Abstract
The Asian brush-clawed shore crab Hemigrapsus takanoi was introduced to the northern Wadden Sea (southeastern North Sea) in 2009 and now represents one of the most abundant brachyuran crab species. Abundance studies revealed an increase of mean crab densities on mixed reefs of native blue mussels (Mytilus edulis) and Pacific oysters (Magallana gigas) from 18 individuals m−2 in 2011 to 216 individuals m−2 in 2020. Despite its current high densities only little is known about the feeding habits of H. takanoi, its effects on prey populations and on the associated community in the newly invaded habitat. We summarize results of individual field and laboratory experiments that were conducted to assess feeding habits and consumption effects caused by Asian brush-clawed shore crabs and, additionally, compare the feeding ecology of H. takanoi with the one of the native shore crab Carcinus maenas. Field experiments manipulating crab densities revealed that both crab species affected the recruitment success of blue mussels, Pacific oysters and Australian barnacles (Austrominius modestus) with highest number of recruits at crab exclusion. However, endobenthic polychaetes within the reefs were differently affected. Only the native C. maenas caused a significant reduction in polychaete densities, whereas the introduced H. takanoi had no effect. Additional comparative laboratory studies revealed that single C. maenas consume more juvenile blue mussels than Asian brush-clawed shore crabs of the same size class. When offering amphipods as a mobile prey species, we found the same pattern with higher consumption rates by C. maenas than by H. takanoi. For Asian but not for native shore crabs, we detected a sex-dependent feeding behavior with male H. takanoi preferring blue mussels, while females consumed more amphipods. Considering mean crab densities and feeding behavior, our results suggest that despite lower consumption rates of single crabs, Asian brush-clawed shore crabs can cause stronger impacts on prey organisms than the native C. maenas, because H. takanoi exceeds their densities manifold. A strong impact of the invader on prey populations is supported by low amphipod occurrence at sites where H. takanoi density is high in the study area. Thus, the introduced Asian brush-clawed shore crab is an additional consumer with significant effects on the associated community of mixed reefs of mussels and oysters in the Wadden Sea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, the introduction and spread of non-native species cause changes in diversity and species interactions in recipient ecosystems (Pyšek and Richardson 2010; Lowry et al. 2013), and this is also well known for coastal habitats (Ruiz et al. 1997; Cohen and Carlton 1998; Gollasch 2006; Williams and Grosholz 2008). Effects on native coastal communities can be caused by direct and indirect interactions between introduced and native species (Lohrer and Whitlatch 2002; Buschbaum et al. 2016; Reise et al. 2017a). For example, introduced predators directly prey on indigenous species or may compete with native predators for limited food sources (Ruiz et al. 1999; Grosholz 2002; Gregory and Quijón 2011; David et al. 2017; Howard et al. 2017). Introduced predators can also cause facilitative effects on native species by preying on snails and, therefore, indirectly reducing grazing pressure on native macroalgae (Trussell et al. 2002; Griffen and Byers 2009).
Predatory brachyuran crab species represent one of the most common taxonomic group of introduced species in coastal ecosystems (Galil 2009; Karatayev et al. 2009; Brockerhoff and McLay 2011; Hänfling et al. 2011; Rato et al. 2021). A prominent example is the European shore crab Carcinus maenas (L.), which is a globally successful invader of coastal ecosystems and can cause ecological but also economic effects in the recipient ecosystems (Grosholz and Ruiz 1995; Grosholz et al. 2000; Carlton and Cohen 2003; Klassen and Locke 2007; Lovell et al. 2007; Kimbro et al. 2009). Further examples are the Asian brush-clawed shore crab Hemigrapsus takanoi Asakura & Watanabe, 2005 and the Asian shore crab Hemigrapsus sanguineus (de Haan, 1835), which have been introduced to coastal areas of northern Europe in the 1990s, presumably by shipping (Gollasch 1999; Obert et al. 2007; Landschoff et al. 2013; Geburzi et al. 2018). Both species originate from the northwestern Pacific Ocean, and in the southeastern North Sea, crabs have first been detected in 2004 in the southern and in 2009 in the northern part (Gollasch 1999; Gittenberger et al. 2010; Obert et al. 2007; Landschoff et al. 2013). Investigations on the spatial occurrence of both crab species in the intertidal zone of the Wadden Sea revealed that H. sanguineus is most abundant in artificial habitats such as coastal protection structures (Landschoff et al. 2013; Jungblut et al. 2017; Geburzi et al. 2018). By contrast, H. takanoi achieves highest abundances in natural epibenthic mixed reefs of native blue mussel (Mytilus edulis Linnaeus, 1758) and introduced Pacific oysters (Magallana gigas Thunberg, 1793), which is also a preferred habitat for native shore crabs C. maenas (Kochmann et al. 2008; Van den Brink et al. 2012; Markert et al. 2014).
Due to the co-occurrence of both species in mixed reefs of mussels and oysters, the focus of this study is on the feeding ecology of H. takanoi and the comparison with that of C. maenas. This allows the identification of potential overlap in food preferences of both crab species with resulting cumulative effects on prey populations. Existing comparative investigations on the feeding ecology of Asian brushed-shore crabs in the Wadden Sea mainly deal with prey selection of differently sized native blue mussels (Bouwmeester et al. 2020) and reveal an increase in prey size in relation to carapace width for both the non-native H. takanoi and for C. maenas. Additionally, it was shown that crabs of both species within one size class prefer the same sized mussels. Consumption rate experiments with H. takanoi in the Baltic Sea report a higher feeding rate of males on blue mussels compared to females (Nour et al. 2020) indicating that food preferences of males and females can be different. Such sex-dependent consumption behavior is also known for C. maenas and other decapod crabs (Ropes 1968; Elner 1980; Eggleston 1990). However, further detailed information on feeding ecology and prey preferences of the introduced Asian brushed-shore crab as well as consequences on native coastal communities in northwestern Europe are limited.
Therefore, the aim of this study was to investigate prey preferences of female and male Asian brush-clawed shore crabs H. takanoi in comparison to the European shore crab C. maenas in the European Wadden Sea (coastal area of the southeastern North Sea). In laboratory experiments, we offered selected key species associated with mixed reefs of mussels and oysters and quantified the consumption rates of both crab species. Additionally, we conducted crab density manipulating experiments in the field to study predation effects of H. takanoi on the associated community of oyster reefs under natural conditions. Finally, to gain insights into the population development of H. takanoi in comparison to the native C. maenas, we compared the density trajectories of both species on a mixed reef of mussels and oysters from 2011, i.e., shortly after the successful establishment of H. takanoi in the survey area, to 2020.
Material and methods
Study area
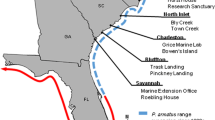
All surveys and experiments were conducted in intertidal mixed reefs of native mussels M. edulis and introduced Pacific oysters M. gigas near the northern part of the island of Sylt in the northern Wadden Sea (German Bight, southeastern North Sea, Fig. 1). The area is located in the cold temperate region with a mean annual water temperature of about 9 °C, a summer average of 15 °C, and a winter average of 4 °C. The tides are semidiurnal and average tidal range is about 1.8 m. Salinity ranges between 31 psu in summer and 28 psu in winter. Further information about hydrography, geology, sediments, and biota of the study site is given in Gätje and Reise (1998). In the Wadden Sea, the biogenic epibenthic reef structures are the preferred natural habitat of the native European shore crab C. maenas and the introduced Asian brush-clawed shore crab, H. takanoi, which is an established member of this community since 2009 (Landschoff et al. 2013).
