Abstract
Heat-altered bones are a common occurrence in the archaeological record, and their analysis can provide detailed insights into past fire use behaviors and subsistence strategies. Heat-altered bones, however, may also result from natural fire events such as wildfires that are unrelated to human activity. We currently lack robust reference materials from natural fire events, analyzed using the same methodological approaches as we apply them to archaeological assemblages, that can be used to differentiate between natural and anthropogenic origins of heated materials. Here, we studied an assemblage of 50 tortoises that perished in a brushfire in Cape Point, South Africa. We used a combination of (1) zooarchaeological assessments of heating pattern and (2) infrared spectroscopy including a heating experiment to reconstruct heating temperatures with the aim to document the fire impact on the tortoise remains. For both approaches, we used statistical models to develop and test predictions that can also be applied to archaeological material. Our analyses suggest a quickly moving and low temperature brushfire in the study region with a generally low and superficial heating impact on the tortoise remains. However, we also observed several high-temperature alterations with calcination and speculate that naturally occurring fuel sources controlled the severity of the fire impact. The evidence of heating on the tortoise was unpatterned. We conclude that temperature alone presents a low confidence deciding factor between wildfires and campfires while skeletal heating pattern, in concert with other contextual analysis, may be able to facilitate this distinction with more localized heating signatures for campfires.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wildfires presented a great danger to our early ancestors but also held a great evolutionary potential (Herzog et al. 2022; Pruetz and LaDuke 2010). Exploitation of natural fires and fire use would have provided our ancestors with a range of critical benefits: increased food range and competitiveness, new technology and improvement of materials, increased independence from natural sources of light and warmth, and the manipulation of environments (see, e.g., Ahler 1983; Bellomo 1994; Brain 1981; Goldberg et al. 2009; Goudsblom 1986; Oakley 1956; Wrangham et al. 1999). Researchers have therefore invested much effort into identifying the development of fire use behaviors and their significance for human evolution. The exploitation of wildfires for resources, for example collecting fire-affected or recently perished animals or other heated food sources, probably presented the first active fire use behavior.
Typically, researchers distinguish between three stages of early fire use behaviors: (1) fire conceptualization and use, (2) control/maintenance, and (3) making (Gowlett 2016; James et al. 1989; Pruetz and LaDuke 2010; Sandgathe et al. 2011; Sandgathe 2017; Stahlschmidt et al. 2015), but in the Palaeolithic reality, these three stages would have most likely been represented by a process of increased habituation instead of singular events or discoveries (Chazan 2017). Avoidance followed by conceptualization of fire (Pruetz and Herzog 2017) would have preceded the first opportunistic fire use stage, marked by harvesting or scavenging for resources in areas affected by wildfires (Geist 1978; Hoare 2019; Parker et al. 2016). Such wildfire exploitation could have included the collection of exposed living (harvesting) or deceased (scavenging) tortoises as a food source; the wildfire would have increased their visibility, limited their mobility, or killed them (Avery et al. 2004). Early exploitation of wildfires would then have progressed into managing small wildfires, by controlling their intensity and direction via fuel control and by moving material to be burnt towards the fire (see Chazan 2017, limited fire maintenance), eventually evolving into transporting embers and fuel to a campsite to construct a localized combustion feature. Only this last step would have left clear evidence in the archaeological record in the form of hearth features (Goldberg et al. 2017). These early fire use behaviors still relied on harvesting and conserving natural fires and only the production of fire, using wood friction or pyrite strike-a-lights (Sorensen et al. 2014; Stapert and Johansen 1999), would have granted our ancestors independence from natural fire sources. The evolutionary impact of fire use behaviors thus depended on the specific benefits of fire use stages, but also on fire availability and increased independence from natural sources of fire (Shimelmitz et al. 2014).
Despite the significance of early fire use there is rarely clear and direct evidence of early fire use behaviors. Early humans’ harvesting, scavenging and managing of a wildfire would have resulted in a dispersion of fire affected materials from the wildfire to the locale of processing, but this dispersion can also result from natural processes, the mere association of heated materials with archaeological materials or heat-altered archaeological materials do not present direct evidence of fire use (James et al. 1989). Hearth features present the best evidence for controlled use of fire (Roebroeks and Villa 2011), but these features rarely preserve, especially in open-air contexts (Mallol et al. 2007; Sergant et al. 2006); their sedimentary evidence may overlap with natural evidence of in situ burning events, such as tree stumps fires (Isaac 1982), and other natural processes can mimic combustion layers (Stahlschmidt et al. 2015). Instead, researchers have been using different classes of heated materials, such as charcoal (Asscher and Boaretto 2019; Pop et al. 2016; Davies et al 2022; Marchenko et al 2022), heated lithics (Sergant et al. 2006; Shimelmitz et al. 2014), heated bones (Brain and Sillen 1988; Pickering et al. 2017), heated ostrich eggshell (Collins and Steele 2017; Mackay et al. 2022), sediment (Gowlett et al. 1981; Isaac 1982), or their co-occurrence (Berna et al. 2012; Hlubik et al. 2019; Preece et al. 2006) at archaeological sites to explore fire use and in situ combustion events. These studies analyzed the context, spatial patterning of the material or their heating parameters, including temperature, duration, and heating pattern, in order to tell natural and anthropogenic heating apart, or more precisely wildfires and campfires. The use of campfires infers access to cooked food, including cooked meat (Carmody et al. 2011; Thompson and Henshilwood 2014a) and plant food (Henry et al. 2011; Larbey et al. 2019), which would have enhanced food range and increased the energy yield of food. Heated bones present potential evidence for consumption of cooked meat, but they can enter the archaeological record by variable processes: natural fires, scavenging and managing of natural fires, discarding or accidental inclusion in a campfire created for heat or warmth or post-depositional heating below an unrelated fire event (Stiner et al 1995). Gallo et al (2021) report that bones heated to low temperatures are more susceptible to diagenesis than unheated or calcined bones. At the same time calcined bones are unlikely to result from cooking behaviors, but instead from accidental exposure to fire (Blasco 2008; Shipman et al. 1984) or the use of bone as fuel (Théry-Parisot 2002). Additionally, macroscopic identification of heated bones can be misleading with organic (Turner et al. 2018) or mineral staining (Shahack-Gross et al. 1997) and weathering (Stiner et al. 1995) macroscopically mimicking heating alterations of bones. The crucial questions then are how to reliably identify heat-altered bones and how to distinguish between the different processes creating heated bones.
