. In addition, a wealth of data on changes in species diversity and interactions between species could be incorporated into modelling approaches to assess consequences for trophic networks and to develop future scenarios.
In our synthesis, we focused mainly on effects of warming and sea level rise on abiotic and biotic ecosystem processes. However, the Wadden Sea also has high cultural and economic importance, e.g. for arts, literature and tourism (Ratter and Gee 2012; Döring et al. 2022). Effects of warming and sea level rise on the ecosystem will have consequences for social structures and human welfare of coastal inhabitants, and vice versa. Adaptation and mitigation strategies cannot only focus on ecosystem or social services, but must consider the Wadden Sea as a closely interconnected system. Climate change forces society to look at the Wadden Sea in a new way and to develop innovative solution strategies with regard to coastal protection measures and tourism, but also species and habitat protection. This can be best achieved through interdisciplinary research developed together by social and natural scientists, for which initial promising approaches already exist (e.g. Daschkeit and Schottes 2002; Reise 2015; Jordan et al. 2023; Kötter-Lange et al. 2023). Strategies to cope with climate change in coastal ecosystems can benefit from the foundation of research conducted over the last 100 years at the AWI Wadden Sea station Sylt, with the shared goal of preserving this unique ecosystem and world heritage site.
References
Adrian-Kalchhauser I, Sultan SE, Shama LNS, Spence-Jones H, Tiso, S, Keller Valsecchi CI, Weissing FJ (2020) Understanding “non-genetic” inheritance: Insights from molecular-evolutionary crosstalk. Trends Ecol Evol 35:1078–1089. https://www.sciencedirect.com/science/article/pii/S0169534720302263
Al-Janabi B, Kruse I, Graiff A, Karsten U, Wahl M (2016) Genotypic variation influences tolerance to warming and acidification of early life‑stage Fucus vesiculosus L. (Phaeophyceae) in a seasonally fluctuating environment. Mar Biol 163:14. https://doi.org/10.1007/s00227-015-2804-8
Albrecht AS (1998) Soft bottom versus hard rock: Community ecology of macroalgae on intertidal mussel beds in the Wadden Sea. J Exp Mar Biol Ecol 229(1):85–109. https://doi.org/10.1016/S0022-0981(98)00044-6
Albrecht A, Reise K (1994) Effects of Fucus vesiculosus covering intertidal mussel beds in the Wadden Sea. Helgoländer Meeresuntersuchungen 48:243–256. https://link.springer.com/article/10.1007/BF02367039
Alfred-Wegener-Institut Helmholtz-Zentrum für Polar-und Meeresforschung (2023) Marine Stations Helgoland and Sylt operated by the Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research. J Large-Scale Res Facil 8:A184. https://doi.org/10.17815/jlsrf-8-184
Armonies W (2017) Long-term change of meiofaunal species composition in a sandy beach, with description of 7 new species of Platyhelminthes. Helgol Mar Res 71:12
Armonies W, Asmus H, Buschbaum C, Lackschewitz D, Reise K, Rick J (2018) Microscopic species make the diversity: a checklist of marine fora and fauna around the Island of Sylt in the North Sea. Helgol Mar Res 72:11
Armonies W, Buschbaum C, Mielck F, Rick J (2023) Mollusc shell detritus affects benthic subtidal community dynamics in the Northern Wadden Sea. Mar Biodivers 53:2
Amorim FdeLL, Wiltshire KH, Lemke P, Carstens K, Peters S, Rick J, Gimenez L, Scharfe M (2023) Investigation of marine temperature changes across temporal and spatial gradients: providing a fundament for studies on the effects of warming on marine ecosystem function and biodiversity. Prog Oceanogr 216:1–24. https://doi.org/10.1016/j.pocean.2023.103080
Androsov A, Fofonova V, Kuznetsov I, Danilov S, Rakowsky N, Harig S, Brix H, Wiltshire KH (2019) FESOM-C vol 2: coastal dynamics on hybrid unstructured meshes. Geosci Model Dev 12:1009–1028. https://doi.org/10.5194/gmd-12-1009-2019
Allen JRL (2000) Morphodynamics of Holocene salt marshes: a review sketch from the Atlantic and Southern North Sea coasts of Europe. Quatern Sci Rev 19:1155–1231. https://doi.org/10.1016/S0277-3791(99)00034-7
Andersen TJ, Svinth S, Pejrup M (2011) Temporal variation of accumulation rates on a natural salt marsh in the 20th century — the impact of sea level rise and increased inundation frequency. Mar Geol 279:178–187. https://doi.org/10.1016/j.margeo.2010.10.025
Arbic BK, Garrett CA (2010) Coupled oscillator model of shelf and ocean tides. Cont Shelf Res 30:564–574. https://doi.org/10.1016/j.csr.2009.07.008
Arns A, Dangendorf S, Jensen J, Talke S, Bender J, Pattiaratchi C (2017) Sea-level rise induced amplification of coastal protection design heights. Sci Rep 7:40171. https://doi.org/10.1038/srep40171
Arns A, Wahl T, Dangendorf S, Jensen J (2015) The impact of sea level rise on storm surge water levels in the northern part of the German Bight. Coast Eng 96:118–131. https://doi.org/10.1016/j.coastaleng.2014.12.002
Armonies W, Herre E, Sturm M (2001) Effects of the severe winter 1995/96 on the benthic macrofauna of the Wadden Sea and the coastal North Sea near the island of Sylt. Helgol Mar Res 55:170–175. https://doi.org/10.1007/s101520100077
Armonies W, Reise K (2003) Empty habitat in coastal sediments for populations of macrozoobenthos. Helgol Mar Res 56:279–287. https://doi.org/10.1007/s10152-002-0129-8
Armonies W, Hellwig-Armonies M (1992) Passive settlement of Macoma balthica spat on tidal flats of the Wadden Sea and subsequent migration of juveniles. Neth J Sea Res 29:371–378. https://doi.org/10.1016/0077-7579(92)90076-Q
Asmus H, Asmus R (1985) The importance of grazing food chain for energy flow and production in three intertidal sand bottom communities of the northern Wadden Sea. Helgoländer Meeresun 39:273–301. https://doi.org/10.1007/BF01992775
Baird D, Asmus H, Asmus R (2004) Energy flow of a boreal intertidal ecosystem, the Sylt-Rømø Bight. Mar Ecol Progress Ser 279:45–61. https://www.jstor.org/stable/24867824. Accessed 28 Sep 2004
Baird D, Ulanowicz RE (1989) The seasonal dynamics of the Chesapeake Bay ecosystem. Ecol Monogr 59:329–364. https://doi.org/10.2307/1943071
Baird D, Asmus H, Asmus R (2012) Effect of invasive species on the structure and function of the Sylt-Rømø Bight ecosystem, northern Wadden Sea, over three time periods. Mar Ecol Prog Ser 462:143–162. https://doi.org/10.3354/meps09837
Baird D, Asmus H, Asmus R, Horn S, de la Vega C (2019) Ecosystem response to increasing ambient water temperatures due to climate warming in the Sylt-Rømø Bight, northern Wadden Sea, Germany. Estuar Coast Shelf Sci 228:106322. https://doi.org/10.1016/j.ecss.2019.106322
Barneche DR, Hulatt CJ, Dossena M, Padfield D, Woodward G, Trimmer M, Yvon-Durocher G (2021) Warming impairs trophic transfer efficiency in a long-term field experiment. Nature 592:76–79. https://doi.org/10.1038/s41586-021-03352-2
Becherer J, Hofstede J, Gräwe U, Purkiani K, Schulz E, Burchard H (2018) The Wadden Sea in transition – consequences of sea level rise. Ocean Dyn 68:131–151. https://doi.org/10.1007/s10236-017-1117-5
Behre K-E (2002) Landscape development and occupation history along the southern North Sea coast. In: Climate development and history of the North Atlantic Realm. Springer Berlin Heidelberg, pp 299–312. https://doi.org/10.1007/978-3-662-04965-5_18
Beng KC, Tomlinson KW, Shen XH, Surget-Groba Y, Hughes AC, Corlett RT, Ferry Slik JW (2016) The utility of DNA metabarcoding for studying the response of arthropod diversity and composition to land-use change in the tropics. Sci Rep 6:24965. https://doi.org/10.1038/srep24965
Benninghoff M, Winter C (2019) Recent morphologic evolution of the German Wadden Sea. Sci Rep 9:9293. https://doi.org/10.1038/s41598-019-45683-1
Beukema J, Dekker R (2014) Variability in predator abundance links winter temperatures and bivalve recruitment: correlative evidence from long-term data in a tidal flat. Mar Ecol Prog Ser 513:1–15. https://doi.org/10.3354/meps10978
Beukema JJ, Dekker R (2020) Half a century of monitoring macrobenthic animals on tidal flats in the Dutch Wadden Sea. Mar Ecol Prog Ser 656:1–18. https://doi.org/10.3354/meps13555
Beukema JJ, Dekker R, Drent J (2017) Dynamics of a Limecola (Macoma) balthica population in a tidal flat area in the western Wadden Sea: effects of declining survival and recruitment. Helgol Mar Res 71:18. https://doi.org/10.1186/s10152-017-0498-7
Beukema JJ, Dekker R, van Stralen MR, de Vlas J (2015) Large-scale synchronization of annual recruitment success and stock size in Wadden Sea populations of the mussel Mytilus edulis L. Helgol Mar Res 69:327–333. https://doi.org/10.1007/s10152-015-0440-9
Billerbeck M, Werner U, Polerecky L, Walpersdorf E, de Beer D, Huettel M (2006) Surficial and deep pore water circulation governs spatial and temporal scales of nutrient recycling in intertidal sand flat sediment. Mar Ecol Prog Ser 326:61–76. https://doi.org/10.3354/meps326061
Bleile N, Thieltges DW (2021) Prey preferences of invasive (Hemigrapsus sanguineus, H. takanoi) and native (Carcinus maenas) intertidal crabs in the European Wadden Sea. J Mar Biol Assoc UK 101:811–817. https://doi.org/10.1017/S0025315421000655