Survey
Population development of Carcinus maenas and Hemigrapsus takanoi at site A
Densities of both crab species were annually surveyed at site A (Oddewatt, Fig. 1) in early summer (May–June) from 2017 to 2020. We randomly placed a frame (25 cm × 25 cm) on the bottom, and all substrate and organisms within the frame (including the upper approximately 5 cm of sediment) were transported to the laboratory, where the samples (n = 6) were sieved (mesh size 0.5 mm). The remaining crabs in the sieve were identified to species level. Afterwards, crabs of both species were counted and carapace width was measured to the nearest 0.1 mm with digital calipers. Additionally, we used density data of both species provided by Landschoff et al. (2013), which have been gained with the same method and at the same site in 2011.
Field survey on relationship between Hemigrapsus takanoi and mobile prey density at site A and B
To study potential effects of H. takanoi on mobile prey species in mixed oyster reefs, we simultaneously quantified the density of H. takanoi and amphipods Gammarus spp. at study sites A (Oddewatt) and B (Puan Klent) at low tide conditions in June 2018 (Fig. 1). Amphipods represent abundant and mobile organisms within epibenthic structures in the Wadden Sea and are a potential prey of Hemigrapsus spp. (Blasi and O’Connor 2016).
Crabs and amphipods were collected from parts of the mixed oyster reefs, which were overgrown by the bladder wrack Fucus vesiculosus forma mytili (Nienburg) because amphipods show a preference for algal covered bivalve beds (Albrecht and Reise 1994). Six random samples were taken from each oyster reef by using a 25 × 25 cm steel frame (625 cm2). All organism within the frame and the top 5 cm of sediment were transported to the laboratory and each sample was sieved (mesh size 1.0 mm). The algae F. vesiculosus forma mytili within each sample was washed over the sieve with fresh water to remove all attached amphipods. All remaining H. takanoi and amphipods in the sieve were counted. Algal biomass was weighted after removing amphipods and crabs.
Field predation experiments at site A
To study predation effects of native C. maenas and introduced H. takanoi on the associated species community of mixed oyster reefs, three field caging experiments were conducted between 2017 and 2019 at mixed mussel and oyster reefs at site A (Oddewatt, Fig. 1). An overview on the field experiments with detailed information on the specific approaches and methods is given in Table 1.
Predation experiment on blue mussel recruits
The effects of both crab species on blue mussel recruitment (settlement and early post-settlement survival) were investigated with a cage enclosure experiment in the field. We used cylindrical cages (15 cm in diameter, 15 cm high) with walls and roofs made of plastic mesh with 3-mm mesh size. On 28 May 2019, the cages were carefully fixed to the mixed reefs of mussel and oysters using three iron rods per cage (50 cm in length, 6 mm in diameter).
The experiment included five treatments with six replicates each: (1) no crabs in the cages, (2) cages with two male C. maenas with a carapace width of 10–50 mm, and (3) cages with two female and four male H. takanoi with a carapace width of 10–40 mm. The crab densities in the cages correspond to the natural ratio of C. maenas and H. takanoi in the surrounding oyster reef quantified in spring 2019. However, used densities in the experiment were about twofold higher than natural densities in order to determine a measurable effect. Additionally, we adapted the size range of both crab species in the cages to the natural size distribution at the time of the experiment and also considered the comparatively high numbers of female H. takanoi in our experimental set up to simulate the natural conditions as well as possible. Two treatments served as a control of possible cage artefacts: (4) open cages with holes of 10 × 10 cm in the walls so that crabs could freely pass into and out of the treated area and (5) untreated areas of the same size as cages.
To provide natural reef conditions and a suitable settlement substate for M. edulis, each cage contained four living M. edulis and two M. gigas. All bivalves were cleaned from any epigrowth before the beginning of the experiment. The experimental set up was randomly organized, and a distance of 2 m was kept between the cages to enable independency of the experimental units. After an experimental period of 14 weeks on 4 September 2019, all remaining crabs and blue mussel recruits in the cages were counted. The mean survival rates of H. takanoi (44 ± 4%) and C. maenas (44 ± 16%) in the cages were largely the same.
Predation experiment on barnacles and juvenile oysters
To investigate predation effects of C. maenas and H. takanoi on the non-native Australian barnacle Austrominius modestus (Darwin) and juvenile Pacific oysters M. gigas, we conducted an enclosure field experiment in September 2018 by using the same oyster reefs and cages as in the predation experiment on blue mussel recruitment (see the “Predation experiment on blue mussel recruits” section). The Australian barnacle was the most abundant barnacle species in the study area at the time of investigation. We used six different treatments with six replicates each: (1) cages with a single male H. takanoi and a defined number of barnacles, (2) cages with a single male C. maenas and a defined number of barnacles, (3) cages with single male H. takanoi and a defined number of juvenile oysters, and (4) cages with a single male C. maenas and a defined number of juvenile oysters. For this experiment with one crab per cage, only male individuals of both crab species with intact claws were used, because of the morphological differences between male and female crabs, which may result in different feeding behavior (Klassen 2012; Bouwmeester et al. 2020). Two treatments checked for natural mortality of barnacles and oysters: (5) cages with barnacles but without crabs and (6) cages with juvenile oysters but without crabs. All crabs of both species in the cages had a carapace width of 15–20 mm. To offer a defined number of barnacles and juvenile oysters in the cages, we collected oysters with a maximal shell length of 12–15 cm, which were overgrown with recently settled A. modestus (size 1–3 mm in diameter) and juvenile M. gigas (shell length 2–5 mm). From these oysters, we removed any epigrowth except either for juvenile barnacles or oysters. All remaining barnacles or oysters were counted. At the beginning of the experiment on 6 September 2018, each cage contained a single oyster with either 40–80 attached A. modestus or 50–75 attached juvenile M. gigas. After an experimental time period of 5 days on 11 September 2018, we quantified survival in the cages by counting all remaining living barnacles and juvenile M. gigas. The survival rate of H. takanoi and C. maenas in the cages was 100% in this experiment.
Predation experiment on endobenthic polychaetes
To examine consumption effects of C. maenas and H. takanoi on endobenthic polychaetes in a mixed reef of mussels and oysters, we conducted an enclosure field experiment by using the same mixed oyster reefs and cages as in the predation experiments on blue mussel recruits, barnacles, and oysters (see the “Predation experiment on blue mussel recruits” and “Predation experiment on barnacles and juvenile oysters” sections). With this experiment, we aimed to imitate natural population structures (density and sex ratio) of both crab species in the cages at the time of the experiment in spring 2017. Therefore, we used both female and male individuals and adapted the number of crabs in the cages, sex, and size structure to the natural conditions at the study site. We used five treatments with six replicates each: (1) no crabs in the cages, (2) cages with three female and three male H. takanoi, and (3) cages with two female and one male C. maenas. Two treatments served as a control of possible cage artefacts: (4) open cages with holes of 10 × 10 cm in the walls so that crabs could freely pass into and out of the treated area and (5) untreated areas of the same size as cages. All H. takanoi had a carapace width of 11–20 mm and C. maenas of 25–50 mm. To keep the conditions as similar as possible in the treatments, each cage contained three blue mussels with a shell length of 3–4 cm and three oysters with a shell length of 8–10 cm, which were cleaned from any epigrowth at the beginning of the experiment on 23 March 2017. After 8.5 weeks on 21 May 2017, the remaining crabs in the cages were counted. The survival rate in the cages was 89 ± 5% for H. takanoi and 72 ± 14% for C. maenas, respectively. Afterwards, sediment samples were taken with a tube cover (8 cm in diameter) to a depth of 10 cm at the center of each experimental plot. The samples were sieved (mesh size 0.5 mm), and all remaining polychaetes in the sieve were counted.