In this study, we analyzed tortoise remains from a recent wildfire in Cape Point, South Africa (Fig. 1), to explore the following intersecting questions regarding early evidence for human use of fire:
-
How can we differentiate between heated tortoise bones resulting from a wildfire, cooking, garbage disposal into hearths, and unintentional heating in archaeological contexts?
-
Is there a characteristic heating pattern on the tortoise skeletal remains resulting from wildfires?
-
What is the IR signature of tortoise bones heated during a wildfire and what temperatures are recorded in the heated bone?
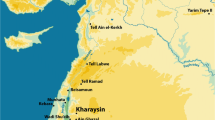
Map of the study region at Cape Point, Table Mountain National Park, Western Cape Province, South Africa. Detailed map of Cape Point showing the vegetation and extent of the 2015 wildfire with the tortoise specimens plotted individually. Details of the A Gifkommetiji and B Circular Drive collection areas. Tortoise burning data and their location coordinates are provided in SI Tables 1 to 3. Map created with ArcMap by ESRI and using 72 Class GTI South African National Land Cover Dataset (2013–2014) (©GEOTERRAIMAGE – 2014) for the Landcover source information. The images used to generate this dataset were from Landset 8 from July 28, 2013, to February 4, 2014, and each square of data is equivalent to 30 × 30 m squares. The overall accuracy of the dataset is 82.5%, but the Fynbos region accuracy was higher at 93.3%
To answer these questions, we collected data on tortoises that were heat impacted during a wildfire, coupling zooarchaeological analyses, including macroscopic observations, with Fourier transform infrared (FTIR) analyses. Studies have suggested that wildfires leave minor traces of heating alterations on animal remains (Avery et al. 2004; Bellomo 1994, 1993; Brain and Sillen 1988; Gowlett et al. 2017) and that wildfire will leave different heating patterns on the skeletons compared to cooking (Blasco 2008; Speth and Tchernov 2002; Thompson 2010; Thompson and Henshilwood 2014a). This study will test both models for tortoises by comparing the signature of a wildfire left on tortoise bone with literature on wildfires and ethnographic and archaeological observations of cooking tortoises. To this aim, we:
-
I
Macroscopically evaluated heating intensity of tortoises killed by a wildfire
-
II
Analyzed the skeletal heating patterns created on tortoise skeletons that were heat altered in the wildfire
-
III
Experimentally heated tortoise bones to track structural and chemical alterations using FTIR analysis
-
IV
Tested the reliability of macroscopic heating identification by comparison with the FTIR data
-
V
Compared heating signatures (temperature and heating pattern) created through anthropogenic fire use, such as cooking, garbage disposal, and accidental post-depositional heating, described in the literature to those created by wildfires
This approach allows us to make robust inferences about the origin of heat impacted animal remains in the archaeological record.
Background
Wildfires
Researchers have used heating temperature as one discriminating factor between natural wildfires and campfires (Backhouse and Johnson 2007; Bellomo 1994, 1993; Brain and Sillen 1988). For example, Bellomo (1994, 1993) suggested that campfires usually exhibit temperatures of 400–600 °C and that wildfires would not reach such temperatures, especially over extended time periods. But a number of wildfire studies have shown that there is a large variability in heating intensity depending on the setting and nature of the fire. Stinson and Wright (1969) observed prairie fires in Texas, USA, and measured temperatures of up to 700 °C at the soil surface but with short durations lasting for only 1–10 min. They also observed limited heating intensity for grass fires, with temperatures of 100–225 °C, and only slightly higher intensities for burning tree stumps with temperatures of 200–250 °C (see also Bellomo and Harris 1990). Gowlett et al. (2017) reported on wild grass and shrub fires in East and South Africa that lasted only a few minutes but reached temperatures above 600 °C. Davis (1959) and Scott (2000, 1989) observed that tree crown fires even reached temperatures of up to 800–900 °C (see also Buenger 2003; David 1990). Pyne et al. (1996) reported low temperatures of 300–600 °C for ground fires, fuelled by soil humus and peat.