Boersma M, Malzahn AM, Greve W, Javidpour J (2007) The first occurrence of the ctenophore Mnemiopsis leidyi in the North Sea. Helgol Mar Res 61:153–155. https://doi.org/10.1007/s10152-006-0055-2
Büttger H, Christoph S, Buschbaum C, Gittenberger A, Jensen K, Kabuta S, Lackschewitz D (2022) Alien species. In: Kloepper S et al. (eds) Common Wadden Sea Secretariat, Wilhelmshaven, Germany
Büttger H, Nehls G, Stoddard P (2014) The history of intertidal blue mussel beds in the North Frisian Wadden Sea in the 20th century: can we define reference conditions for conservation targets by analysing aerial photographs? J Sea Res 87:91–102. https://doi.org/10.1016/j.seares.2013.12.001
Buschbaum C, Chapman AS, Saier B (2006) How an introduced seaweed can affect epibiota diversity in different coastal systems. Mar Biol 148:743–754. https://doi.org/10.1007/s00227-005-0128-9
Buschbaum C, Cornelius A, Goedknegt MA (2016) Deeply hidden inside introduced biogenic structures–Pacific oyster reefs reduce detrimental barnacle overgrowth on native blue mussels. J Sea Res 117:20–26. https://doi.org/10.1016/j.seares.2016.09.002
Buschbaum C, Lackschewitz D, Reise K (2012) Nonnative macrobenthos in the Wadden Sea ecosystem. Ocean Coast Manag 68:89–101. https://doi.org/10.1016/j.ocecoaman.2011.12.011
Buschbaum C, Horn S (2024) Community and trophic effects of introduced species in the European Wadden Sea. In: Baird D, Elliot M (eds) Treatise on estuarine and coastal science, vol 4, 2nd edn. Elsevier, Oxford, pp 645–668
Chernetsky AS, Talke SHM, SA, (2010) The effect of tidal asymmetry and temporal settling lag on sediment trapping in tidal estuaries. Ocean Dyn 60:1219–1241. https://doi.org/10.1007/s10236-010-0329-8
Chmura GL, Anisfeld SC, Cahoon DR, Lynch JC (2003) Global carbon sequestration in tidal, saline wetland soils. Glob Biogeochem Cycles 17:1111. https://doi.org/10.1029/2002gb001917
Chmura GL (2013) What do we need to assess the sustainability of the tidal salt marsh carbon sink? Ocean Coast Manag 83:25–31. https://doi.org/10.1016/j.ocecoaman.2011.09.006
Christensen V, Pauly D (1992) ECOPATH II—a software for balancing steady-state ecosystem models and calculating network characteristics. Ecol Model 61:169–185. https://doi.org/10.1016/0304-3800(92)90016-8
Clark NJ, Kerry JT, Fraser CI (2020) Rapid winter warming could disrupt coastal marine fish community structure. Nat Clim Chang 1:862–867. https://doi.org/10.1038/s41558-020-0838-5
Cornelius A, Buschbaum C (2020) Introduced marine ecosystem engineers change native biotic habitats but not necessarily associated species interactions. Estuar Coast Shelf Sci 245:106936. https://doi.org/10.1016/j.ecss.2020.106936
Cornelius A, Wagner K, Buschbaum C (2021) Prey preferences, consumption rates and predation effects of Asian shore crabs (Hemigrapsus takanoi) in comparison to native shore crabs (Carcinus maenas) in nortwestern Europe. Mar Biodivers 51:75. https://doi.org/10.1007/s12526-021-01207-7
Daschkeit A, Schottes P (2002) Klimafolgen für Mensch und Küste am Beispiel der Nordseeinsel Sylt. Umweltnatur- & Umweltsozialwissenschaften. Springer, Berlin, Heidelberg, p 335
Daufresne M, Lengfellner K, Sommer U (2009) Global warming benefits the small in aquatic ecosystems. PNAS 106:12788–12793. https://doi.org/10.1073/pnas.0902080106
Deiner K, Bik HM, Mächler E et al (2017) Environmental DNA metabarcoding: transforming how we survey animal and plant communities. Mol Ecol 26:5872–5895. https://doi.org/10.1111/mec.14350
de Beer D, Wenzhöfer F, Ferdelman TG, Boehme SE, Huettel M (2005) Transport and mineralization rates in North Sea sandy intertidal sediments, Sylt-Rømø Basin, Wadden Sea. Limnology and Oceanography 50(1): 113–127. https://doi.org/10.4319/lo.2005.50.1.0113
De Jong F (2007) Marine eutrophication in perspective: on the relevance of ecology for environmental policy. Springer Science and Business Media. https://doi.org/10.1007/3-540-33648-6
De Jonge VN, Schückel U (2019) Exploring effects of dredging and organic waste on the functioning and the quantitative biomass structure of the Ems estuary food web by applying Input Method balancing in Ecological Network Analysis. Ocean Coast Manag 174:38–55. https://doi.org/10.1016/j.ocecoaman.2019.03.013
De Jonge VN, Schückel U (2021) A comprehensible short list of ecological network analysis indices to boost real ecosystem-based management and policy making. Ocean Coast Manag 208:105582. https://doi.org/10.1016/j.ocecoaman.2021.105582
De la Vega C, Horn S, Baird D, Hines D, Borret S, Jensen LF, Schwemmer P, Asmus R, Siebert U, Asmus H (2018a) Seasonal dynamics and functioning of the Sylt-Rømø Bight, northern Wadden Sea. Estuar Coast Shelf Sci 203:100–118. https://doi.org/10.1016/j.ecss.2018.01.021
De la Vega C, Schückel U, Horn S, Kröncke I, Asmus R, Asmus H (2018b) How to include ecological network analysis results in management? A case study of three tidal basins of the Wadden Sea, south-eastern North Sea. Ocean Coast Manag 163:401–416. https://doi.org/10.1016/j.ocecoaman.2018.07.019
Demann F, Wegner KM (2019) Infection by invasive parasites increases susceptibility of native hosts to secondary infection via modulation of cellular immunity. J Anim Ecol 88:427–438. https://doi.org/10.1111/1365-2656.12939
Dethlefsen V (1975) The influence of Mytilicola intestinalis STEUER on meat content of mussel Mytilus edulis. Aquaculture 6:83–97. https://doi.org/10.1016/0044-8486(75)90091-5
Devlin TA, Pan J (2020) Tidal evolution related to changing sea level; worldwide and regional surveys, and the impact to estuaries and other coastal zones. Estuaries and Coastal Zones - Dynamics and Response to Environmental Changes, Intech Open. https://doi.org/10.5772/intechopen.91061
Diederich S (2005) Differential recruitment of introduced Pacific oysters and native mussels at the North Sea coast: coexistence possible? J Sea Res 53:269–281. https://doi.org/10.1016/j.seares.2005.01.002
Diederich S, Nehls G, van Beusekom JE, Reise K (2005) Introduced Pacific oysters (Crassostrea gigas) in the northern Wadden Sea: invasion accelerated by warm summers? Helgol Mar Res 59:97–106. https://doi.org/10.1007/s10152-004-0195-1
Dissanayake DMPK, Ranasinghe R, Roelvink JA (2009) Effect of sea level rise in tidal inlet evolution: a numerical modelling approach. J Coast Res 56:942–946. JSTOR. https://www.jstor.org/stable/25737925
Dissanayake DMPK, Ranasinghe R, Roelvink JA (2012) The morphological response of large tidal inlet/basin systems to relative sea level rise. Clim Chang 113:253–276. https://doi.org/10.1007/s10584-012-0402-z
Dolch T, Hass HC (2008) Long-term changes of intertidal and subtidal sediment compositions in a tidal basin in the northern Wadden Sea (SE North Sea). Helgol Mar Res 62:3–11. https://doi.org/10.1007/s10152-007-0090-7
Dolch T, Reise K (2010) Long-term displacement of intertidal seagrass and mussel beds by expanding large sandy bedforms in the northern Wadden Sea. J Sea Res 63:93–101. https://doi.org/10.1016/j.seares.2009.10.004
Dolch T, Buschbaum C, Reise K (2013) Persisting intertidal seagrass beds in the northern Wadden Sea since the 1930s. J Sea Res 82:134–141. https://doi.org/10.1016/j.seares.2012.04.007
Dolch T, Folmer EO, Frederiksen MS, Herlyn M, van Katwijk MM, Kolbe K, Krause-Jensen D, Schmedes P, Westerbeek EP (2017) Seagrass. In: Kloepper S et al (eds) Wadden sea quality status report 2017. Common Wadden Sea Secretariat, Wilhelmshaven. qsr.waddensea-worldheritage.org/reports/seagrass
Donatelli C, Duran-Matute M, Gräwe U, Gerkema T (2022) Residual circulation and freshwater retention within an event-driven system of intertidal basins. J Sea Res 186:102242. https://doi.org/10.1016/j.seares.2022.102242
Donelson JM, Salinas S, Munday PL, Shama LNS (2018) Transgenerational plasticity and climate change experiments: Where do we go from here? Glob Chang Biol 24:13–34. https://doi.org/10.1111/gcb.13903
Döring M, Walsh C, Ratter B (2022) Emplaced climate imaginaries: The regional construction of climate futures on the German Wadden Sea Coast. Geoforum 137:222–229. https://doi.org/10.1016/j.geoforum.2022.02.010
Dunne JA (2009) Food webs. In: Meyers R (eds) Encyclopedia of complexity and systems science. Springer, New York. https://doi.org/10.1007/978-0-387-30440-3_216
Dunne JA, Lafferty KD, Dobson AP, Hechinger RF, Kuris AM, Martinez ND, McLaughlin JP, Mouritsen KN, Poulin R, Reise K, Stouffer DB, Thieltges DW, Williams RJ, Zander CD (2013) Parasites affect food web structure primarily through increased diversity and complexity. PLoS Biol 11:e1001579. https://doi.org/10.1371/journal.pbio.1001579
Dulvy NK, Rogers SI, Jennings S, Stelzenmüller V, Dye SR, Skjoldal HR (2008) Climate change and deepening of the North Sea fish assemblage: a biotic indicator of warming seas. J Appl Ecol 45:1029–1039. https://doi.org/10.1111/j.1365-2664.2008.01488.x
Durant JM, Hjermann DØ, Ottersen G, Stenseth NC (2007) Climate and the match or mismatch between predator requirement and resource availability. Clim Res 33:271–283. https://doi.org/10.3354/cr033271
Duran-Matute M, Gerkema T, Sassi MG (2016) Quantifying the residual volume transport through a multiple-inlet system in response to wind forcing: the case of the Western Dutch Wadden Sea. J Geophys Res Oceans 121:8888–8903. https://doi.org/10.1002/2016JC011807