Laboratory predation experiments
To focus on specific aspects of the feeding ecology of H. takanoi in comparison to the native C. maenas and to investigate differences in prey choice of female and male crabs of both species, we conducted three laboratory experiments. Before starting the experiments, all organisms were collected in the field and kept in aquaria with seawater in climate chambers. All experiments were conducted using a natural light dark cycle and constant temperature conditions (15°C). Organisms (crabs, blue mussels, and amphipods) were acclimated to experimental conditions for at least 3 days and were fed daily. Mussels were fed with algae suspension (Instant Algae Iso 1800TM, Shellfish Diet 1800 TM), crabs were fed with mussel, and oyster flesh and amphipods were fed with fucoid macroalgae.
All crabs were starved for 24 h prior to the experiment to standardize hunger levels. Only intact and not freshly moulted crabs were used. All feeding trials lasted for 24 h. The respective crabs and prey items were added to aquaria (11 cm length, 11 cm width, 18 cm height), and after 24 h, the remaining prey organisms were counted. In pilot studies, the maximum feeding rate of both crab species was determined for blue mussels and amphipods to avoid food limitation in the course of the experiments. For all experiments, we used a control treatment, i.e., aquaria with prey organisms but without crabs. Survival rate of prey organisms was 100% in all controls of all laboratory experimental approaches. An overview on the three laboratory experiments with detailed information on the specific approaches and methods is given in Table 2.
Consumption of Mytilus edulis and the amphipod Gammarus locusta by Hemigrapsus takanoi
To compare consumption rates of introduced H. takanoi on amphipods (Gammarus locusta Linnaeus, 1758) and juvenile blue mussels (M. edulis), we conducted a no-choice experiment. Single H. takanoi individuals were separately offered 30 blue mussels (shell length 8–11 mm) and 10 G. locusta (body length 5–14 mm) within one aquarium, respectively. To explore potential gender differences in consumption, we offered both prey items to female and male H. takanoi.
The experiment with blue mussels as prey was 30 times replicated for both sexes. The experiment with amphipods as prey was 13 times replicated for female crabs and 15 times replicated for male H. takanoi. Crab carapace width of both sexes was 15–20 mm. Six control treatments were performed without any crab to control the natural mortality of both prey species.
Prey preference of Hemigrapsus takanoi between Mytilus edulis and Gammarus locusta
To investigate whether H. takanoi prefers juvenile blue mussels or amphipods (G. locusta) as prey, we conducted a choice experiment. Within one aquarium, we simultaneously offered a single crab 30 juvenile mussels (shell length 8–11 mm) and 10 amphipods (body length 5–14 mm). Again, we tested for gender differences in H. takanoi and performed this experiment with female and male crabs (carapace width 15–22 mm for both sexes), with 15 replicates each. Six control treatments were performed without any crab to control the natural mortality of both prey species.
Comparison of consumption rates of Hemigrapsus takanoi and Carcinus maenas on Mytilus edulis and Gammarus locusta
To compare consumption rates of H. takanoi and C. maenas on blue mussels and amphipods under the same experimental conditions, we conducted an additional no-choice experiment. Single crab individuals were separately offered 30 blue mussels (H. takanoi) or 60 blue mussels (C. maenas) (shell length 8–11 mm) and 10 amphipods (body length 5–14 mm) within one aquarium, respectively. To explore potential gender differences in consumption, we offered both prey items to female and male H. takanoi as well as to female and male C. maenas. Crab carapace width of both species and sexes was 13–38 mm. Replicates for the respective treatments varied from five to nine (Table 2). Six control treatments per prey species were performed without any crab to control the natural mortality of M. edulis and G. locusta.
Statistical analysis
All statistical analyses were conducted in the statistical software R (version 3.6.3, R Development Core Team 2017), and the predefined functions from the “stats” (R Core Team 2013); “ppcor” (Kim 2015); “MASS” (Venables and Ripley 2002); “ggplot2” (Wickham 2009); “car”; and “carData” (Fox and Weisberg 2019) packages were used to perform the analyses. The data are given as arithmetic means with standard error (SE). Effects were considered to be statistically significant if p value was < 0.05.
To test, whether there is a relationship between the densities of H. takanoi and amphipods Gammarus spp. in our field density survey, we pooled all our samples from both study sites (A Oddewatt, B Puan Klent) and correlated mean crab density per m2 to amphipod densities with a Spearman correlation test.
All field experiments were analyzed by using a one-way analysis of variance ANOVA, and the Levene test was used to test for homoscedasticity of variances. Data of all experiments were heterogeneous in variances and, therefore, log transformed to achieve homoscedasticity of variances. In one case (predation experiment on barnacles), variances remained heterogeneous despite transformation. Therefore, we used the non-parametric Kruskal–Wallis test and analyzed different survival rate of barnacles between the experimental treatments by using a Wilcoxon signed rank test. Data of all other experiments were homogenous in variances after transformation, and we used the Turkey’s Honest Significant Difference (HSD) multiple comparison test for pairwise comparisons between the treatments.
All laboratory experiments were analyzed by using non-parametric Mann–Whitney U-tests for all pairwise comparisons.
Results
Field density surveys
Density development of Carcinus maenas and Hemigrapsus takanoi at site A
The survey data revealed different density patterns for the native C. maenas and the non-native H. takanoi on an intertidal mussel reef in the northern Wadden Sea in the time period from 2011 to 2020 (Fig. 2). The two species showed contrasting developments: The maximum density of C. maenas was 38 ± 7 individuals m−2 in 2011 and decreased to 5 ± 3 individuals m−2 in 2020. The density of H. takanoi, by contrast, increased from a mean density of 18 ± 5 individuals m−2 in 2011 to a maximum of 248 ± 49 individuals m−2 in 2018 and remained at about 200 individuals m−2 until 2020.
Density of Gammarus spp. and Hemigrapsus takanoi at sites A and B
In June 2018, six samples on mixed oyster reefs were taken at two different sites to quantify amphipod and H. takanoi densities. At site A (Oddewatt), the mean density of Gammarus spp. was 45 ± 25 individuals m−2, while at site B (Puan Klent), amphipod densities were higher with 261 ± 38 individuals m−2 (Fig. 3). The densities of H. takanoi showed an opposite pattern with a mean density of 248 ± 49 individuals m−2 at site A and 53 ± 7 individuals m−2 at site B. We found a negative correlation between densities of H. takanoi and Gammarus spp. in the pooled samples of both study sites (n = 12, Spearman’s rank correlation: r = -0.828, p < 0.001). The mean algal biomass within the samples did not significantly differ between the two study sites (Mann–Whitney -U-test: p = 0.262) and was 310 ± 37 g m−2 at site A and 408 ± 25 g m−2 at site B, respectively.
Field experiments
Predation experiment on blue mussel recruits
The number of mussel recruits significantly differed between the treatments (one-way ANOVA: F = 9.489, df = 4, p < 0.001, Fig. 4). Most blue mussels recruited in the absence of crabs (exclusion treatment) with a mean density of 2994 ± 684 individuals m−2, which was significantly higher than recruitment of M. edulis in all other treatments (Tukey’s test: p < 0.001, for all comparisons). The number of juvenile mussels in the treatment with enclosed native C. maenas (450 ± 168 individuals m−2) was higher than in the treatment with enclosed non-native H. takanoi (185 ± 102 individuals m−2), but differences were not significantly different. We also found no significant differences between crab inclusion treatments, open cages (397 ± 112 individuals m−2), and untreated areas (291 ± 76 individuals m−2) (Tukey’s test: p > 0.3 for all comparisons).