Campfires, including experimental ones, have been reported to range from 400-1000 °C (Aldeias 2017; Aldeias et al. 2016; Bellomo 1994, 1993; Karkanas 2021) overlapping with the wildfire temperatures given above. So instead, Gowlett et al. (2017) and Hlubik et al. (2019) suggest that heating duration may present a more reliable discriminating factor between campfires and wildfires, with wildfires having been reported to last only a few seconds to minutes (see also Stinson and Wright 1969). It is important to distinguish here between the residence time of a fire with open flames, which is followed by lower temperatures in the smouldering state (David 1990). David (1990) suggests that both the residence time and smouldering conditions are rather short in natural settings, lasting from a few seconds to minutes. However, some natural, organic-rich materials can burn for over several hours at temperatures above 300 °C, fuelled by for example duff (DeBano et al. 1977), dung (Gowlett et al. 2017), tree stumps (Isaac 1982) and logs (Buenger 2003; David 1990). The latter two can even leave in situ combustion features behind that mimic hearths (Isaac 1982). This overview shows that wildfires can reach variable temperatures and burn for variable durations, mostly depending on fuel availability and variability on the landscape. The next question then is what impact wildfires will have on cultural materials and what temperatures the cultural materials would record, as fire affected materials will present the archaeological evidence of fire events. Here, we focus on animal remains.
Animals will typically react to wildfires by avoidance, with some species, e.g., chimps, having been observed to monitor wildfires and other animals to exploit them as a hunting grounds and food sources, e.g. for injured or dead animasl and insects (Bonta et al. 2017; Goudsblom 1986; Hoare 2019; Pruetz and Herzog 2017; Wrangham 2009). Wild animals get trapped or injured inside wildfires or even die in the flames (Smith et al. 2001) or due to smoke inhalation (Howard et al. 1959). Small animals including tortoises are especially prone to getting trapped inside wildfires (Avery et al. 2004; Esque et al. 2003; Sanz-Aguilar et al. 2011). Avery et al. (2004) report on a wildfire in South Africa, which covered an area of about 18,400 ha, which was estimated to have killed between 90 000 to 280 000 tortoises. They also noted that most specimens only show light traces of heat alteration. A number of mammals of all sizes also died in this wildfire, and their remains were encountered during the survey. Animal remains may also become heat altered in a wildfire postmortem. Avery et al. (2004) observed that tortoise skeletal remains sitting on the land surface previous to the wildfire were more heavily heat impacted, with impacts ranging from charring to calcination. Several other studies report on macroscopic heating alterations of animal remains due to wildfires. David (1990) reports on a controlled low intensity and slow burning bushfire in Australia with a temperature estimation of 450–500 °C and flaming combustion lasting for only 20–30 s followed by a few minutes of smoldering. Bones placed in this bushfire carbonized and exhibited superficial cracking and longitudinal collapsing of mid-shafts. In contrast, Bellomo and Harris (1990) observed no thermal alteration of bones after a low temperature grass fire in Zaire. Gowlett et al. (2017) placed fleshed animal remains and green bones into the way of wildfires in East and South Africa and monitored the fire intensity using thermocouples. The studied wildfires included bush and grassfires and observations were limited to macroscopic heating alterations noted as degree of charring. Gowlett et al. (2017) found that due to the short duration of the fires, charring on the animal remains was low and limited to areas directly exposed to flames. Contrary to these studies reporting no or minor heating alterations, Alvarez and colleagues (2017) observed a high incidence of heating alterations on animal remains after a grass fire in Argentina. In a systematic survey of the wildfire affected region, they noted an incidence of 70% of animal remains as heat altered and a majority of these as calcined. They also observed that the heating pattern on the animal remains was rather homogenous in contrast to other studies showing heterogeneous patterns, where protruding body elements and elements with little tissue attachment exhibited the highest heating impact (Lloveras et al. 2009; Medina et al. 2012).
Buenger (2003) presents a comprehensive overview on the effect of wildfires, including grass fires, forest fires, and fires in the riparian zones, on open-air archaeological sites and exposed materials, including bones in various settings. Buenger (2003) observed that the severity of heating alteration on the bones depends on temperature and exposure time, both driven by fuel availability and type. Observed heating alterations ranged from slight discoloration, charring, combustion and fissuring to extreme friability. The highest temperatures and impact on bones were observed during a pine and juniper forest fire with temperature reaching above 800 °C and smoldering temperatures of up to 400 °C for at least an hour, after which, monitoring equipment failed. We found no studies on the effect of wildfire on subsurface bones. There are, however, several experimental studies on bones buried below campfires showing that these will experience heating alterations ranging from charring to complete calcination depending on burial depth, campfire temperature and duration, as well as sediment type (Aldeias et al. 2016; Bennett 1999; De Graaff 1961; Mallol et al. 2013; Pérez et al 2017; Stiner et al. 1995; Téllez et al 2022; Wadley et al. 2019). Buenger (2003) suggests that because wildfires have on average a shorter burning duration than campfires, only very limited subsurface heating alterations of bones would occur due to wildfires.