Duarte CM, Losada IJ, Hendriks IE, Mazarrasa I, Marbà N (2013) The role of coastal plant communities for climate change mitigation and adaptation. Nat Clim Chang 3:961–968. https://doi.org/10.1038/nclimate1970
Elliott M, Hemingway KL (2002) Fishes in estuaries. Blackwell Sci. https://doi.org/10.1002/9780470995228
Elliott M, Whitfield AK, Potter IC, Blaber SJM, Cyrus DP, Nordlie FG, Harrison TD (2007) The guild approach to categorizing estuarine fish assemblages: a global review. Fish Fish 8:241–268. https://doi.org/10.1111/j.1467-2679.2007.00253.x
Elschot K, Bakker JP, Temmerman S, van de Koppel J, Bouma TJ (2015) Ecosystem engineering by large grazers enhances carbon stocks in a tidal salt marsh. Mar Ecol Prog Ser 537:9–21. https://doi.org/10.3354/meps11447
Eschweiler N, Christensen HT (2011) Trade-off between increased survival and reduced growth for blue mussels living on Pacific oyster reefs. J Exp Mar Biol Ecol 403(1-2):90–95. https://doi.org/10.1016/j.jembe.2011.04.010
Esselink P, van Duin WE, Bunje J, Cremer J, Folmer EO, Frikke J, Glahn M, de Groot AV, Hecker N, Hellwig U, Jensen K, Körber P, Petersen J, Stock M (2017) Salt marshes. Wadden Sea Quality Status Report. Common Wadden Sea Secretariat, Wilhelmshaven, Germany. qsr.waddensea-worldheritage.org/reports/salt-marshes
Fath BD, Scharler UM, Ulanowicz RE, Hannon B (2007) Ecological network analysis: network construction. Ecol Model 208:49–55. https://doi.org/10.1016/j.ecolmodel.2007.04.029
Fath BD, Patten BC (1999) Review of the foundations of network environ analysis. Ecosystems 2:167–179. https://doi.org/10.1007/s100219900067
Feis ME, Goedknegt MA, Thieltges DW, Buschbaum C, Wegner KM (2016) Biological invasions and host-parasite coevolution: different coevolutionary trajectories along separate parasite invasion fronts. Zoology 119:366–374. https://doi.org/10.1016/j.zool.2016.05.012
Feis ME, John U, Lokmer A, Luttikhuizen PC, Wegner KM (2018) Dual transcriptomics reveals co-evolutionary mechanisms of intestinal parasite infections in blue mussels Mytilus edulis. Mol Ecol 27:1505–1519. https://doi.org/10.1111/mec.14541
Feis ME, Goedknegt MA, Arzul I, Chenuil A, Boon OD, Gottschalck L, Kondo Y, Ohtsuka S, Shama LNS, Thieltges DW, Wegner KW, Luttikhuizen PC (2019) Global invasion genetics of two parasitic copepods infecting marine bivalves. Sci Rep 9:12730. https://doi.org/10.1038/s41598-019-48928-1
Feis ME, Gottschalck L, Ruf LC, Theising F, Demann F, Wegner KM (2022) Invading the occupied niche: how a parasitic copepod of introduced oysters can expel a congener from native mussels. Front Mar Sci 9:915841. https://doi.org/10.3389/fmars.2022.915841
Fellous A, Wegner KM, John U, Mark FC, Shama LNS (2022) Windows of opportunity: ocean warming shapes temperature-sensitive epigenetic reprogramming and gene expression across gametogenesis and embryogenesis in marine stickleback. Glob Chang Biol 28:54–71. https://doi.org/10.1111/gcb.15942
Ferguson RMW, O’Gorman EJ, McElroy DJ, McKew BA, Coleman RA, Emmerson MC, Dumbrell AJ (2021) The ecological impacts of multiple environmental stressors on coastal biofilm bacteria. Glob Chang Biol 27:3166–3178. https://doi.org/10.1111/gcb.15626
Feser F, Barcikowska M, Krueger O, Schenk F, Weisse R, Xia L (2015) Storminess over the North Atlantic and Northwestern Europe—a review. Q J R Meteorol Soc 141:350–382. https://doi.org/10.1002/qj.2364
Flemming B, Davis RA Jr (1994) Holocene evolution, morphodynamics and sedimentology of the Spiekeroog barrier island system (southern North Sea). Senckenb Marit 24:117–155
Flemming BW, Nyandwi N (1994) Land reclamation as a cause of fine-grained sediment depletion in backbarrier tidal flats (southern North Sea). Neth J Aquat Ecol 28:299–307
Flemming BW, Bartholomä A (1997) Response of the Wadden Sea to a rising sea level: a predictive empirical model. Deutsche Hydrographische Zeitschrift 49:343–353
Fofonova V, Androsov A, Sander L, Kuznetsov I, FdeLL A, Hass HC, Wiltshire KH (2019) Non-linear aspects of the tidal dynamics in the Sylt-Rømø Bight, south-eastern North Sea. Ocean Sci 15:1761–1782. https://doi.org/10.5194/os-15-1761-2019
Fossheim M, Primicerio R, Johannesen E, Ingvaldsen RB, Aschan MM, Dolgov AV (2015) Recent warming leads to a rapid borealization of fish communities in the Arctic. Nat Clim Chang 5:673–677. https://doi.org/10.1038/nclimate2647
Fourqurean JW, Duarte CM, Kennedy H, Marbà N, Holmer M, Mateo MA, Apostolaki ET, Kendrick GA, Krause-Jensen D, McGlathery KJ, Serrano O (2012) Seagrass ecosystems as a globally significant carbon stock. Nat Geosci 5(7):505–509. https://doi.org/10.1038/ngeo1477
Fruergaard M, Sander L, Goslin J, Andersen TJ (2021) Temporary late Holocene barrier-chain deterioration due to insufficient sediment availability, Wadden Sea, Denmark. Geology 49:162–167. https://doi.org/10.1130/G47978.1
Fuxjager L, Wanzenböck S, Ringler E, Wegner KM, Ahnelt H and Shama LNS (2019) Within-generation and transgenerational plasticity of mate choice in oceanic stickleback under climate change. Philos Trans R Soc 374:20180183. https://doi.org/10.1098/rstb.2018.0183
Ganske A, Tinz B, Rosenhagen G, Heinrich H (2016) Interannual and multidecadal changes of wind speed and directions over the North Sea from climate model results. Meteorol Z 25:463–478. https://doi.org/10.1127/metz/2016/0673
Geburzi JC, Brandis D, Buschbaum C (2018) Recruitment patterns, low cannibalism and reduced interspecific predation contribute to high invasion success of two Pacific crabs in northwestern Europe. Estuar Coast Shelf Sci 200:460–472. https://doi.org/10.1016/j.ecss.2017.11.032
Gerkema T, Duran-Matute M (2017) Interannual variability of mean sea level and its sensitivity to wind climate in an inter-tidal basin. Earth Syst Dyn 8:1223–1235. https://doi.org/10.5194/esd-8-1223-2017
Gimenez L, Boersma M, Wiltshire K (2024) A multiple baseline approach for marine heatwaves. Limnol Oceanogr 69:638–651. https://doi.org/10.1002/lno.12521
Goedknegt MA, Welsh JE, Drent J, Thieltges DW (2015) Climate change and parasite transmission: how temperature affects parasite infectivity via predation on infective stages. Ecosphere 6:9. https://doi.org/10.1890/es15-00016.1
Goedknegt MA, Feis ME, Wegner KM, Luttikhuizen PC, Buschbaum C, Camphuysen K et al (2016a) Parasites and marine invasions: ecological and evolutionary perspectives. J Sea Res 113:11–27. https://doi.org/10.1016/j.seares.2015.12.003
Goedknegt MA, Schuster A-K, Buschbaum C, Gergs R, Jung AS, Luttikhuizen PC et al (2016b) Spillover but no spillback of two invasive parasitic copepods from invasive Pacific oysters (Crassostrea gigas) to native bivalve hosts. Biol Invasions 19:365–379. https://doi.org/10.1007/s10530-016-1285-0
Goedknegt MA, Thieltges DW, van der Meer J, Wegner KM, Luttikhuizen PC (2018) Cryptic invasion of a parasitic copepod: compromised identification when morphologically similar invaders co-occur in invaded ecosystems. PLoS ONE 13:e0193354. https://doi.org/10.1371/journal.pone.0193354
Gräwe U, Burchard H, Müller M, Schuttelaars HM (2014) Seasonal variability in M2 and M4 tidal constituents and its implications for the coastal residual sedimane transport. Geophys Res Lett 41:5563–5570. https://doi.org/10.1002/2014GL060517
Gräwe U, Flöser G, Gerkema T, Duran-Matute M, Badewien TH, Schulz E, Burchard H (2016) A numerical model for the entire Wadden Sea: skill assessment and analysis of hydrodynamics. J Geophys Res Oceans 121:5231–5251. https://doi.org/10.1002/2016JC011655
Goss-Custard JD, Le V, Dit Durel SEA, McGrorty S, Reading CJ (1982) Use of mussel Mytilus edulis beds by oystercatchers Haematopus ostralegus according to age and population size. J Anim Ecol 51:543–554. https://doi.org/10.2307/3983
Gutierrez JL, Jones CG, Strayer DL, Iribarne OO (2003) Mollusks as ecosystem engineers: the role of shell production in aquatic habitats. Oikos 101:79–90. https://doi.org/10.1034/j.1600-0706.2003.12322.x
Graiff A, Dankworth M, Wahl M, Karsten U, Bartsch I (2017) Seasonal variations of Fucus vesiculosus fertility under ocean acidification and warming in the western Baltic Sea. Bot Mar 60(3):239–255. https://doi.org/10.1515/bot-2016-0081
Graversen AEL, Banta GT, Masque P, Krause-Jensen D (2022) Carbon sequestration is not inhibited by livestock grazing in Danish salt marshes. Limnol Oceanogr 67:19–35. https://doi.org/10.1002/lno.12011
Hagen R, Winter C, Kösters F (2022) Changes in tidal asymmetry in the German Wadden Sea. Ocean Dyn 72:325–340. https://doi.org/10.1007/s10236-022-01509-9
Hagmeier A, Kändler R (1927) Neue Untersuchungen im nordfriesischen Wattenmeer und auf den fiskalischen Austembänken. Wissenschaftliche Meeresun (Helgoland) 16:1–90
Hansen MM, Olivieri I, Waller DM, Nielsen EE, Ge MWG (2012) Monitoring adaptive genetic responses to environmental change. Mol Ecol 21:1311–1329. https://doi.org/10.1111/j.1365-294X.2011.05463.x
Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, Grimes DJ et al (1999) Emerging marine diseases–climate links and anthropogenic factors. Science 285:1505–1510. https://doi.org/10.1126/science.285.5433.1505
Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, Ostfeld RS et al (2002) Climate warming and disease risks for terrestrial and marine biota. Science 296:2158–2162. https://doi.org/10.1126/science.1063699
Hermans THJ, Malagón-Santos V, Katsman CA, Jane RA, Rasmussen DJ, Haasnoot M, Garner GG, Kopp RE, Oppenheimer M, Slangen ABA (2023) The timing of decreasing coastal flood protection due to sea-level rise. Nat Clim Chang 13:359–366. https://doi.org/10.1038/s41558-023-01616-5
Herrmann JP, Jansen S, Temming A (1998) Consumption of fish and decapod crustaceans and their role in the trophic relations of the Sylt-Rømø Bight. In: Ökosystem Wattenmeer-Austausch-Transport- Und Stoffumwandlungsprozesse. Springer Berlin Heidelberg, pp 81–88
Himes-Cornell A, Pendleton L, Atiyah P (2018) Valuing ecosystem services from blue forests: a systematic review of the valuation of salt marshes, sea grass beds and mangrove forests. Ecosyst Serv 30:36–48. https://doi.org/10.1016/j.ecoser.2018.01.006
Hiscock K, Southward A, Tittley I, Hawkins S (2004) Effects of changing temperature on benthic marine life in Britain and Ireland. Aquat Conserv Mar Freshwat Ecosyst 14:333–362. https://doi.org/10.1002/aqc.628
Hofstede JLA (2015) Theoretical considerations on how Wadden Sea tidal basins may react to accelerated sea level rise. Z Geomorphol 59:377–391. https://doi.org/10.1127/zfg/2014/0163
Hofstede JLA, Becherer J, Burchard H (2018) Are Wadden Sea tidal systems with a higher tidal range more resilient against sea level rise? J Coast Conserv 22:71–78. https://doi.org/10.1007/s11852-016-0469-1
Hofstede J, Becherer J, Burchard H (2019) Morphologische Projektionen für zwei Tidensysteme im Wattenmeer von Schleswig-Holstein: SH-Trend. Die Küste 87. https://doi.org/10.18171/1.087101
Holleman RC, Stacey MT (2014) Coupling of sea level rise, tidal amplification, and inundation. J Phys Oceanogr 44:1439–1455. https://doi.org/10.1175/JPO-D-13-0214
Horn S, de la Vega C, Asmus R, Schwemmer P, Enners L, Garthe S, Binder K, Asmus H (2017) Interaction between birds and macrofauna within food webs of six intertidal habitats of the Wadden Sea. PLoS ONE 12:e0176381. https://doi.org/10.1371/journal.pone.0176381
Horn S, de la Vega C, Asmus R, Schwemmer P, Enners L, Garthe S, Haslob H, Binder K, Asmus H (2019) Impact of birds on intertidal food webs assessed with ecological network analysis. Estuar Coast Shelf Sci 219:107–119. https://doi.org/10.1016/j.ecss.2019.01.023
Horn S, Coll M, Asmus H, Dolch T (2021a) Food web models reveal potential ecosystem effects of seagrass recovery in the northern Wadden Sea. Restor Ecol 29:e13328. https://doi.org/10.1111/rec.13328
Horn S, Meunier CL, Fofonova V, Wiltshire KH, Sarker S, Pogoda B, Asmus H (2021b) Toward improved model capacities for assessment of climate impacts on coastal bentho-pelagic food webs and ecosystem services. Front Mar Sci 8:567266. https://doi.org/10.3389/fmars.2021.567266
Huismans Y, van der Spek A, Lodder Q, Zijlstra R, Elias E, Wang ZB (2022) Development of intertidal flats in the Dutch Wadden Sea in response to a rising sea level: spatial differentiation and sensitivity to the rate of sea level rise. Ocean Coast Manag 216:105969. https://doi.org/10.1016/j.ocecoaman.2021.105969
Idier D, Paris F, Cozannet GL, Boulahya F, Dumas F (2017) Sea-level rise impacts on the tides of the European Shelf. Cont Shelf Res 137:56–71. https://doi.org/10.1016/j.csr.2017.01.007
IPCC (2022) Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Pörtner H-O, Roberts DC, Tignor M, Poloczanska ES, Mintenbeck K, Alegría A, Craig M, Langsdorf S, Löschke S, Möller V, Okem A, Rama B (eds) Cambridge University Press. Cambridge University Press, Cambridge, UK and New York, NY, USA, p 3056. https://doi.org/10.1017/9781009325844
Jänicke L, Ebener A, Dangendorf S, Arns A, Schindelegger M, Niehüser S et al (2021) Assessment of tidal range changes in the North Sea from 1958 to 2014. J Geophys Res Oceans 126:e2020JC016456. https://doi.org/10.1029/2020JC016456
Jansen S, Walpersdorf E, Werner U, Billerbeck M, Böttcher ME, de Beer D (2009) Functioning of intertidal flats inferred from temporal and spatial dynamics of O2, H2S and pH in their surface sediments. Ocean Dyn 59:317–332. https://doi.org/10.1007/s10236-009-0179-4
Jensen KT (1992a) Dynamics and growth of the cockle, Cerastoderma edule, on an intertidal mud-flat in the Danish Wadden Sea: effects of submersion times and density. Neth J Sea Res 28:335–345. https://doi.org/10.1016/0077-7579(92)90035-D
Jensen KT (1992b) Macrozoobenthos on an intertidal mudflat in the Danish Wadden Sea. comparisons of surveys made in the 1930s, 1940s and 1980s. Helgoländer Meeresun 46:363–376. https://doi.org/10.1007/BF02367204
Jensen KT, Mouritsen KN (1992) Mass mortality in two common soft-bottom invertebrates, Hydrobia ulvae and Corophium volutator - the possible role of trematodes. Helgoländer Meeresun 46:329–339. https://doi.org/10.1007/BF02367103
Jiang L, Gerkema T, Idier D, Slangen ABA, Soetaert K (2020) Effects of sea-level rise on tides and sediement dynamics in a Dutch tidal bay. Ocean Sci 16:307–321. https://doi.org/10.5194/os-16-307-2020
Jordan C, Visscher J, Schlurmann T (2021) Projected responses of tidal dynamics in the North Sea to sea-level rise and morphological changes in the Wadden Sea. Front Mar Sci 8:685758. https://doi.org/10.3389/fmars.2021.685758
Jordan P, Döring M, Fröhle P, Ratter BMW (2023) Exploring past and present dynamics of coastal protection as possible signposts for the future? J Coast Conserv 27:2. https://doi.org/10.1007/s11852-022-00921-z
Jung AS, van der Veer HW, Philippart CJ, Waser AM, Ens BJ, de Jonge VN, Schückel U (2020) Impacts of macrozoobenthic invasions on a temperate coastal food web. Mar Ecol Prog Ser 653:19–39. https://doi.org/10.3354/meps13499
Kay JJ, Graham LA, Ulanowicz RE (1989) A detailed guide to network analysis. Network analysis in marine ecology. Springer, pp 15–61. https://doi.org/10.1007/978-3-642-75017-5_2
Kerimoglu O, Voynova VG, Chegini F, Brix H, Callies U, Hofmeister R, Klingbeil K, Schrum C, van Beusekom JEE (2020) Interactive impacts of meteorological and hydrological conditions on the physical and biogeochemical structure of a coastal system. Biogeosciences 2020:5097–5127. https://doi.org/10.5194/bg-17-5097-2020
Keshta A, Koop-Jakobsen K, Titschack J, Mueller P, Jensen K, Baldwin A, Nolte S (2020) Ungrazed salt marsh has well connected soil pores and less dense sediment compared with grazed salt marsh: a CT scanning study. Estuar Coast Shelf Sci 245:106987. https://doi.org/10.1016/j.ecss.2020.106987
Kirwan ML, Guntenspergen GR (2012) Feedbacks between inundation, root production, and shoot growth in a rapidly submerging brackish marsh. J Ecol 100(3):764–770. https://doi.org/10.1111/j.1365-2745.2012.01957.x
Koffijberg K, Bregnballe T, Frikke J, Hälterlein B, Bentzon Hansen M, Meyer J, Reichert G, Umland J, van der Meij T (2022) Breeding birds. In: Kloepper S, Meise K (eds) Wadden sea quality status report. Common Wadden Sea Secretariat, Wilhelmshaven
Koop-Jakobsen K, Dolch T (2023) Future climate conditions alter biomass of salt marsh plants in the Wadden Sea. Mar Biodivers 53:41. https://doi.org/10.1007/s12526-023-01347-y
Korringa P (1950) De aanval van de parasiet Mytilicola intestinalis op de Zeeuwse mosselcultuur. Viss-Nieuws 7:1–7
Kötter-Lange K, Lienhoop N, Buschbaum C (2023) Effects and perception of marine introduced species by stakeholders in the Wadden Sea - an exploratory approach. Mar Biodivers 53:58. https://doi.org/10.1007/s12526-023-01358-9
Kuris AM, Hechinger RF, Shaw JC, Whitney KL, Aguirre-Macedo ML, Boch CA et al (2008) Ecosystem energetic implications of parasite and free-living biomass in three estuaries. Nature 454:515–518. https://doi.org/10.1038/nature06970
Kuwae T, Hori M (2019) Blue carbon in shallow coastal ecosystems. Blue carbon shallow coastal ecosystems 1. Springer, Singapore. https://doi.org/10.1007/978-981-13-1295-3
Lackschewitz D, Reise K, Buschbaum C, Karez R (2022) Neobiota der deutschen Nord- und Ostseeküste. LLUR-SH-Gewässer D25, Flintbek, Germany
Lang AC, Buschbaum C (2010) Facilitative effects of introduced Pacific oysters on native macroalgae are limited by a secondary invader, the seaweed Sargassum muticum. J Sea Res 63(2):119–128. https://doi.org/10.1016/j.seares.2009.11.002
Lang A, Mikolajewicz U (2020) Rising extreme sea levels in the German Bight under enhanced CO2 levels: a regionalized large ensemble approach for the North Sea. Clim Dyn 55:1829–1842. https://doi.org/10.1007/s00382-020-05357-5
Larkum WD, Orth RJ, Duarte CM (2006) Seagrasses, biology and conservation. Springer, Heidelberg
Le Roux F, Wegner KM, Polz MF (2016) Oysters and Vibrios as a model for disease dynamics in wild animals. Trends Microbiol 24:568–580. https://doi.org/10.1016/j.tim.2016.03.006
Leonardi N, Fagherazzi S (2015) Effect of local variability in erosional resistance on large-scale morphodynamic response of salt marshes to wind waves and extreme events. Geophys Res Lett 42(14):5872–5879. https://doi.org/10.1002/2015GL064730