Mean densities m−2 (± SE) of juvenile blue mussels Mytilus edulis in a field cage experiment at an intertidal mixed oyster reef after an experimental period of 14 weeks in spring/summer 2019. Five treatments were used: one predator exclusion treatment; two inclusion treatments with Carcinus maenas and Hemigrapsus takanoi in the cages, respectively; two control treatments with open cages and untreated areas
Predation experiment on barnacles and juvenile oysters
The survival rate of barnacles A. modestus differed between the three experimental treatments (Kruskal–Wallis test: p < 0.001, Fig. 5). Highest barnacle survival rate was detected in the absence of crabs with a mean survival of 99.38% ± 0.62% cage−1, and survival rate was significantly lower in the treatments with enclosed native C. maenas (77.40% ± 3.89% cage−1, Wilcoxon signed rank test: p = 0.004) and non-native H. takanoi (83.14% ± 3.13% cage−1, Wilcoxon signed rank test: p = 0.004) in the cages, respectively.
Mean survival percentage cage−1 (± SE) of juvenile Austrominius modestus and juvenile Magallana gigas in a field caging experiment with three different treatments: two inclusion treatments (Carcinus maenas, Hemigrapsus takanoi) and one control (without predators) at the end of 5 days exposure in September 2018
Survival rate of juvenile Pacific oysters M. gigas was also significantly different between cages with native C. maenas (70.95% ± 4.60% cage−1), cages with non-native H. takanoi (67.45 ± 2.47% cage−1), and cages without crabs (96.74 ± 1.46% cage−1; one-way ANOVA: F = 54.28, df = 2, p < 0.001, Fig. 5). We found significantly lower survival rates of juvenile oysters in cages with native C. maenas and non-native H. takanoi (Turkey’s test p < 0.001, respectively) in comparison to cages without crabs.
The survival rate of A. modestus and M. gigas in cages with native C. maenas was not significantly different from the survival rate in cages with non-native H. takanoi (A. modestus Turkey’s test, p = 0.273; C. gigas, Turkey’s test, p = 0.515).
Predation experiment on endobenthic polychaetes
The mean number of polychaetes in the sediment samples significantly differed between the treatments (one-way ANOVA: df = 4, F = 2.877, p = 0.043, Fig. 6) and was lowest in cages with native C. maenas (2817 ± 1237 individuals m−2). The number of polychaetes in cages with non-native H. takanoi (6266 ± 15 individuals m−2) was not significantly different from cages without crabs (6863 ± 1884 individuals m−2; Tukey’s test: p = 0.986). Interestingly, polychaete densities were 2.8-fold higher in open cages (8122 ± 2359 individuals m−2) and 3.5-fold higher in untreated areas (10,112 ± 1810 individuals m−2) than in cages with the native C. maenas, although this species also had access to both control treatments. Maybe, our used density of C. maenas in the cages was higher than the natural density in the surrounding environment, although this has been adjusted before the experiment. In our analysis, we did not differentiate between different polychaete species but most abundant were Tharyx killariensis (Southern, 1914), Capitella capitata (Fabricius, 1780), and Scoloplos armiger (Müller, 1776).
Mean densities (± SE) of polychaetes m−2 in a field cage experiment at an intertidal mixed oyster reef after an experimental period of 8.5 weeks in spring 2017. Five treatments were used: two inclusion treatments (Carcinus maenas, Hemigrapsus takanoi), one exclusion treatment (without predators), and two control treatments (open cages and untreated areas)
Laboratory experiments
Consumption rate of Mytilus edulis and Gammarus locusta by Hemigrapsus takanoi
In a no-choice experiment, female H. takanoi consumed significantly more amphipods G. locusta than M. edulis (Mann–Whitney U-test: p < 0.001), while male H. takanoi consumed more blue mussels than amphipods (Mann–Whitney U-test: p < 0.001, Fig. 7). Comparing the sexes, the consumption of amphipods G. locusta by female and male H. takanoi was significantly different (Mann–Whitney U-test: p < 0.001). Female crabs consumed about twice as many amphipods (8.00 ± 0.37 amphipods crab−1 d−1) than male individuals (4.06 ± 0.47 amphipods crab−1 d−1). The consumption of blue mussels by female and male crabs was also significantly different (Mann–Whitney U-test: p < 0.001). Male crabs consumed about 10 times more blue mussels (14.83 ± 0.96 blue mussels crab−1 d−1) than female conspecifics (1.43 ± 0.22 blue mussels crab−1 d−1).
Prey preference of Hemigrapsus takanoi between Mytilus edulis and Gammarus locusta
In this choice experiment, female H. takanoi consumed significantly more amphipods G. locusta (7.13 ± 0.42 amphipods crab−1 d−1) than blue mussels M. edulis (1.40 ± 0.37 blue mussels crab−1 d−1, Mann–Whitney U-test: p < 0.001, Fig. 8). Male H. takanoi showed an opposite pattern with lower consumption of G. locusta (3.53 ± 0.72 amphipods crab−1 d−1) in comparison to M. edulis (23.2 ± 2.43 blue mussels crab−1 d−1, Mann–Whitney U-test: p < 0.001, Fig. 8). Thus, the choice experiment confirmed the pattern from the no-choice experiment (see the “Consumption rate of M. edulis and G. locusta by H. takanoi” section).
Personal observations during the course of the experiment revealed that female and male H. takanoi use different techniques to open blue mussels. Male Asian brush-clawed shore crabs crushed the mussels into many small pieces by using their powerful claws (Fig. 9a,c). Female conspecifics opened the shells at the posterior end and removed the mussel flesh from the opening, leaving the mussel shell largely intact (Fig. 9b,d).
The strategy of Hemigrapsus takanoi for preying on mussels is different in male (a) and female (b) Asian brush-clawed shore crabs. Male crabs crack the whole shell, resulting in many shell fragments (c). Female crabs open Mytilus edulis shells at the posterior end of the mussels, leaving the shells largely intact (d)
Comparison of consumption rates of Mytilus edulis and Gammarus locusta by Hemigrapsus takanoi and Carcinus maenas
In an additional no choice experiment, native C. maenas consumed significantly more M. edulis than non-native H. takanoi (analyzed over both sexes: Mann–Whitney U-test: p < 0.012, Fig. 10a). While female (28.0 ± 7.85 blue mussels crab−1 d−1) and male (26.62 ± 7.97 blue mussels crab−1 d−1) C. maenas did not consume different numbers of M. edulis, female H. takanoi (3.88 ± 1.88 blue mussels crab−1 d−1) consumed fewer blue mussels than male Asian brush-clawed shore crabs (9.0 ± 2.88 blue mussels crab−1 d−1), although in this experiment, the difference was not significantly different (Mann–Whitney U-test: p = 0.182; compare the “Consumption rate of M. edulis and G. locusta by H. takanoi” section).