According to the studies above, the effect of wildfires on bones and animal remains can vary quite largely, from no visible change to charring then calcination with patchy alterations on the skeletons. For complete carcasses, more extensive heating alterations at the more exposed parts can be expected, e.g., extremities, but for partial remains and bones exposed on the surface, the pattern would be more random or homogenous. The intensity of the wildfires and their effect on animal remains and bones depends on fuel type and load, fire behavior, proximity of the bones to fuels and the fire (Álvarez et al. 2017; Buenger 2003) and characteristics of the material exposed to the fire. All listed actualistic studies employ macroscopic analysis of fire alterations, but studies on early fire evidence rely on sophisticated techniques to identify thermally altered bone (Berna et al. 2012; Hlubik et al. 2019; Walker et al. 2016). Our study fills this methodological gap between Pleistocene archaeological and actualistic studies and adds to the wildfire research body by contributing zooarchaeological and heating temperature estimations for tortoises that died in wildfires.
Cooking tortoises and other anthropogenic processes creating heat-altered tortoises
Tortoise remains are a common occurrence in South African sites (Cruz-Uribe and Schrire 1991; Klein and Cruz-Uribe 1983; Orton 2012; Steele et al. 2016; Steele and Klein 2013; Thompson 2010; Thompson and Henshilwood 2014b) and often show heating alterations indicating related fire use (Thompson and Henshilwood 2014a). Potential behaviors resulting in the accumulation of heat impacted tortoise bones at archaeological sites include cooking of tortoises, disposal of tortoise residues into a fire, accidental or post-depositional heating of tortoise bones during unrelated later fire events and the collection of tortoises that were fire affected in a wildfire. To tell these processes apart in an archaeological assemblage, it is necessary to first differentiate between natural and anthropogenic burning events. Sampson (2000) compared accumulations of tortoise bones by raptors with archaeological deposits containing tortoise remains from Later Stone Age and historic occupation of the site Haaskraal, South Africa. This study observed a diverging elemental distribution with more shell elements in archaeological sites and more cranial and vertebral elements at roosts. The study also noted a higher frequency of heat-altered materials in the archaeological deposits, with 30–40% charring (but see Álvarez et al. (2017), for a much higher incidence of heating alterations and degree in a wildfire), and this observation has been referenced by other studies as an indicator for anthropogenic fire use (Blasco et al. 2016; Campmas et al. 2016; del Papa 2016; Thompson and Henshilwood 2014a). Similarly, Avery et al. (2004) observed that bushfires will contribute tortoises to the taphonomic stream but with generally only low heating alterations on the skeletal remains. However, they also observed that dry tortoise bones exposed on the landscape show a high incidence of heating including charring and calcination and they emphasize the scavenging potential of bushfires for early humans. In this latter case, if hominins picked up and consumed wildfire-roasted tortoises, the nature of the heating would be natural but their final deposition anthropogenic.
Schneider and Everson (1989) use ethnographic references to recognize evidence of cooking tortoises in the past. They report on ethnographic observations of a tortoise cooking strategy that involves putting them top shell down into a fireplace (Möllhausen 1958), sometimes with the plastron opened and filled with hot stones (Felger et al. 1981; White and Stevens 1980). Based on this, archaeological studies have used the distribution of heating on tortoise skeletal elements to differentiate cooking from accidental heating (Blasco 2008; Blasco et al. 2016, 2011; Lupo and Schmitt 2005; Schneider and Everson 1989; Speth and Tchernov 2002; Thompson and Henshilwood 2014a). These studies use the following criteria to identify cooking of tortoises: a high incidence of heated material, usually above 30% (following Sampson 2000), but of low intensity; more evidence of heating of the carapace compared to plastron elements as well as more heating traces on the exterior compared to the interior shells; and more shell than limb heating alterations. A random distribution pattern on the skeletal elements as well as on the dorsal or ventral sides of the shell is interpreted as accidental or post-depositional heating. However, all these studies rely on macroscopic identification of heating and heating intensity and they lack direct wildfire comparisons on heating patterns on the skeletal remains.
We here (1) investigate the patterns of heating on a sample of tortoises collected after a wildfire, specifically testing predictions about the interaction of the specimen’s position (upright, sideways, upside down) and element group for presence of heating, while considering element preservation, or lack thereof, referred to as “missingness”; (2) test the reliability of macroscopic identification of heating alterations of tortoise bones using infrared analysis and modelling of the spectral data to infer heating temperature ranges; and (3) qualitatively consider how heating patterns of the tortoises fire impacted in a wildfire compare to those created by cooking in a campfire.
Heating alterations of bone
Fresh bone is composed of three main components, water (free and structural, ~ 10%), organics (protein and collagen, ~ 20%), and bone mineral (~ 70%), poorly crystalline bioapatite with carbonate substitutions for phosphate and to a lesser degree hydroxyl (Munro et al. 2007; Pasteris et al. 2004; Pate and Hutton 1988; Weiner and Wagner 1998; Wopenka and Pasteris 2005). Studies of the impacts of heating on bone are centered on these three components while being almost exclusively focussed on mammalian bones, specifically ungulates. There is limited to no research on heated bones from other animal classes (see, e.g., Butler and Shahack-Gross 2017, Nicholson 1993) while zooarchaeological research has shown that a wide variety of animals was used in the Paleolithic (Zilhão et al. 2020; Blasco et al. 2016).
There are four main overlapping stages that impact mammalian bone when heated under oxidizing conditions (Ellingham et al. 2016; Etok et al. 2007; Shipman et al. 1984; Thompson 2004; Thompson et al. 2013; van Hoesel et al. 2019; Zazzo et al. 2009) (Fig. 2):
-
1.