Lindhorst S, Fürstenau J, Hass C, Betzler C (2010) Anatomy and sedimentary model of a hooked spit (Sylt, southern North Sea). Sedimentology 57:935–955. https://doi.org/10.1111/j.1365-3091.2009.01126.x
Lodder QJ, Wang ZB, Elias EPL, van der Spek AJF, de Looff H, Townend IH (2019) Future response of the Wadden Sea tidal basins to relative sea-level rise—an aggregated modelling approach. Water 11:2198. https://doi.org/10.3390/w11102198
Lokmer A, Wegner KM (2015) Hemolymph microbiome of Pacific oysters in response to temperature, temperature stress and infection. ISME J 9:670–682. https://doi.org/10.1038/ismej.2014.160
MacFadden DR, McGough SF, Fisman D et al (2018) Antibiotic resistance increases with local temperature. Nat Clim Chang 8:510–514. https://doi.org/10.1038/s41558-018-0161-6
Madsen AT, Murray AS, Andersen TJ, Pejrup M (2010) Spatial and temporal variability of sediment accumulation rates on two tidal flats in Lister Dyb tidal basin, Wadden Sea, Denmark. Earth Surf Process Landforms 35:1556–1572. https://doi.org/10.1002/esp.1999
Mai S, Bartholomä A (2000) The missing mud flats of the Wadden Sea: a reconstruction of sediments and accommodation space lost in the wake of land reclamation. Proceedings in Marine Science 2:257–272. https://doi.org/10.1016/S1568-2692(00)80021-2
Markert A, Wehrmann A, Kröncke I (2010) Recently established Crassostrea-reefs vs. native Mytilus-beds: differences in habitat engineering affects the macrofaunal communities (Wadden Sea of Lower Saxony, southern German Bight). Biol Invasions 12(1):15–32
Martens P, van Beusekom JEE (2008) Zooplankton response to a warmer northern Wadden Sea. Helgol Mar Res 62:67–75. https://doi.org/10.1007/s10152-007-0097-0
Mayer B, Mathis M, Mikolajewicz U, Pohlmann T (2022) RCP8.5-Projected changes in German Bight storm surge characteristics from regionalized ensemble simulations for the end of the twenty-first century. Front Clim 4: 992119. https://www.frontiersin.org/articles/10.3389/fclim.2022.992119
Mayr T (2009) Die invasive Rotalge Gracilaria vermiculophylla und die heimische Braunalge Fucus vesiculosus forma mytili im Eulitoral des Sylter Wattenmeeres. Diploma-thesis, University of Oldenburg, Germany, p 81
McQuatters-Gollop A, Mitchell I, Vina-Herbon C, Bedford J, Addison PF, Lynam CP, Geetha P, Vermeulan EA, Smit K, Bayley DT (2019) From science to evidence–how biodiversity indicators can be used for effective marine conservation policy and management. Front Mar Sci 6:109. https://doi.org/10.3389/fmars.2019.00109
McLeod E, Chmura GL, Bouillon S, Salm R, Björk M, Duarte CM, Lovelock CE, Schlesinger WH, Silliman BR (2011) A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front Ecol Environ 9:552–560. https://doi.org/10.1890/110004
Mensch B, Neulinger SC, Graiff A, Pansch A, Künzel S, Fischer MA, Schmitz RA (2016) Restructuring of epibacterial communities in Fucus vesiculosus forma mytili in response to elevated pCO2 and increased temperature levels. Front Micobiol 7:434. https://doi.org/10.3389/fmicb.2016.00434
Meyer J, Kröncke I, Bartholomä A, Dippner JW, Schückel U (2016) Long-term changes in species composition of demersal fish and epifauna species in the Jade area (German Wadden Sea/ North Sea) since 1972. Estuar Coast Shelf Sci 181:284–293. https://doi.org/10.1016/j.ecss.2016.08.047
Moehler J, Wegner KM, Reise K, Jacobsen S (2011) Invasion genetics of Pacific oyster Crassostrea gigas shaped by aquaculture stocking practices. J Sea Res 66(3):256–262. https://doi.org/10.1016/j.seares.2011.08.004
Möbius KA (1877) Die Auster und die Austernwirtschaft. Wiegandt, Hempel and Parey, Berlin, 126 pp
Möller I, Kudella M, Rupprecht F, Spencer T, Paul M, van Wesenbeeck B, Wolters G, Jensen K, Bouma TJ, Miranda-Lange M, Schimmels S (2014) Wave attenuation over coastal salt marshes under storm surge conditions. Nat Geosci 7:727–731. https://doi.org/10.1038/NGEO2251
Montero-Serra I, Edwards M, Genner MJ (2015) Warming shelf seas drive the subtropicalization of European pelagic fish communities. Glob Chang Biol 21:144–153. https://doi.org/10.1111/GCB.12747
Moreno HD, Köring M, Di Pane J, Tremblay N, Wiltshire KH, Boersma M, Meunier CL (2022) An integrated multiple driver mesocosm experiment reveals the effect of global change on planktonic food web structure. Communications Biology 5:179. https://www.nature.com/articles/s42003-022-03105-5
Mueller P, Granse D, Nolte S, Do HT, Weingartner M, Hoth S, Jensen K (2017) Top-down control of carbon sequestration: grazing affects microbial structure and function in salt marsh soils. Ecol Appl 27:1435–1450. https://doi.org/10.1002/eap.1534
Mueller P, Kutzbach L, Mozdzer TJ, Jespersen E, Barber DC, Eller F (2023) Minerogenic salt marshes can function as important inorganic carbon stores. Limnol Oceanogr 68(4):942–952. https://doi.org/10.1002/lno.12322
Mueller P, Ladiges N, Jack A, Schmiedl G, Kutzbach L, Jensen K, Nolte S (2019) Assessing the long-term carbon-sequestration potential of the semi-natural salt marshes in the European Wadden Sea. Ecosphere 10:e02556. https://doi.org/10.1002/ecs2.2556
Munday PL, Warner RR, Monro K, Pandolfi JM, Marshall DJ (2013) Predicting evolutionary responses to climate change in the sea. Ecol Lett 16:1488–1500. https://doi.org/10.1111/ele.12185
Nehls G, Thiel M (1993) Large-scale distribution patterns of the mussel Mytilus edulis in the Wadden Sea of Schleswig-Holstein: do storms structure the ecosystem? Neth J Sea Res 31:181–187. https://doi.org/10.1016/0077-7579(93)90008-G
Nehls G, Diederich S, Thieltges DW, Strasser M (2006) Wadden Sea mussel beds invaded by oysters and slipper limpets: competition or climate control? Helgol Mar Res 60:135–143. https://doi.org/10.1007/s10152-006-0032-9
Nolte S, Müller F, Schuerch M, Wanner A, Esselink P, Bakker JP, Jensen K (2013) Does livestock grazing affect sediment deposition and accretion rates in salt marshes? Estuar Coast Shelf Sci 135:296–305. https://doi.org/10.1016/j.ecss.2013.10.026
Odongo V, Asmus H, Ahnelt H, Boersma M, Rick J, Wiltshire KH, Horn S (2024) Seasonal variations of a coastal fish community in relation to environmental parameters - a case study of the Sylt-Rømø Bight, southeastern North Sea. Estuar Coast Shelf Sci 300:108723. https://doi.org/10.1016/j.ecss.2024.108723
Ojaveer H, Galil BS, Carlton JT, Alleway H, Goulletquer P, Lehtiniemi M, Marchini A, Miller W, Occhipinti-Ambrogi A, Peharda M, Ruiz GM, Williams SL, Zaiko A (2018) Historical baselines in marine bioinvasions: implications for policy and management. PLoS ONE 13:e0202383. https://doi.org/10.1371/journal.pone.0202383
Oost P, Hofstede J, Weisse R, Baart F, Janssen G, Zijlstra R (2017) Climate change. In: Kloepper et al (eds) Wadden sea quality status report. Common Wadden Sea Secretariat, Wilhelmshaven. Last updated 21.12.2017
Oppenheimer M, Glavovic BC, Hinkel J, Van De Wal R, Magnan AK, Abd-Elgawad A et al (2019) Sea level rise and implications for low-lying islands, coasts and communities. IPCC Special Report on the Ocean and Cryosphere in a Changing Climate, vol 123. IPCC, Geneva
Oprandi A, Atzori F, Azzola A, Bianchi CN, Cadoni N, Carosso L, Desiderà E, Frau F, Garcia Gutiérrez ML, Guidetti P (2023) Multiple indices on different habitats and descriptors provide consistent assessments of environmental quality in a marine protected area. Front Mar Sci 10:1111592. https://doi.org/10.3389/fmars.2023.1111592
Ossó A, Allan RP, Hawkins E, Shaffrey L, Maraun D (2022) Emerging new climate extremes over Europe. Clim Dyn 58:487–501. https://doi.org/10.1007/s00382-021-05917-3
Ouyang X, Lee SY (2014) Updated estimates of carbon accumulation rates in coastal marsh sediments. Biogeosciences 11:5057–5071. https://doi.org/10.5194/bg-11-5057-2014
Pansch A (2019) The effects of ocean acidification, warming and enhanced nutrients on a Wadden Sea community - Examined with Mesocosm experiments. PhD/Doctoral thesis. Christian-Albrechts-Universität Kiel, Kiel, Germany, p 149. https://macau.uni-kiel.de/receive/macau_mods_00000157
Pansch A, Winde A, Asmus R, Asmus H (2016) Tidal benthic mesocosms simulating future climate change scenarios in the field of marine ecology. Limnol Oceanogr Methods 14:257–267. https://doi.org/10.1002/lom3.10086
Paul M, Bischoff C, Koop-Jakobsen K (2022) Biomechanical traits of salt marsh vegetation are insensitive to future climate scenarios. Sci Rep 12:21272. https://doi.org/10.1038/s41598-022-25525-3
Pejrup M, Larsen M, Edelvang K (1997) A fine-grained sediment budget for the Sylt-Romo tidal basin. Helgoländer Meeresuntersungen 51:253–268
Philippart CJM, Gerkema T, van der Veer HW (2017b) North Sea coastal ecology: future challenges. J Sea Res 127:227–230. https://doi.org/10.1016/j.seares.2017.07.004
Philippart CJM, Mekkes L, Buschbaum C, Wegner KM, Laursen K (2017a) Climate ecosystems. In: Kloepper et al (eds) Wadden Sea quality status report. Common Wadden Sea Secretariat, Wilhelmshaven
Pickering MD, Wells NC, Horsburgh KJ, Green JAM (2012) The impact of future sea-level rise on the European Shelf tides. Cont Shelf Res 35:1–15. https://doi.org/10.1016/j.csr.2011.11.011
Plüß A (2003). Das Nordseemodell der BAW zur Simulation der Tide in der Deutschen Bucht. Die Küste 67:84–127. https://hdl.handle.net/20.500.11970/101497