The consumed number of amphipods was also significantly different between native C. maenas and introduced H. takanoi with higher consumption rates in C. maenas than in H. takanoi (analyzed over both sexes: Mann–Whitney U-test: p = 0.006, Fig. 10b). Female and male C. maenas consumed no significant different numbers of amphipods. Female H. takanoi (6.00 ± 0.57 amphipods crab−1 d−1) consumed similar numbers of amphipods than female (6.67 ± 0.92 amphipods crab−1 d−1) and male C. maenas (6.80 ± 0.73 amphipods crab−1 d−1), but consumption was significantly higher than of male H. takanoi (1.83 ± 0.40 amphipods per crab d−1, Mann–Whitney U-test: p < 0.003). In this case, the low consumption rate of amphipods by male H. takanoi mainly caused the significant difference between both crab species. Comparison of consumption rate of mussels and amphipods by female C. maenas as well as male C. maenas revealed no significant difference between the sexes (Mann–Whitney U-test: p > 0.136, for both comparisons). Female Asian brush-clawed shore crabs consumed more amphipods than blue mussels although the difference was not significantly different (Mann–Whitney U-test: p < 0.085). Male H. takanoi showed a contrasting pattern with higher consumption of M. edulis than G. locusta, but this difference was also not significantly different (Mann–Whitney U-test: p < 0.064). However, the pattern of prey preferences for female and male H. takanoi was the same as in the previous experiments.
Discussion
The results of this study reveal a strong density increase of introduced Asian brush-clawed crabs in the northern Wadden Sea during the last decade, whereas densities of native C. maenas have decreased. Both crab species show an overlapping food spectrum of epibenthic prey organisms with higher consumption rates per crab for C. maenas. The occurrence of endobenthic polychaeta worms, by contrast, was only affected by native shore crabs.
Population development of Asian and native shore crabs
Since its first detection in 1993, the Asian brush-clawed shore crab H. takanoi now occurs at many coastal habitats in western Europe (Gollasch 1999) and strongly increased in abundance of more than 100 individuals m−2 at different sites in the Wadden Sea (Schückel et al. 2013; Geburzi et al. 2018; Bleile 2019). Shortly after the introduction into a new environment, invasion trajectories of non-native species regularly show a lag phase with low abundances, followed by an exponential density increase (Mack et al. 2000; Blackburn et al. 2011; Reise et al. 2017b). A subsequent adjustment phase is triggered by behavioral and evolutionary adaptations and often results in population dynamics with fluctuating density levels (Reise et al. 2017a). Such a pattern was also shown by H. takanoi in our study area at site A with still low abundances of about 20 individuals m−2 in 2011 and a subsequent strong density increase until 2018. Since then, densities remain relatively stable with values of about 225 individuals m−2. Our study focused on one site, but since 2011, a strong density increase of H. takanoi has also been observed at many further oyster reefs in the northern Wadden Sea (Mölle 2017), suggesting that the investigated oyster reef and found invasion trajectory of H. takanoi are representative for the study area.
Asian brush-clawed shore crabs need epibenthic structures, and mixed reefs of M. edulis and M. gigas offer such a habitat with ideal growth and living conditions for the newcomer (Landschoff et al. 2013). Furthermore, low intraspecific competition and reduced predation pressure by native shore crabs facilitated the high recruitment success and the comparatively high survival probability of juvenile H. takanoi on the reefs (Geburzi et al. 2018). Since the introduction of H. takanoi, the native C. maenas has to share one of its preferred habitats with the invader. In 2011, densities of C. maenas in mixed reefs were still higher than of the non-native H. takanoi, but decreased in the following years, while densities of H. takanoi strongly increased. At site A in 2020, densities of H. takanoi were finally many times higher than of. C. maenas. This development may be caused by high predation pressure of adult Asian brush-clawed shore crabs on native C. maenas recruits (Lohrer and Whitlatch 2002; van den Brink and Hutting 2017; Geburzi et al. 2018) and competition for food sources. However, also other factors or multiple as well as combined effects may have been responsible, because a decline in C. maenas was already observed in the southern Wadden Sea before the introduction of H. takanoi (Van den Brink et al. 2012). Reasons for the previous decrease of C. maenas are still unknown, but it also may have made the enormous increase in Asian brush-clawed shore crabs possible in the first place (Van den Brink et al. 2012). The current pattern with a dominance of H. takanoi in mixed reefs will presumably persist because the presence of Asian brush-clawed shore crabs enhances the recruitment of juvenile conspecifics (Geburzi et al. 2018).
However, despite decreasing densities in oyster reefs, it seems not likely that the occurrence of native shore crabs is endangered by the spread of H. takanoi on a Wadden-Sea wide scale. In contrast to Asian brush-clawed shore crabs, C. maenas also achieves high densities in other habitats than mixed reefs of mussels and oysters such as bare sedimentary tidal flats and seagrass beds that also serve as nursery grounds for native shore crabs (Polte et al. 2005; van den Brink and Hutting 2017). Additionally, a large part of the adult C. maenas population lives in the subtidal zone and provides a high number of planktonic larvae, which settle in the above-mentioned habitats.
Prey preferences of introduced and native crabs
Beside direct predation effects between introduced H. takanoi and native C. maenas, also competition for food may affect the occurrence of both species in mixed reefs of mussels and oysters (van den Brink and Hutting 2017). Both species show an overlapping food spectrum but with partly different prey preferences. While the native C. maenas feeds on both epibenthic and endobenthic prey items such as polychaetes, the introduced H. takanoi mainly consumes epibenthic organisms. The broader food spectrum of C. maenas may offer a competitive advantage over H. takanoi. Despite the competitive advantage, however, densities of C. maenas have decreased on the oyster reef at site A in the last 10 years, suggesting that competition for food with H. takanoi has presumably not caused the density development of C. maenas. This assumption is supported by the fact that both crab species can also use other food sources such as algae and carrion (Knudsen 1964; Moore and Howarth 1996). Indeed, we regularly observed Asian brush-clawed shore crabs and native shore crabs feeding on just died mussels and oysters being common on the reefs.
The introduction of H. takanoi and its preferred consumption of epibenthic organisms cause an increased predation pressure on sessile and mobile organisms above the sediment surface within an oyster reef. Therefore, associated species such as sessile barnacles attached to the bivalve shells and motile amphipods may suffer from high Asian brush-clawed shore crab densities and may show lower densities in comparison to the situation before the establishment of H. takanoi. The increased consumption of epibenthic organisms could also explain the negative correlation between the densities of H. takanoi and amphipods. Although this pattern was also observed at other sites in the northern Wadden Sea (K. Reise, personal communication), this result has to be considered with caution. We have only investigated a limited number of oyster reefs, and we have no additional information on other predators and the sex ratio of H. takanoi at these sites, which also may affect amphipod densities. Generally, associated species of epibenthic structures in the Wadden Sea often reveal high interannual variations (Buschbaum 2000), and whether densities of barnacles, amphipods, and further species will decrease in the long run cannot be yet answered by our investigations but should be subject in forthcoming more long-lasting studies.