Dehydration with loss of free water at 100 to 300 °C and loss of structural water linked to the decomposition of organic matter (see next stage) from 300 to 600/700 °C.
-
2.
Decomposition of organic matter, starting with charring followed by combustion, from 200 to 600 °C.
-
3.
Inversion of the inorganics or bone mineral starts at 500 °C with loss of carbonate and intake of hydroxyl groups and phosphates.
-
4.
Fusion of the crystalline structure with complete loss of organics, formation of secondary carbonate and interlocking of mineral crystals, also called calcination or sintering (> 700 °C).
Reidsma et al. (2016) reports that under reducing conditions, all these processes are slowed down and show a different heating alteration pattern, enabling the differentiation between bones heated under oxidizing or reducing conditions. Similarly, taphonomy, specifically high or low pH, can impact in what temperature brackets changes to the bone mineral and organics occur (Reidsma 2022).
Early on researchers used color changes of bones to investigate and identify heating alterations reflecting these processes (Shipman et al. 1984; Stiner et al. 1995). These color coding schemes generally start with tan colors for not heated bone, then include blackened bones due to charring of the organics, and finally gray colors with combustion to white colors by calcination at high temperatures. Additionally, fissuring and friability have been used as further indicators of heating (Stiner et al. 1995). However, studies have shown that mineral or humic staining and weathering can easily mimic such alterations in Pleistocene contexts because dehydration and decomposition will also occur over time with weathering (Shahack-Gross et al. 1997; Stiner et al. 1995). Consequently, researchers have been employing a wealth of microscopic and laboratory analysis to unambiguously identify heating and to reconstruct heating temperature, including structural and molecular changes observed with a scanning electron microscope and X-ray diffraction (Gallo et al. 2021; Shipman et al. 1984), transmission electron microscopy (Koon 2012; Koon et al. 2003), and Raman spectroscopy (Marques et al. 2018). By far the most prevalent approach to documenting molecular changes to heated bone is infrared spectroscopy (see references below). Infrared analyses are relatively low cost and can readily be used to detect the crystalline and compositional changes occurring in bone with increasing temperatures during heating with minimal sample preparation (Figs. 2 and 3). The changes are often described by appearance and disappearance of peaks (positions marked in wavenumbers: cm−1), or changes in the ratios of two peak heights or areas:
-
1.
Dehydration is reflected in the disappearance of the broad band at 2600 to 3600 cm−1 (van Hoesel et al. 2019).
-
2.
Decomposition is reflected in (a) loss of collagen (amide I and II peaks at 1630 cm−1 and 1545 cm−1, respectively) (Ellingham et al. 2016; Shahack-Gross et al. 1997; Weiner 2010) and (b) the loss of organics, carbonyl, compared to inorganics, here carbonates, with an increasing C/C height ratio (1455 cm−1/ 1415 cm−1) (Thompson et al. 2009) or decreasing CO/CO3 height ratio (1650 cm−1/1415 cm−1) (Thompson et al. 2013).
-
3.
Inversion is reflected in (a) the increase of hydroxyl and replacement of carbonates, resulting in an increasing OH/P (630 cm−1/605 cm−1) height ratio (Snoeck et al. 2014) and the appearance of a new hydroxyl peak (sometimes referred to as the phosphate high temperature peak or PHT) at 630 cm−1 (Larbey et al. 2019; Mentzer 2014; Schiegl et al. 2003; Snoeck et al. 2014; Thompson et al. 2013), (b) phosphate substitutions of carbonate with a decreasing C/P height ratio (1415 cm−1/1035 cm−1) (Olsen et al. 2008; Thompson et al. 2009) or CO3/P height ratio (1650 cm−1/1035 cm−1) (Thompson et al. 2013), broadening of the phosphate peak reflected in the full width at half maximum of the phosphate peak at 1035 cm−1 (FWHM) (Ellingham et al. 2016; Thompson et al. 2009), in the appearance of a right shoulder developing into a peak at ~ 1088 cm−1, the peak at 960 cm−1 changing its shape (Larbey et al. 2019; Mentzer 2014) and finally the shift of the center of the main phosphate peak to lower wavelength numbers (Weiner 2010).
-
4.
Coalescence, increased crystal order and organization with decreased crystal strain, is reflected in an increasing splitting factor (SF or CI = crystallinity index), measured by the sum of the absorbance of the 565 cm−1 and 605 cm−1 peaks and divided by the absorbance of the 595 cm−1 trough between them (Stiner et al. 1995; Surovell and Stiner 2001; Weiner and Bar-Yosef 1990)
FTIR spectra of the experimentally heated tortoise bones exhibiting different heating signals with increasing temperatures. Note that the temperature range 300–600 °C shows less clear changes compared to what has been reported for mammalian bones, e.g., no or an only very weakly expressed shoulder and peak at 1088 cm.−1
Note, however, that the exact position of the peaks may slightly change from instrument to instrument, that the use of an attenuated total reflectance (ATR) accessory will cause a shift in peak positions to slightly lower wavenumbers, and that the heating alteration processes will influence each other and spectral changes may therefore also reflect on multiple stages. In addition, sensitivity in certain regions of the spectrum can also vary from instrument to instrument, which impacts the absolute values of peak height ratios (Pothier-Bouchard et al. 2019). There are three further caveats to heating temperature estimations based on infrared analyses. First, the presence of flesh will impact heating alterations of bone as the presence of skin will first impede heating alterations, but with higher temperatures, this organic tissue will combust and increase heating alterations (Ellingham et al. 2016). Second, Snoeck et al. (2014) observed that exposure time also positively influences the inversion but not coalescence stage resulting in calcination. Gallo et al. (this issue) using heating durations of 10 min, 9 h, and 48 h observed macroscopic difference between the different duration modes but not in their IR analyses (see also (Ellingham et al. 2016). Third, Surovell and Stiner (2001), using the KBr pellet sample preparation method, observed that grinding intensity affects the SF, but Thompson et al. (2013, 2009) noted that the effect of the grinding intensity using the ATR accessory is negligible (see also Marques et al. 2018).