QSR (1993) Quality status report of the north sea (QSR). Subregion 10. The Wadden Sea. Common Wadden Sea Secretariat, Wilhelmshaven, Geffen Druck, Bremen, p 174
Pugh DT (1987) Tides, surges and mean sea level: a handbook for engineers and scientists. Wiley, Chichester, p 472
Ramler D, Mitteroecker P, Shama LNS, Wegner KM, Ahnelt H (2014) Non-linear effects of temperature on body form and developmental canalization in the threespine stickleback. J Evol Biol 27:497–507. https://doi.org/10.1111/jeb.12311
Ratter BMW, Gee K (2012) Heimat – A German concept of regional perception and identity as a basis for coastal management in the Wadden Sea. Ocean Coast Manag 68:127–137. https://doi.org/10.1016/j.ocecoaman.2012.04.013
Reef KRG, Lipari G, Roos PG, Hulscher SJMH (2018) Time-varying storm surges on Lorentz’s Wadden Sea Networks. Ocean Dyn 68:1051–1065. https://doi.org/10.1007/s10236-018-1181-5
Reents S, Möller I, Evans BR, Schoutens K, Jensen K, Paul M, Bouma TJ, Temmerman S, Lustig J, Kudella M, Nolte S (2022) Species-specific and seasonal differences in the resistance of salt-marsh vegetation to wave impact. Front Mar Sci 9:898080. https://doi.org/10.3389/fmars.2022.898080
Reid PC, Hari RE, Beaugrand G, Livingstone DM, Marty C, Straile D, Barichivich J, Goberville E, Adrian R, Aono Y, Brown R, Foster J, Groisman P, Hélaouët P, Hsu H-H, Kirby R, Knight J, Kraberg A, Li J, Lo T-T, Myneni RB, North RP, Pounds JA, Sparks T, Stübi R, Tian Y, Wiltshire KH, Xiao D, Zhu Z (2016) Global impacts of the 1980s regime shift. Glob Chang Biol 22:682–703. https://doi.org/10.1111/gcb.13106
Reise K (1977) Predator exclusion experiments in an intertidal mud flat. Helgoländer Meeresun 30:263–271. https://doi.org/10.1007/BF02207840
Reise K (1978) Experiments on epibenthic predation in the Wadden Sea. Helgoländer Meeresun 31:55–101. https://doi.org/10.1007/BF02296991
Reise K (1982) Long-term changes in the macrobenthic invertebrate fauna of the Wadden Sea: are polychaetes about to take over? Neth J Sea Res 16:29–36. https://doi.org/10.1016/0077-7579(82)90014-X
Reise K (1985) Tidal flat ecology - an experimental approach to species interactions. Springer, Berlin, Heidelberg, New York
Reise K (1998) Pacific oysters invade mussel beds in the European Wadden Sea. Senckenb Marit 28:167–175. https://doi.org/10.1007/BF03043147
Reise K (2003) Metapopulation structure in the lagoon cockle Cerastoderma lamarcki in the northern Wadden Sea. Helgol Mar Res 56:252–258. https://doi.org/10.1007/s10152-002-0125-z
Reise K (2005) Coast of change: habitat loss and transformations in the Wadden Sea. Helgol Mar Res 59:9–21. https://doi.org/10.1007/s10152-004-0202-6
Reise K (2015) Kurswechsel Küste. Was tun, wenn die Nordsee steigt? Wachholtz Verlag – Murmann Publishers. Kiel/Hamburg, p 199
Reise K, Schubert A (1987) Macrobenthic turnover in the subtidal Wadden Sea: the Norderaue revisited after 60 years. Helgoländer Meeresuntersuchungen 41:69–82. https://doi.org/10.1007/BF02365100
Reise K, Herre E, Sturm M (1989) Historical changes in the benthos of the Wadden Sea around the island of Sylt in the North Sea. Helgoländer Meeresun 43:417–433. https://doi.org/10.1007/BF02365901
Reise K, Herre E, Sturm M (1994) Biomass and abundance of macrofauna in intertidal sediments of Königshafen in the northern Wadden Sea. Helgoländer Meeresun 48:201–215. https://doi.org/10.1007/BF02367036
Reise K, van Beusekom JEE (2008) Interactive effects of global and regional change on a coastal ecosystem. Helgol Mar Res 62:85–91. https://doi.org/10.1007/s10152-007-0102-7
Reise K, Buschbaum C, Büttger H, Wegner MK (2017) Invading oysters and native mussels: from hostile takeover to compatible bedfellows. Ecosphere 8:e01949. https://doi.org/10.1002/ecs2.1949
Reise K, Michaelis R, Rybalka N (2022a) Invading grass-like alga transforms rippled sand bars into bumpy muddy flats: arrival of a game changer in the Wadden Sea? Aquat Invasions 17:1–20. https://doi.org/10.3391/ai.2022.17.1.01
Reise K, Lackschewitz D, Wegner KM (2022b) Marine turf of an invasive alga expels lugworms from the lower shore. Mar Biol 169:16. https://doi.org/10.1007/s00227-021-04004-9
Reise K, Buschbaum C, Lackschewitz D, Thieltges DW, Waser AM, Wegner KM (2023) Introduced species in a tidal ecosystem of mud and sand: curse or blessing? Mar Biodivers 53:5. https://doi.org/10.1007/s12526-022-01302-3
Reise K, Lackschewitz D (2023) Strangers at the German shores. Klaas Jarchow Hamburg, Germany, 203 pages
Reise K, Simon M, Herre E (2001) Density-dependent recruitment after winter disturbance on tidal flats by the lugworm Arenicola marina. Helgol Mar Res 55:161–165. https://doi.org/10.1007/s101520100076
Rick JJ, Scharfe M, Romanova T, van Beusekom JEE, Asmus R, Asmus H, Mielck F, Kamp A, Sieger R, Wiltshire KH (2023) An evaluation of long-term physical and hydrochemical measurements at the Sylt Roads Marine Observatory (1973–2019), Wadden Sea, North Sea. Earth Syst Sci Data 15:1037–1057. https://doi.org/10.5194/essd-15-1037-2023
Ricklefs K, Büttger H, Asmus H (2020) Occurrence, stability, and associated species of subtidal mussel beds in the North Frisian Wadden Sea (German North Sea Coast). Estuar Coast Shelf Sci 233:106549. https://doi.org/10.1016/j.ecss.2019.106549
Rodionov SN, Krovnin AS (1992) The 1980s in the context of climatic changes in the North Atlantic region. - ICES mar. Sei Symp 195:93–102
Røy H, Seong J, Jansen S, de Beer D (2008) Tide-driven deep pore-water flow in intertidal sand flats. Limnol Oceanogr 53(4):1521–1530. https://doi.org/10.4319/lo.2008.53.4.1521
Rubinetti S, Fofonova V, Arnone E, Wiltshire KH (2023) A complete 60-year catalog of wind events in the German Bight (North Sea) derived from ERA5 reanalysis data. Earth Space Sci 10:e2023EA003020. https://doi.org/10.1029/2023EA003020
Ruppert KM, Kline RJ, Rahman MS (2019) Past, present, and future perspectives of environmental DNA (eDNA) metabarcoding: a systematic review in methods, monitoring, and applications of global eDNA. Glob Ecol Conserv 17:e00547
Rupprecht F, Möller I, Paul M, Kudella M, Spencer T, van Wesenbeek BK, Wolters G, Jensen K, Bouma TJ, Miranda-Lange M, Schimmels S (2017) Vegetation-wave interactions in salt marshes under storm surge conditions. Ecol Eng 100:301–315. https://doi.org/10.1016/j.ecoleng.2016.12.030
Rutterford LA, Simpson SD, Bogstad B, Devine JA, Genner MJ (2023) Sea temperature is the primary driver of recent and predicted fish community structure across Northeast Atlantic shelf seas. Glob Chang Biol. https://doi.org/10.1111/gcb.16633
Rybalka N, Epkes S, Wegner KM, Michaelis R, Reise K (2022) Invasive Vaucheria (Xanthophyceae) at the lower shore of the Wadden Sea. Phycologia 61:274–283. https://doi.org/10.1080/00318884.2022.2035532
Safi G, Giebels D, Arroyo NL, Heymans JJ, Preciado I, Raoux A, Schückel U, Tecchio S, de Jonge VN, Niquil N (2019) Vitamine ENA: a framework for the development of ecosystem-based indicators for decision makers. Ocean Coast Manag 174:116–130. https://doi.org/10.1016/j.ocecoaman.2019.03.005
Saintilan N, Horton B, Törnquist TE, Ashe EL, Khan NS, Schuerch M, Perry C, Kopp RE, Garner GG, Murray N, Rogers K, Albert S, Kelleway J, Shaw TA, Woodroffe CD, Lovelock CE, Goddard MM, Hutley LB, Kovalenko K, Feher L, Guntenspergen G (2023) Widespread retreat of coastal habitats is likely at warming levels above 1.5°C. Nature 62. https://doi.org/10.1038/s41586-023-06448-z
Scanes E, Parker LM, Seymour JR, Siboni N, King WL, Danckert NP, Wegner KM, Dove MC, O´Connor WA, Ross PM (2021) Climate change alters the haemolymph microbiome of oysters. Mar Pollut Bull 164:111991. https://www.sciencedirect.com/science/article/pii/S0025326X21000254
Schade FM, Raupach MJ, Wegner KM (2015) Seasonal variation in parasite infection patterns of marine fish species from the Northern Wadden Sea in relation to interannual temperature fluctuations. J Sea Res 113:73–84. https://doi.org/10.1016/j.seares.2015.09.002
Schade FM, Shama LNS and Wegner KM (2014) Impact of thermal stress on evolutionary trajectories of pathogen resistance in three-spined stickleback (Gasterosteus aculeatus). BMC Evol Biol 14:164. https://doi.org/10.1186/s12862-014-0164-5
Schanz A, Polte P, Asmus H (2002) Cascading effects of hydrdynamics on an epiphyte-grazer system in intertidal seagrass beds in the Wadden Sea. Mar Biol 141:287–297
Schanz A, Asmus H (2003) Impact of hydrodynamics on development and morphology of intertidal seagrasses in the Wadden Sea. Mar Ecol Prog Ser 261:123–134
Scharfe M, Wiltshire KH (2019) Modeling of intra-annual abundance distributions: constancy and variation in the phenology of marine phytoplankton species over five decades at Helgoland Roads (North Sea). Ecol Model 404:46–60
Schiller J, Lackschewitz D, Buschbaum C, Reise K, Pang S, Bischof K (2018) Heading northward to Scandinavia: Undaria pinnatifida in the northern Wadden Sea. Bot Mar 61:365–371. https://doi.org/10.1515/bot-2017-0128
Schoutens K, Heuner M, Minden V, Schulte Ostermann T, Silinski A, Belliard J-P, Temmerman S (2019) How effective are tidal marshes as nature-based shoreline protection throughout seasons? Limnol Oceanogr 64(4):1750–1762. https://doi.org/10.1002/lno.11149