The increased predation pressure may not only affect oyster reef associated organisms but also the habitat-forming species itself. In our field experiments, H. takanoi and C. maenas revealed a significant reduction of native M. edulis and non-native M. gigas with lower densities of both bivalve species at crab presence in the cages. This treatment also excluded further potential consumers of juvenile blue mussels such as starfish. However, potential other predators than crabs did not additionally reduce the number of mussel recruits in untreated areas and open cages. This result indicates that crab predation was the main factor, which affected blue mussel recruitment success. However, not only the cumulative impact of H. takanoi and C. maenas is important but also the temporal occurrence and recruitment of both crab species in oyster reefs. The native C. maenas occurs from late spring to autumn on intertidal oyster reefs, but spends the winter months in the sublittoral zone and shows a temporal restricted recruitment phase in July and August. By contrast, the introduced H. takanoi inhabits intertidal oyster reefs all year round with continuous recruitment that peaks in September (Geburzi et al. 2018). Thus, predation effects by Asian brush-clawed shore crabs on mussels and oysters are not limited in time and may also reduce the number of bivalves during the winter months. Additionally, temporal mismatch between bivalve recruits and predatory juvenile C. maenas may cause high recruitment success in M. edulis (Strasser and Günther 2001). With the permanent presence of juvenile and adult H. takanoi on oyster reefs, this temporal refuge for bivalve recruits seems to have ceased. The resulting higher predation pressure must be compensated by mussel and oyster recruitment. Otherwise, the structure and spatial extent of oyster reefs will be affected in the long term, but this can only be determined by ongoing studies.
Differences between sexes
In our laboratory experiments, both crab species consumed amphipods and blue mussels. While the sex of C. maenas did not significantly influence the consumption rate, the food preference of H. takanoi was different between female and male individuals. Male Asian brush-clawed shore crabs consumed fewer amphipods than female crabs of the same size class, but consumption of blue mussels was higher in male compared to female H. takanoi.
The different consumption patterns of female and male H. takanoi can be explained by sexual dimorphism in the shape of the chelae (Mingkid et al. 2006; Markert et al. 2016). Male H. takanoi have larger and stronger claws than similar-sized females (Nour et al. 2020). The filigree and tweezer-like claw shape of female crabs likely facilitate the catching of small mobile prey items, while the big claw of males is less suited for catching amphipods but advantageous for cracking mussels. The different food preferences of female and male H. takanoi show that not only the total density of a predator species but also the sex ratio is important when considering the effects on specific prey species. For example, in our laboratory experiments, the consumption of blue mussels by female H. takanoi was quite low, and, therefore, mainly male Asian brush-clawed shore crabs should be considered to exert the predation pressure on M. edulis (see Fig. 7). In the northern Wadden Sea, densities of male H. takanoi were not significantly higher than of female conspecifics, although slight differences in the sex ratio may occur (Schückel et al. 2013; Goedknegt et al. 2017) that we considered in our field experiments by adjusting the number of females and males in the cages to field densities at the respective times of the experiments. Interestingly, Nour et al. (2020) investigated the sex ratio of H. takanoi in the Western Baltic Sea and revealed that females were dominant over males (mean sex ratio of 1.4:1), but this ratio also shows strong seasonal changes with resulting predatory effects on population dynamics of blue mussels M. edulis. Therefore, sex specific investigations of introduced predatory species are essential to assess their effects on native prey organisms in invaded habitats.
Consumption rates and synthesis
Our results show that H. takanoi is nowadays a well-established and permanent non-native predator on oyster reefs in the Wadden Sea and will presumably have a persistent impact on the associated community of mixed reefs of mussels and oysters but also on the habitat structuring bivalves itself.
In our field experiments, we aimed to adapt the densities and sex ratios of H. takanoi and C. maenas to their natural occurrences within oyster reefs and did not find significant differences in the effects on the studied prey species (see M. edulis Fig. 4; A. modestus and C. gigas Fig. 5). However, for consumption rate comparisons between Asian brush-clawed shore crabs and native C. maenas, our more controlled laboratory experiments were much more suitable. They revealed that the consumption rate of single C. maenas is generally higher than of single H. takanoi, but the net effect on the number of consumed prey organisms in the field may be different when considering crab densities on oyster reefs. For example, our comparative experiments on consumption rates of C. maenas and H. takanoi (see Fig. 10) revealed that the mean consumption of a single C. maenas was about 7 amphipods d−1, which was almost two times higher than 4 amphipods d−1 consumed by a single H. takanoi (mean of both sexes). Assuming the highest densities found for C. maenas (38 individuals m−2 in 2011) and H. takanoi (248 individuals m−2 in 2018), this corresponds to a total consumption of 266 amphipods m−2 d−1 by C. maenas but of 992 amphipods m−2 d−1 by H. takanoi, respectively. A similar pattern was found when considering the habitat building blue mussels as prey. The mean consumption rate of a single C. maenas was 27 mussels d−1 (see Fig. 10) and about 4.5 times higher than 6 mussels d−1 consumed by a single H. takanoi (mean of both sexes). This corresponds to a total consumption rate of 1026 mussels m−2 d−1 by C. maenas and 1488 mussels m−2 d−1 by H. takanoi, respectively, when again using the highest densities of both crab species during the study period. In both cases, the total predation pressure of H. takanoi is much higher than that of the native C. maenas. Although natural consumption rates might be lower, these examples demonstrate how the predation conditions may have changed by the introduction of H. takanoi and its successful establishment on oyster reefs in the Wadden Sea (Fig. 11). Whether and to what extent these changed consumption patterns and the cumulative predation pressure of both crab species will affect the habitat and its associated organisms in the long term remains to be investigated.
Schematic illustration of predator prey interactions and densities of prey organisms in mixed reefs of mussels and oysters before (a) and after the introduction (b) of Asian brush-clawed shore crabs. It is suggested that the establishment and high densities of Hemigrapsus takanoi have caused higher predation pressure on epibenthic prey organisms such as barnacles, amphipods, as well as juvenile Pacific oysters and blue mussels in mixed reefs of mussels and oysters, while predation pressure on endobenthic polychaetes is reduced due to decreased densities of native Carcinus maenas
This study combined a series of small case experiments to provide the most comprehensive overall picture of potential predation effects of the introduced Asian brush-clawed shore crab H. takanoi in comparison to the native C. maenas that may explain the current invasion status of the Asian brush-clawed shore crab.