Despite a wealth of experimental infrared studies on heat altered bone, the application to material from Pleistocene archaeological contexts has been limited and researchers have been using variable indicators to identify heating and heating temperature (see, e.g., Berna et al. 2012; Gallo et al. 2021; Larbey et al. 2019; Mentzer 2014; Shahack-Gross et al. 2014; Walker et al. 2016). Further complicating things are weathering alterations that mirror heating. This is especially problematic for SF values below 4 (Stiner et al. 1995; Weiner and Bar-Yosef 1990). Similarly, weathering that mirrors heating may also impact indices proposed by Thompson et al. (2013, 2009), which rely heavily on loss of organics and water, processes that may also result from weathering instead of heating (Fig. 2). Consequently, temperature estimations for heated Pleistocene archaeological bones are less exact and researchers employ broad categories for heating temperature reconstruction. For example, a recent analysis of bone and tusk from the site of Evron Quarry, Israel, utilized only the PHT peak to identify evidence of heating above ~ 600 °C (Stepka et al. 2022). The analysis yielded a small but significant population of tusk fragments that had been heated to relatively high temperatures. However, to gain further information into wildfires and fire use behaviors, we need more detailed information on past fires including their temperature range. Here, in this study, we set out to provide a model independent of animal species that can still provide information on temperature range.
Materials and methods
Tortoise collection
A lightning strike started a wildfire in the Cape Point Region of Table Mountain National Park, South Africa, on the afternoon March 4, 2015. Winds with gusts over 30 km/h spread the fire northwards, burning a little over 976 ha by the time it was extinguished by fire fighters on March 6 (Chad Cheney, personal communication) (Fig. 1). The Cape Point vegetation can be described as strandveld and coastal fynbos on a nutrient-poor, sandy, and calcareous substrate (Dubay 2018; Manning 2018). Fynbos is often characterized as containing hard-leafed plants in a relatively open shrubland, with 1–3 m tall shrubs that are richly branched with twisted trunks and contain a diversity of species (Manning 2018). The fynbos landscape of the Cape Point Peninsula is generalized as medium dense with tall proteoid shrubland over a dense, moderately tall ericaceous shrubland with no trees (Mucina and Rutherford 2006). Areas of the landscape which are considered to be fynbos: thicket (Fig. 1) have both the tall proteas and the moderately tall ericas, but these are underlaid by mostly soft, broad-leafed shrubs (Manning 2018). Fynbos: open bush regions are similar to the areas of thicket, but they lack the understory of softer shrubs while areas of a fynbos: low shrub are populated by low and spreading protea mixed with restios and other low small-leafed shrubs, including short ericas (Manning 2018) The fynbos ecosystem is a fire-adapted vegetation that requires burning intervals of between 6 and 30 years for optimal growth due to the infertility of fynbos soils which require the replenishment of the important nutrients the fire provides (Avery et al. 2004; Dubay 2018; Etok et al. 2007; Kruger 1977). At the same time, low fuel connectivity protects the fynbos from completely burning through and allows its recovery (Gillson et al. 2020).
Shortly after the fire, two regions were investigated to collect tortoises that had perished in the fire; we did not systematically survey but thoroughly walked regions were park rangers earlier had seen an abundance of deceased tortoises (Fig. 1). On March 20, 2015, Teresa Steele and Marisa De Kock (South African National (SAN) Parks) collected 36 tortoises (Table 1) from the Gifkommetiji Region, and on March 25, 2015, Steele and Fagan Goodheart (also of SAN Parks) collected 14 tortoises from the Circular Drive region, while seeing a few surviving tortoises, some with charred shells (Fig. 4E). During collection, each specimen was numbered and photographed in the position in which it was found, and its GPS coordinates were recorded (Fig. 4A–D). In the field, we also noted heavily skeletonized specimens that appeared to have died before this fire event as “landscape” specimens, because they appeared to have been part of the landscape before this fire (Fig. 4C). These specimens were identified as such because their skeletons were sun bleached, their keratin scutes were missing, and in one case plant roots were invading the skeleton; Stuart and Meakin (1983) also reported previously deceased specimens in their post-fire survey. After recording, each specimen was placed in a plastic or mesh bag with its identification tag. At the end of each day, the specimens were stored in a chest freezer.