Schrum C (1997) Thermohaline stratification and instabilities at tidal mixing fronts: results of an eddy resolving model for the German Bight. Cont Shelf Res 17(6):689–716
Schulze D, Jensen K, Nolte S (2021) Livestock grazing reduces sediment deposition and accretion rates on a highly anthropogenically altered marsh island in the Wadden Sea. Estuar Coast Shelf Sci 251:107191. https://doi.org/10.1016/j.ecss.2021.107191
Schückel U, Kröncke I, Baird D (2015) Linking long-term changes in trophic structure and function of an intertidal macrobenthic system to eutrophication and climate change using ecological network analysis. Mar Ecol Prog Ser 536:25–38. https://doi.org/10.3354/meps11391
Schückel U, Nogues Q, Brito J, Niquil N, Blomqvist M, Sköld M, Hansen J, Jakobsen H, Morato T (2023). Pilot assessment of ecological network analysis indices. In: OSPAR, 2023: the 2023 Quality Status Report for the North-East Atlantic. OSPAR Commission, London. https://oap.ospar.org/en/ospar-assessments/quality-status-reports/qsr-2023/indicator-assessments/pilot-assessment-ecological-network-analysis-indices/
Schuerch M, Dolch T, Bisgwa J, Vafeidis AT (2018) Changing sediment dynamics of a mature backbarrier salt marsh in response to sea-level rise and storm events. Front Mar Sci 5:155. https://doi.org/10.3389/fmars.2018.00155
Schuerch M, Vafeidis A, Slawig T, Temmerman S (2013) Modeling the influence of changing storm patterns on the ability of a salt marsh to keep pace with sea level rise. J Geophys Res: Earth Surf 118:84–96. https://doi.org/10.1029/2012JF002471
Schulz G, van Beusekom JEE, Jacob J, Bold S, Schöl A, Ankele M, Sanders T, Dähnke K (2023) Low discharge intensifies nitrogen retention in rivers – a case study in the Elbe River. Sci Total Environ 904:166740. https://doi.org/10.1016/j.scitotenv.2023.166740
Schutte CA, Ahmerkamp S, Wu CS, Seidel M, de Beer D, Cook PLM, Joye SB (2019) Biogeochemical dynamics of coastal tidal flats. Coastal Wetlands 2:407–440. https://doi.org/10.1016/B978-0-444-63893-9.00012-5
Schwimmer RA (2001) Rates and processes of marsh shoreline erosion in Rehoboth Bay, delaware, U.S.A. J Coast Res 17(3):672–683
Seaman MNL, Ruth M (1997) The molluscan fisheries of Germany. NOAA Technical Report NMFS, Europe, pp 57–84
Serrano O, Lovelock CE, Atwood TB, Macreadie PI, Canto R, Phinn S, Arias-Ortiz A, Bai L, Baldock J, Bedulli C, Carnell P, Connolly RM, Donaldson P, Esteban A, Ewers Lewis CJ, Eyre BD, Hayes MA, Horwitz P, Hutley LB, Kavazos CRJ, Kelleway JJ, Kendrick GA, Kilminster K, Lafratta A, Lee S, Lavery PS, Maher DT, Marbà N, Masque P, Mateo MA, Mount R, Ralph PJ, Roelfsema C, Rozaimi M, Ruhon R, Salinas C, Samper-Villarreal J, Sanderman J, Sanders JC, Santos I, Sharples C, Steven ADL, Cannard T, Trevathan-Tackett SM, Duarte CM (2019) Australian vegetated coastal ecosystems as global hotspots for climate change mitigation. Nat Commun 10:4313. https://doi.org/10.1038/s41467-019-12176-8
Séveno NA, Kallifidas D, Smalla K, van Elsas JD, Collard J-M, Karagouni AD, Wellington EMH (2002) Occurrence and reservoirs of antibiotic resistance genes in the environment. Rev Med Microbiol 13(1):15–27
Shama LNS (2017) The mean and variance of climate change in the oceans: hidden evolutionary potential under stochastic environmental variability in marine sticklebacks. Sci Rep 7:8889. https://doi.org/10.1038/s41598-017-07140-9
Shama LNS, Wegner KM (2014) Grandparental effects in marine sticklebacks: transgenerational plasticity across multiple generations. J Evol Biol 27:2297–2307. https://doi.org/10.1111/jeb.12490
Shama LNS (2015) Bet hedging in a warming ocean: predictability of maternal environment shapes offspring size variation in marine sticklebacks. Glob Chang Biol 21:4387–4400. https://doi.org/10.1111/gcb.13041
Shama LNS, Mark FC, Strobel A, Lokmer A, John U, Wegner KM (2016) Transgenerational effects persist down the maternal line in marine sticklebacks: gene expression matches physiology in a warming ocean. Evol Appl 9:1096–1111. https://doi.org/10.1111/eva.12370
Shama LNS, Strobel A, Mark FC and Wegner KM (2014) Transgenerational plasticity in marine sticklebacks: maternal effects mediate impacts of a warming ocean. Funct Ecol 28:1482–1493. https://doi.org/10.1111/1365-2435.12280
Sieck K, Nam C, Bouwer LM, Rechid D, Jacob D (2021) Weather extremes over Europe under 1.5 and 2.0° C global warming from HAPPI regional climate ensemble simulations. Earth Syst Dyn 12:457–468. https://doi.org/10.5194/esd-12-457-2021
Sigismund F, Schrum C (2001) Decadal changes in the wind forcing over the North Sea. Climate Res 18:39–45. https://doi.org/10.3354/cr018039
Singer A, Bijleveld AI, Hahner AF, Holthuijsen S, Hubert K, Kerimoglu O, Kleine Schaars L, Kröncke I, Lettmann K, Rittweg T, Scheiffarth G, van der Veer H, Wurpts A (2023) Long-term response of coastal macrofauna communities to de-eutrophication and sea level rise mediated habitat changes (1980s and 2018). Front Mar Sci 9:963325
Song J, Liang D (2023) Community structure of zooplankton and its response to aquatic environmental changes based on eDNA metabarcoding. J Hydrol 622:129692
Spear MJ, Walsh JR, Ricciardi A, van der Zanden MJ (2021) The invasion ecology of sleeper populations: prevalence, persistence, and abrupt shifts. Bioscience 71:357–369. https://doi.org/10.1093/biosci/biaa168
Spence-Jones HC, Pein CM, Shama LNSS (in press) Intergenerational effects of ocean temperature variation: early life benefits are short-lived in threespine stickleback. Plos One
Strasser M (2002) Reduced epibenthic predation on intertidal bivalves after a severe winter in the European Wadden Sea. Mar Ecol-Prog Ser 241:113–123
Strasser M, Günther C-P (2001) Larval supply of predator and prey: temporal mismatch between crabs and bivalves after a severe winter in the Wadden Sea. J Sea Res 46:57–67. https://doi.org/10.1016/S1385-1101(01)00063-6
Strasser M, Dekker R, Essink K, Günther C-P, Jaklin S, Kröncke I, Madsen PB, Michaelis H, Vedel G (2003) How predictable is high bivalve recruitment in the Wadden Sea? J Sea Res 49:47–57. https://doi.org/10.1016/S1385-1101(02)00198-3
Suchrow S, Pohlmann N, Stock M, Jemnsen K (2012) Long-term surface elevation changes in German North Sea salt marshes. Estuar Coast Shelf Sci 98:71–83. https://doi.org/10.1016/j.ecss.2011.11.031
Thieltges DW, Strasser M, van Beusekom JEE, Reise K (2004) Too cold to prosper-winter mortality prevents population increase of the introduced American slipper limpet Crepidula fornicata in northern Europe. J Exp Mar Biol Ecol 311:375–391. https://doi.org/10.1016/j.jembe.2004.05.018
Thieltges DW, Krakau M, Andresen H, Fottner S, Reise K (2006) Macroparasite community in molluscs of a tidal basin in the Wadden Sea. Helgol Mar Res 60:307–316. https://doi.org/10.1007/s10152-006-0046-3
Troost K (2010) Causes and effects of a highly successful marine invasion: case-study of the introduced Pacific oyster Crassostrea gigas in continental NW European estuaries. J Sea Res 64:145–165. https://doi.org/10.1016/j.seares.2010.02.004
Tulp I, van der Veer HW, Walker P, van Walraven L, Bolle LJ (2017) Can guild- or site-specific contrasts in trends or phenology explain the changed role of the Dutch Wadden Sea for fish? J Sea Res 127:150–163. https://doi.org/10.1016/j.seares.2016.10.001
Ulanowicz RE (2004) Quantitative methods for ecological network analysis. Comput Biol Chem 28:321–339. https://doi.org/10.1016/j.compbiolchem.2004.09.001
van Beusekom JEE, Weigelt-Krenz S, Martens P (2008) Long-term variability of winter nitrate concentrations in the northern Wadden Sea driven by freshwater discharge, decreasing riverine loads and denitrification. Helgol Mar Res 62:49–57. https://doi.org/10.1007/s10152-007-0092-5
van Beusekom JEE, Buschbaum C, Reise K (2012) Wadden Sea tidal basins and the mediating role of the North Sea in ecological processes: scaling up of management? Ocean Coast Manag 68:69–78. https://doi.org/10.1016/j.ocecoaman.2012.05.002
van Beusekom JEE, Carstensen J, Dolch T, Grage A, Hofmeister R, Lenhart H, Kerimoglu O, Kolbe K, Pätsch J, Rick J, Rönn L, Ruiter H (2019) Wadden Sea eutrophication: long-term trends and regional differences. Front Mar Sci 6:370. https://doi.org/10.3389/fmars.2019.00370
Van der Veer H, Dapper R, Henderson PA, Jung AJ, Philippart CJ, Witte JI, Zuur AF (2015) Changes over 50 years in fish fauna of a temperate coastal sea: degradation of trophic structure and nursery function. Estuar Coast Shelf Sci 155:156–166. https://doi.org/10.1016/j.ecss.2014.12.041
Van De Pol M, Ens BJ, Heg D, Brouwer L, Krol J, Maier M, Exo K-M, Oosterbeek K, Lok T, Eising CM, Koffijberg K (2010) Do changes in the frequency, magnitude and timing of extreme climatic events threaten the population viability of coastal birds? J Appl Ecol 47:720–730. https://doi.org/10.1111/j.1365-2664.2010.01842.x
Vermeersen B, Slangen A, Gerkema T, Baart F, Cohen K, Dangendorf S, Van der Wegen M (2018) Sea-level change in the Dutch Wadden Sea. Neth J Geosci 97:79–127. https://doi.org/10.1017/njg.2018.7
Vorberg R, Breckling P (1999) Atlas der Fische im Schleswig-Holsteinischen Wattenmeer. Schriftenr. des Natl. Schleswig-Holsteinisches Wattenmeer