References
Albrecht A, Reise K (1994) Effects of Fucus vesiculosus covering intertidal mussel beds in the Wadden Sea. Helgol Meeresunters 48:243–256. https://doi.org/10.1007/BF02367039
Blackburn TM, Pyšek P, Bacher S, Carlton JT, Duncan RP, Jarošík V, Wilson JRU, Richardson DM (2011) A proposed unified framework for biological invasions. Trends Ecol Evol 26(7):333–339. https://doi.org/10.1016/j.tree.2011.03.023
Blasi JC, O’Connor NJ (2016) Amphipods as potential prey of the Asian shore crab Hemigrapsus sanguineus: laboratory and field experiments. J Exp Mar Biol Ecol 474:18–22. https://doi.org/10.1016/j.jembe.2015.09.011
Bleile N (2019) Effects of predator competition and prey preference of C. maenas and invasive Hemigrapsus spp. on prey communities in the Wadden Sea. Master-Thesis University of Groningen, The Netherlands
Bouwmeester MM, Waser AM, van der Meer J, Thieltges DW (2020) Prey size selection in invasive (Hemigrapsus sanguineus and H. takanoi) compared with native (Carcinus maenas) marine crabs. J Mar Biol Assoc UK 100:73–77. https://doi.org/10.1017/S0025315419000985
Brockerhoff A, McLay C (2011) Human-mediated spread of alien crabs. In: Galil B, Clark P, Carlton J (eds) The Wrong Place - Alien Marine Crustaceans: Distribution, Biology and Impacts. Invading Nature - Springer Series in Invasion Ecology, vol 6. Springer, Dordrecht
Buschbaum C (2000) Direct and indirect effects of Littorina littorea (L.) on barnacles growing on mussel beds in the Wadden Sea. Hydrobiologia 440:119–128. https://doi.org/10.1023/A:1004142306396
Buschbaum C, Cornelius A, Goedknegt MA (2016) Deeply hidden inside introduced biogenic structures Pacific oyster reefs reduce detrimental barnacle overgrowth on native blue mussels. J Sea Res 117:20–26. https://doi.org/10.1016/j.seares.2016.09.002
Carlton JT, Cohen AN (2003) Episodic global dispersal in shallow water marine organisms: the case history of the European shore crabs Carcinus maenas and C. aestuarii. J Biogeogr 30:1809–1820. https://doi.org/10.1111/j.1365-2699.2003.00962.x
Cohen AN, Carlton JT (1998) Accelerating invasion rate in a highly invaded estuary. Science 279:555–558. https://doi.org/10.1126/science.279.5350.555
David P, Thébault E, Anneville O, Duyck P-F, Chapuis E, Loeuille N (2017) Impacts of invasive species on food webs: a review of empirical data. Adv Ecol Res 56:1–60. https://doi.org/10.1016/bs.aecr.2016.10.001
Eggleston DB (1990) Functional responses of blue crabs Callinectes sapidus Rathbun feeding on juvenile oysters Crassostrea virginica (Gmelin): effects of predator sex and size, and prey size. J Exp Mar Biol Ecol 143:73–90. https://doi.org/10.1016/0022-0981(90)90112-P
Elner RW (1980) The influence of temperature, sex, and chela size in the foraging strategy of the shore crab, Carcinus maenas (L.). Mar Behav Phys 7:15–24. https://doi.org/10.1080/10236248009386968
Fox J, Weisberg S (2019) An R companion to applied regression, 3rd edn. Sage, Thousand Oaks (https://socialsciences.mcmaster.ca/jfox/Books/Companion/). Accessed 18 May 2021
Gätje C, Reise K (eds) (1998) Ökosystem Wattenmeer: Austausch-,Transport- und Stoffumwandlungsprozesse. Springer-Verlag, Berlin
Galil BS (2009) Taking stock: inventory of alien species in the Mediterranean Sea. Biol Invasions 11:359–372. https://doi.org/10.1007/s10530-008-9253-y
Geburzi JC, Brandis D, Buschbaum C (2018) Recruitment patterns, low cannibalism and reduced interspecific predation contribute to high invasion success of two Pacific crabs in northwestern Europe. Estuar Coast Shelf Sci 200:460–472. https://doi.org/10.1016/j.ecss.2017.11.032
Gittenberger A, Rensing M, Stegenga H, Hoeksema BW (2010) Native and non- native species of hard substrata in the Dutch Wadden Sea. Ned Faun Meded 33:21–76
Goedknegt MA, Havermans J, Waser AM, Pieternella CL, Velilla E, Camphysen KCJ, van der Meer J, Thieltges DW (2017) Cross-species comparison of parasite richness, prevalence, and intensity in native compared to two invasive brachyuran crabs. Aquat Invasions 12(2):201–212. https://doi.org/10.3391/ai.2017.12.2.08
Gollasch S (1999) The Asian decapod Hemigrapsus penicillatus (De Haan, 1835) (Grapsidae, Decapoda) introduced in European waters: status quo and future perspective. Helgol Meeresunters 52:359–366. https://doi.org/10.1007/BF02908909
Gollasch S (2006) Overview on introduced aquatic species in European navigational and adjacent waters. Helgol Mar Res 60:84–89. https://doi.org/10.1007/s10152-006-0022-y
Griffen BD, Byers JE (2009) Community impacts of two invasive crabs: the interactive roles of density, prey recruitment, and indirect effects. Biol Invasions 11:927–940. https://doi.org/10.1007/s10530-008-9305-3
Grosholz E (2002) Ecological and evolutionary consequences of coastal invasions. Trends Ecol Evol 17:22–27. https://doi.org/10.1016/S0169-5347(01)02358-8
Grosholz ED, Ruiz GM, Dean CA, Shirley KA, Maron JL, Connors PG (2000) The impacts of a nonindigenous marine predator in a California bay. Ecology 81:1206–1224. https://doi.org/10.1890/0012-9658(2000)081[1206:TIOANM]2.0.CO;2
Grosholz ED, Ruiz GM (1995) Spread and potential impact of the recently introduced European green crab, Carcinus maenas, in central California. Mar Biol 122:239–247
Gregory GJ, Quijón PA (2011) The impact of a coastal invasive predator on infaunal communities: assessing the roles of density and a native counterpart. J Sea Res 66:181–186. https://doi.org/10.1016/j.seares.2011.05.009
Hänfling B, Edwards F, Gherardi F (2011) Invasive alien Crustacea: dispersal, establishment, impact and control. Biocontrol 56:573–595. https://doi.org/10.1007/s10526-011-9380-8
Howard BR, Therriault TW, Côté IM (2017) Contrasting ecological impacts of native and non-native marine crabs: a global meta-analysis. Mar Ecol Prog Ser 577:93–103. https://doi.org/10.3354/meps12245
Jungblut S, Beermann J, Boos K, Saborowski R, Hagen W (2017) Population development of the invasive crab Hemigrapsus sanguineus (De Haan, 1853) and its potential native competitor Carcinus maenas (Linnaeus, 1758) at Helgoland (North Sea) between 2009 and 2014. Aquat Invasions 12. https://doi.org/10.3391/ai.2017.12.1.09
Karatayev AY, Burlakova LE, Padilla DK, Mastitsky SE, Olenin S (2009) Invaders are not a random selection of species. Biol Invasions 11:2009–2019. https://doi.org/10.1007/s10530-009-9498-0
Kim S (2015) ppcor: An R package for a fast calculation to semi-partial correlation coefficients. Commun Stat Appl Methods 22(6):665–674
Kimbro DL, Grosholz ED, Baukus AJ, Nesbitt NJ, Travis NM, Attoe S, Coleman-Hulbert C (2009) Invasive species cause large-scale loss of native California oyster habitat by disrupting trophic cascades. Oecologia 160:563–575. https://doi.org/10.1007/s00442-009-1322-0
Klassen G, Locke A (2007) A biological synopsis of the European green crab, Carcinus maenas. Can Manuscr Rep Fish Aquat Sci 2818. Fisheries and Oceans Canada, Moncton
Klassen G (2012) Biological synopsis of the Asian shore crab, Hemigrapsus sanguineus. Can Manuscr Rep Fish Aquat Sci 2978. Fisheries and Oceans Canada, Burlington
Knudsen JW (1964) Observations of the reproductive cycles and ecology of the common Brachyura and crablike Agoura of Puget Sound, Washington. Pac Sci 18:3–33
Kochmann J, Buschbaum C, Volkenborn N, Reise K (2008) Shift from native mussels to alien oysters: Differential effects of ecosystem engineers. J Exp Mar Biol Ecol 364:1–10. https://doi.org/10.1016/j.jembe.2008.05.015
Landschoff J, Lackschewitz D, Kesy K, Reise K (2013) Globalization pressure and habitat change: Pacific rocky shore crabs invade armored shorelines in the Atlantic Wadden Sea. Aquat Invasions 8:77–87. https://doi.org/10.3391/ai.2013.8.1.09