Tortoise collection after brush fire. A Burnt vegetation in the Gifkommetijie collection area. B #22 16/150: adult male with extreme heating in the sideways position (carapace length = 175 mm) and #23 16/151: juvenile with light heating in the upright position (carapace length = 91 mm); C #15 16/143: a moderately heated landscape specimen that appeared to have died prior to this fire; D #21 16/149: an adult female with extreme heating in the upside down position (carapace length = 158 mm). E A living tortoise found in the Circular Drive area; it survived, despite the heat-alteration of its shell
Data collection proceeded in two phases. Initially, whole tortoise data were collected on the frozen specimens shortly after collection (SI 4A-B). These specimens were then skeletonized, and after skeletonization, further data were collected on the isolated skeletal elements (SI 4C-D). Specimens were skeletonized by placing them in mesh bags and then leaving them on the roof of the Beattie Building at the University of Cape Town for small scavengers to deflesh them. The fine mesh of the bags would have prevented the loss of all but the smallest elements; for this reason, we have excluded carpals, tarsals, metapodials, and phalanges from our analyses, although we collected data on them when present. After the specimens were defleshed, they were soaked in water with dishwashing detergent, and with bleach when needed; after the bones were cleaned, they were air dried, catalogued, labelled, and curated (Louisa Hutten, Chief Scientific Officer, Department of Archaeology, University of Cape Town, personal communication). They are now available in the Faunal Laboratory in the Department of Archaeology at the University of Cape Town.
Retrospectively from the photographs taken in the field, we extracted information on the direct association of the specimen (< 5 cm distance) with different natural fuels (charred fine organics, branches or scrubs) and each specimen’s resting position (upside down, upright, sideways) (Table 1). We propose that resting position could be a discriminating attribute with comparisons to tortoises that may have been cooked in hearths, because ethnographic accounts indicate that tortoises were cooked in their shell, placed upside down (carapace down) in hearths (Felger et al. 1981; Möllhausen 1958; White and Stevens 1980).
Zooarchaeological and macroscopic heating assessment
While frozen, we collected data on the whole specimens: a visual assessment of the degree of heating (none, light, moderate, high and extreme); the degree of gular prominence (none, slight, prominent, and very prominent), which indicates the species and sex, where the males have prominent gulars for sparring; the degree of plastron concavity (none, slight, prominent, and very prominent), which also indicates sex where the males have prominent concavities to facilitate mounting females (Branch 2008); and age (juvenile, subadult, adult) based on overall body size (SI 4A-B). We also recorded quantitative morphometrics, but they are not a component of the current analysis.
After skeletonization, we collected data on the skeletons, facilitated by comparative specimens curated in the Department of Archaeology at the University of Cape Town (SI 4C-D). Primarily based on Blasco (2008) and Thompson and Henshilwood (2014a, b), we generated a list of skeletal elements, excluding carpals, tarsals, metapodials, and phalanges. For all 47 tortoise specimens (n = 47), we recorded the degree of heat-alteration in two places: internal and the external surface for each shell element and the proximal and the distal ends for each limb element (see SI 1 and 2). We followed Stiner et al. (1995) for color coding heated bones: stage 0 not heated (cream/tan); stage 1 < half carbonized; stage 2 > half carbonized (black); stage 3 fully carbonized (completely black); stage 4 charred > calcined (more black than white); stage 5 charred < calcined (more white than black); and stage 6 fully calcined (completely white). This generated two scores for every element, ranging from 0 to 6; however, in analysis, these were simplified into heating absence (0) or presence (1–6). All specimens were missing some elements, which were recorded as such.
Element recovery and missing heating scores
As a preliminary step in data analysis, we examined the relationships between the percentage of elements recovered from each tortoise and (1) the specimen-level initial heating assessment and (2) the tortoise’s position at the time of collection. We also examined rates of missing elements by element group and position, to detect relationships between these variables. A qualitative assessment of the missingness pattern and likely causes of missingness helped guide later decisions about statistical modeling of heating scores.
Modeling heating scores
The primary questions concern how heating rates differ by element group and the tortoise’s position at the time of collection, suggesting a factorial model for heating, with element group and position as covariates. Each element side (ventral/dorsal) or end (proximal/distal) has a binary score reflecting the presence or absence of heating, and the sum of these scores over all elements in a group produces an outcome suited to a binomial regression model. The subsequent issues to resolve were how multi-level structure—elements within tortoises—would be incorporated in a model, and how to obtain correct inferences about heating rates when elements were missing.
A multi-level statistical model contains effects for nested groups in a sample and is appropriate when basic sampling units (shell and skeletal elements) are aggregated within larger units (tortoises) (see, e.g., Gelman and Hill 2006). Tortoises varied in their overall heating levels due to variable exposure to fire. A multi-level model containing a unique intercept for each tortoise adjusts for variable exposures and, potentially, other idiosyncratic features of the tortoise, as well as acknowledges that elements are grouped within tortoises: this was the basic model used here. For element e in element group g, and tortoise t collected in position p, define πe,t (g, p) as the probability that the element was heated. The statistical model is then

where logit π = log( π / (1—π)), µ is a common intercept, Zt is a unique intercept for tortoise t, distributed as a Gaussian variable with mean zero and standard deviation\({\sigma }_{Z}\), and the ß's are effects of element group g, position p and their interaction. A model with additional structure, acknowledging the spatial relationships between elements within tortoises, could be entertained. We decided to forgo this, based on size and scale considerations: the largest tortoise’s plastron was just under 300 mm, and all others were under 200 mm; therefore, the distances between their various shell and skeletal elements are arguably inconsequential in comparison to a brushfire’s scale.