Wachler B, Seiffert R, Rasquin C, Kösters F (2020) Tidal response to sea level rise and bathymetric changes in the German Wadden Sea. Ocean Dyn 70:1033–1052. https://doi.org/10.1007/s10236-020-01383-3
van Beusekom JEE, Loebl M, Martens P (2009) Distant riverine nutrient supply and local temperature drive the long-term phytoplankton development in a temperate coastal basin. J Sea Res 61(1-2):26–33. https://doi.org/10.1016/j.seares.2008.06.005
van der Meer J, Dankers N, Ens BJ, van Stralen M, Troost K, Waser AM (2019) The birth, growth and death of intertidal soft-sediment bivalve beds: No need for large-scale restoration programs in the Dutch Wadden Sea. Ecosystems 22:1024–1034. https://doi.org/10.1007/s10021-018-0320-7
van Walraven L, Dapper R, Nauw JJ, Tulp I, Witte JI, van der Veer HW (2017) Long-term patterns in fish phenology in the western Dutch Wadden Sea in relation to climate change. J Sea Res 127:173–181. https://doi.org/10.1016/j.seares.2017.04.001
Wanzenböck S, Fuxjäger L, Ringler E, Ahnelt H and Shama LNS (2022) Temperature-dependent reproductive success of stickleback lateral plate morphs: implications for population polymorphism and range shifts under ocean warming. Front Mar Sci 9:759450. https://www.frontiersin.org/journals/marine-science/articles/10.3389/fmars.2022.759450/full
Ward JR, Lafferty KD (2004) The elusive baseline of marine disease: are diseases in ocean ecosystems increasing? PLoS Biol 2:e120. https://doi.org/10.1371/journal.pbio.0020120
Waser AM, Splinter W, van der Meer J (2015) Indirect effects of invasive species affecting the population structure of an ecosystem engineer. Ecosphere 6:109. https://doi.org/10.1890/ES14-00437.1
Waser AM, Deuzeman S, waKangeri AK, van Winden E, Postma J, de Boer P, van der Meer J, Ens BJ (2016) Impact on bird fauna of a non-native oyster expanding into blue mussel beds in the Dutch Wadden Sea. Biol Conserv 202:39–49. https://doi.org/10.1016/j.biocon.2016.08.007
Waser AM, Lackschewitz D, Knol J, Reise K, Wegner KM, Thieltges DW (2020) Spread of the invasive shell-boring annelid Polydora websteri (Polychaeta, Spionidae) into naturalised oyster reefs in the European Wadden Sea. Mar Biodivers 50:63. https://doi.org/10.1007/s12526-020-01092-6
Watermann BT, Herlyn M, Daehne B, Bergmann S, Meemken M, Kolodzey H (2008) Pathology and mass mortality of Pacific oysters, Crassostrea gigas (Thunberg), in 2005 at the East Frisian coast, Germany. J Fish Dis 31:621–630. https://doi.org/10.1111/j.1365-2761.2008.00953.x
Wegner KM, Volkenborn N, Peter H, Eiler A (2013) Disturbance induced decoupling between host genetics and composition of the associated microbiome. BMC Microbiol 13:252. https://doi.org/10.1186/1471-2180-13-252
Wegner KM, Piel D, Bruto M, John U, Mao Z, Alunno-Bruscia M et al (2019) Molecular targets for coevolutionary interactions between Pacific oyster larvae and their sympatric Vibrios. Front Microbiol 10:2067. https://doi.org/10.3389/fmicb.2019.02067
Wegner KM, Lokmer A, John U (2020) Genomic and transcriptomic differentiation of independent invasions of the Pacific oyster Crassostrea gigas. Front Ecol Evol 8:567049. https://doi.org/10.3389/fevo.2020.567049
Wehrmann A, Herlyn M, Bungenstock F et al (2000) The distribution gap is closed — first record of naturally settled pacific oysters Crassostrea gigas in the East Frisian Wadden Sea, North Sea. Senckenb Marit 30:153–160. https://doi.org/10.1007/BF03042964
Weinert M, Kröncke I, Mathis M, Neumann H, Pohlmann T, Reiss H (2016) Modeling climate change effects on benthos: distributional shifts in the North Sea from 2001 to 2099. Estuar Coast Shelf Sci 175:157–168. https://doi.org/10.1016/j.ecss.2016.03.024
Wendling CC, Batista FM, Wegner KM (2014) Persistence, seasonal dynamics and pathogenic potential of Vibrio communities from Pacific oyster hemolymph. PLoS ONE 9:e94256. https://doi.org/10.1371/journal.pone.0094256
Wendling CC, Wegner KM (2015) Adaptation to enemy shifts: rapid resistance evolution to local Vibrio spp. in invasive Pacific oysters. Proc R Soc B Biol Sci 282:e20142244. https://doi.org/10.1098/rspb.2014.2244
Wendling CC, Fabritzek AG, Wegner KM (2016) Population-specific genotype x genotype x environment interactions in bacterial disease of early life stages of Pacific oyster larvae. Evol Appl 10:338–347. https://doi.org/10.1111/eva.12452
Whitehouse GA, Aydin KY, Hollowed AB, Holsman KK, Cheng W, Faig A, Haynie AC, Hermann AJ, Kearney KA, Punt AE, Essington TE (2021) Bottom–up impacts of forecasted climate change on the eastern Bering Sea food web. Front Mar Sci 8:624301. https://doi.org/10.3389/fmars.2021.624301
Wiltshire KH, Boersma M, Carstens K, Kraberg AC, Peters S, Scharfe M (2015) Control of phytoplankton in a shelf sea: Determination of the main drivers based on the Helgoland Roads Time Series. J Sea Res 105:42–52. https://doi.org/10.1016/j.seares.2015.06.022
Windsor DA (1998) Most of the species on earth are parasites. Int J Parasitol 28:1939–1941. https://doi.org/10.1016/s0020-7519(98)00153-2
Witte S, Buschbaum C, van Beusekom JEE, Reise K (2010) Does climatic warming explain why an introduced barnacle finally takes over after a lag of more than 50 years? Biol Invasions 12:3579–3589. https://doi.org/10.1007/s10530-010-9752-5
Wood CL, Johnson PT (2015) A world without parasites: exploring the hidden ecology of infection. Front Ecol Environ 13:425–434. https://doi.org/10.1890/140368
Zhang Z, Zhang Q, Wang T et al (2022) Assessment of global health risk of antibiotic resistance genes. Nat Commun 13:1553. https://doi.org/10.1038/s41467-022-29283-8
Ziegelmeier E (1964) Einwirkungen des kalten Winters 1962/63 auf das Makrobenthos im Ostteil der Deutschen Bucht. Helgolander Wiss Meeresunters 10:276–282. https://doi.org/10.1007/BF01626113
Acknowledgements
This paper synthesises 100 years of research at the Wadden Sea Station Sylt. The station has been a place of exciting, dynamic and fruitful research for generations of scientists, students and guests, many of whom had their first contact with a marine ecosystem here. Our thanks go to all of our former colleagues for their scientific contributions, as well as to all laboratory technicians, technical staff, administration assistants and crews of our research vessels without whose support this research would not be possible. We also thank Karsten Reise for comments on an earlier version and two reviewers for their helpful suggestions that greatly improved the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. T. Dolch, C. Buschbaum and A. Cornelius would like to thank the Landesbetrieb für Küstenschutz, Nationalpark und Meeresschutz Schleswig–Holstein (LKN), the Landesamt für Umwelt des Landes Schleswig–Holstein (LfU, former LLUR), the Niedersächsischer Landesbetrieb für Wasserwirtschaft, Küste und Naturschutz (NLWKN), the Landesamt für Umwelt, Naturschutz und Geologie Mecklenburg-Vorpommern (LUNG) and the Bundesamt für Naturschutz (BfN) for funding the seagrass monitoring in Schleswig–Holstein and the marine neobiota assessment on German coasts. A. Cornelius was funded by the Deutsche Bundesstiftung Umwelt (DBU, Grant no. 20018/530). K.H. Wiltshire, V. Siderenko, S. Rubinetti, M. Guinard and J. Rick acknowledge support by the Federal German Ministry of Education and Research through the CoastalFutures (Grant no. 03F0911J) and the Bioweb (Grant no. 03F0861A) projects. V. Sidorenko, S. Rubinetti and S. Brand acknowledge support by the Federal Ministry of Education and Research (BMBF) through the project CREATE (03F091A), part of the research mission ‘Protection and Sustainable use of Marine Areas’ within the German Marine Research Alliance (DAM). J. Hoffmann was supported by the Marie Curie action KARST, under the EU H2020 program, project number 101027303. C. Broquard was funded by the BMBF in the framework of the JPI Aquatic Pollutants programme (03F0907A). A.M. Waser acknowledges support from the project MARBEFES of the European Union's Horizon Europe Programme (Grant no. 101060937, www.marbefes.eu).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
No animal testing was performed during this study.
Sampling and field studies
For this review manuscript, no sampling and field studies were conducted.
Data availability
No additional data were collected and all data are available in the original papers.
Author contribution
CB and LNSS conceived the manuscript, and coordinated, revised and edited the text and figures. All authors contributed to the writing and approved the final version.
Additional information
Communicated by H. Hillebrand
This paper is dedicated to the 100th anniversary of the Wadden Sea Station Sylt in 2024.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Christian Buschbaum and L. N. S. Shama shared first authorship.
This article is a contribution to the Topical Collection Biodiversity and Ecology of the Wadden Sea under changing environments.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Buschbaum, C., Shama, L.N.S., Amorim, F.L.L. et al. Climate change impacts on a sedimentary coast—a regional synthesis from genes to ecosystems. Mar. Biodivers. 54, 64 (2024). https://doi.org/10.1007/s12526-024-01453-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-024-01453-5