Lohrer AM, Whitlatch RB (2002) Interactions among Aliens: apparent replacement of one exotic species by another. Ecology 83:719–732. https://doi.org/10.1890/0012-9658(2002)083[0719:IAAARO]2.0.CO;2
Lovell S, Besedin E, Grosholz E (2007) Modeling economic impacts of the European green crab. American Agricultural Economics Association Annual Meeting, Portland. https://doi.org/10.22004/ag.econ.9765
Lowry E, Rollinson EJ, Laybourn AJ, Scott TE, Aiello-Lammens ME, Gray SM, Mickley J, Gurevitch J (2013) Biological invasions: a field synopsis, systematic review, and database of the literature. Ecol Evol 3:182–196. https://doi.org/10.1002/ece3.431
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasion: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710. https://doi.org/10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2
Markert A, Raupach MJ, Segelken-Voigt A, Wehrmann A (2014) Molecular identification and morphological characteristics of native and invasive Asian brush-clawed crabs (Crustacea: Brachyura) from Japanese and German coasts: Hemigrapsus penicillatus (De Haan, 1835) versus Hemigrapsus takanoi Asakura & Watanabe 2005. Org Divers Evol 14:369–382. https://doi.org/10.1007/s13127-014-0176-4
Markert A, Esser W, Frank D, Wehrmann A, Exo K-M (2016) Habitat change by the formation of alien Crassostrea-reefs in the Wadden Sea and its role as feeding sites for waterbirds. Estuar Coast Shelf Sci 131:41–51. https://doi.org/10.1016/j.ecss.2013.08.003
Mölle J (2017) Winter occurrence and feeding habits of introduced Asian shore crabs in the Wadden Sea. Bachelor-Thesis University of Kiel, Germany
Mingkid WM, Akiwa S, Watanabe S (2006) Morphological characteristics, pigmentation, and distribution of the sibling penicillate crabs, Hemigrapsus penicillatus (De Haan, 1835) and H. takanoi Asakura & Watanabe, 2005 (Decapoda, Brachyura, Grapsidae) in Tokyo Bay. Crustaceana 79:1107–1112. https://doi.org/10.1163/156854006778859696
Moore PG, Howarth J (1996) Foraging by marine scavengers: effects of relatedness, bait damage and hunger. J Sea Res 36:267–273. https://doi.org/10.1016/S1385-1101(96)90795-9
Nour OM, Stumpp M, Morón Lugo SC, Barboza FR, Pansch C (2020) Population structure of the recent invader Hemigrapsus takanoi and prey size selection on Baltic Sea mussels. Aquat Invasions 15:297–317. https://doi.org/10.3391/ai.2020.15.2.06
Obert B, Herly M, Grotjahn M (2007) First records of two crabs from the North West Pacific Hemigrapsus sanguineus and H. takanoi at the coast of lower Saxony, Germany. Wadden Sea Newsl 1:21–22
Polte P, Schanz A, Asmus H (2005) The contribution of seagrass beds (Zostera noltii) to the function of tidal flats as a juvenile habitat for dominant, mobile epibenthos in the Wadden Sea. Mar Biol 147:813–822. https://doi.org/10.1007/s00227-005-1583-z
Pyšek P, Richardson DM (2010) Invasive species, environmental change and management, and health. Annu Rev Environ Resour 35:25–55. https://doi.org/10.1146/annurev-environ-033009-095548
R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3–900051–07–0, URL http://www.R-project.org/
Rato L, Crespo D, Lemos MFL (2021) Mechanisms of bioinvasions by coastal crabs using integrative approaches – A conceptual review. Ecol Indic 125:107578. https://doi.org/10.1016/j.ecolind.2021.107578
Reise K, Buschbaum C, Büttger H, Wegner KM (2017a) Invading oysters and native mussels: from hostile takeover to compatible bedfellows. Ecosphere 8:1–14. https://doi.org/10.1002/ecs2.1949
Reise K, Buschbaum C, Büttger H, Rick J, Wegner KM (2017b) Invasion trajectory of Pacific oysters in the northern Wadden Sea. Mar Biol 164:68. https://doi.org/10.1007/s00227-017-3104-2
Ropes JW (1968) The feeding habits of the green crab, Carcinus maenas (L.). Fish Bull 67:183–203
Ruiz GM, Carlton JT, Grosholz ED, Hines AH (1997) Global invasions of marine and estuarine habitats by non-indigenous species: mechanisms, extent, and consequences. Amer Zool 37:621–632. https://doi.org/10.1093/icb/37.6.621
Ruiz GM, Fofonoff P, Hines AH, Grosholz ED (1999) Non-indigenous species as stressors in estuarine and marine communities: assessing invasion impacts and interactions. Limnol Oceanogr 44:950–972. https://doi.org/10.4319/lo.1999.44.3_part_2.0950
Schückel U, Markert A, Neumann H, Kröncke I, Wehrmann A (2013) Neue Krebse auf dem Vormarsch Arealverschiebung und Bioinvasion in der Nordsee. Senckenberg Natur-Forschung-Museum 143(5/6):152–157
Strasser M, Günther C-P (2001) Larval supply of predator and prey: temporal mismatch between crabs and bivalves after a severe winter in the Wadden Sea. J Sea Res 46:57–67. https://doi.org/10.1016/S1385-1101(01)00063-6
Trussell GC, Ewanchuk PJ, Bertness MD (2002) Field evidence of trait-mediated indirect interactions in a rocky intertidal food web. Ecol Lett 5:241–245. https://doi.org/10.1046/j.1461-0248.2002.00304.x
Van den Brink AM, Wijnhoven S, McLay CL (2012) Competition and niche segregation following the arrival of Hemigrapsus takanoi in the formerly Carcinus maenas dominated Dutch delta. J Sea Res 73:126–136. https://doi.org/10.1016/j.seares.2012.07.006
Van den Brink AM, Hutting S (2017) Clash of the crabs: interspecific, inter-cohort competition between the native European green crab, Carcinus maenas and the exotic brush clawed crab Hemigrapsus takanoi on artificial oyster reefs. J Sea Res 128:41–51
Venables WN, Ripley BD (2002) Modern applied statistics with S, Fourth edition. Springer, New York. ISBN 0–387–95457–0, https://www.stats.ox.ac.uk/pub/MASS4/
Wickham H (2009) ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York, p 2009
Williams SL, Grosholz ED (2008) The invasive species challenge in estuarine and coastal environments: marrying management and science. Estuaries Coast 31:3–20. https://doi.org/10.1007/s12237-007-9031-6
Acknowledgements
We are grateful to Silja Bürkle, Sarah Büker, Cassandra Scheibl, Eileen Whitelaw, and Eike Petersen for their extensive field and laboratory work as well as their enthusiasm. We are also grateful to Tobias Dolch and David Thieltges for their helpful comments on the manuscript. We are also grateful for the comments and suggestions of two anonymous reviewers, which significantly improved the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by the German Environmental Foundation (DBU), grant no. 20018/530.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for animal testing, animal care, and use of animals were followed by the authors.
Sampling and field studies
All necessary permits for sampling were obtained by the authors from the competent authorities. The study is compliant with CBD and Nagoya protocols.
Data availability
The datasets generated during and/or analyzed during the current study are submitted to the PANGAEA repository https://www.pangaea.de/.
Author contribution
AC and CB conceived and designed the research. AC and KW conducted the experiments. AC, CB, and KW analyzed the data. CB and AC wrote the manuscript. All authors read and approved the manuscript.
Additional information
Communicated by E. Macpherson
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cornelius, A., Wagner, K. & Buschbaum, C. Prey preferences, consumption rates and predation effects of Asian shore crabs (Hemigrapsus takanoi) in comparison to native shore crabs (Carcinus maenas) in northwestern Europe. Mar. Biodivers. 51, 75 (2021). https://doi.org/10.1007/s12526-021-01207-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-021-01207-7