Exploratory analysis of the pattern of missing heating scores suggested that missingness varied by element group and position but was relatively independent of the specimen-level initial heating assessment. We therefore proceeded with statistical modeling of heating scores under the assumption of covariate-dependent missingness: i.e., that missingness depends on the element group and tortoise’s position, but not on the heating score that would have been observed, had the element been recovered. Covariate-dependent missingness is a form of missing data characterized as missing at random (Little and Rubin 2002; Section 1.3). Little (1992) noted that under this form of missing data, the missing observations do not contain information about the regression of the outcome (the heating score) on the covariates, and therefore analysis based on only the complete cases is valid (see also Little and Rubin 2002, Section 6.2).
Planned comparisons
We focused on five predictions, stated in Table 2, comparing heating rates across different groups of elements (such as shell interior versus exterior) in context of the tortoise’s position at the time of collection. Although shell interior and exterior elements are mutually exclusive, the combined predictions involve non-mutually exclusive groups, making analysis by a single model challenging. A set of four models, each focusing on a comparison of the form “element group A versus element group B,” where A and B are mutually exclusive, was adequate for testing the five predictions.
We tested the predictions by estimating contrasts from the fitted models (Table 2). We calculated a confidence interval for each contrast corrected by the Bonferroni procedure to preserve a 5% error rate over all tests.
Post hoc exploration of proximal and distal limbs
To help understand and interpret the test of shell versus limb elements, we calculated empirical rates of missingness and heating for proximal versus distal limbs, for each tortoise position. The proximal versus distal contrast was not captured by a fitted model under our analysis plan; therefore, we approached this comparison descriptively and informally.
Computing
Data management, model fitting and visualization were performed in R version 4.1.0 (R Core Team 2021). Multi-level regression models were fitted with the library glmmADMB (Skaug et al. 2016). The R code is available in SI 5 and 6.
IR analyses and heating experiment
Sample selection
We selected six specimens for infrared (IR) analyses: two without heating alterations for a heating experiment (#18 and #35) and four with maximum heating damage to explore maximum temperature alteration reached during this wildfire (Table 3). Of these latter four, three specimens (#21, #22, #32) most likely died during the wildfire whereas one (#15) presents as a landscape specimen. We collect 29 subsamples from these four wildfire tortoise specimens, using variable skeletal elements but targeting areas of highest macroscopically visible heat alteration. During the subsampling, we noted that heating alterations mainly pertained to the bone surface and did not penetrate complete bones. Consequently, we collected IR samples by scraping off material from the bone surface to gain information on maximum heating temperature. We collected subsamples (6–9) from each of the 4 selected wildfire specimens to represent multiple macroscopic heating stages and collected 59 IR spectra from these. For the heating experiment, we collected 8 plastron and 8 carapace bones from each heat-unaffected specimen presenting the most common and unique bone types of the tortoise skeleton, not present in mammals.
Heating experiment
Experimental heating studies of bones for infrared analysis dominantly use only mammalian bone (but see Butler and Shahack-Gross 2017 for fish and bird bones). We here conducted a heating experiment with tortoise bones to account for possible difference between reptilian and mammalian bones. We heated the bones in a muffle furnace for 1 h at temperatures of 100 °C, 200 °C, 300 °C, 400 °C, 500 °C, 600 °C, 700 °C, and 800 °C (Fig. 5) and subsequently let them cool down for 24 h in a desiccator before IR analysis. For each heating step, two bones were used, one carapace and one plastron. After heating, we color coded each bone following the color and temperature classification scheme by Stiner et al. (1995), but using a slightly adjusted scheme as the heating alterations impact bones more evenly in the muffle furnace (Fig. 5): stage 1 light brown 100 °C; stage 2 brown 200 °C; stage 3 black 300 °C; stage 4 very light brown 400 °C; stage 5 Gy 500 °C; stage 6 white with bluish streaks 600–800 °C.
FTIR spectroscopy
We collected mid-infrared spectra using a diamond crystal ATR accessory, which has been proposed to give more reliable results than the KBr pellet preparation method for heated bones (Thompson et al. 2009, Marques et al. 2018). For the wildfire specimen, we collected 1 to 4 spectra per sample (alphabetical sublabeling), depending on the amount of available material. For the heating experiment, we took 4 samples per bone and collected four spectra per sample (a, b, c, d). Samples were ground in a mortar and IR spectra of the powder were collected with identical instruments, a Cary 660 FTIR spectrometer at both the University Tübingen and the Max Planck Institute for Evolutionary Anthropology, equipped with the GladiATR Vision single bounce diamond ATR accessory (Pike Technologies), with spectral output to the Resolutions Pro software package (Agilent Technologies). Spectra were recorded at 4 cm−1 resolution with 32 scans in the 4000 and 400 cm−1 wavenumber range. We applied no corrections to the spectra. Prior to analysis for heat-induced change, we analysed each spectrum for manganese presence, marked by peaks at 536 cm−1 and 1080 cm−1 (Potter and Rossman 1979), which may also leave black staining mimicking charring (Shahack-Gross et al. 1997) as well as for the presence of quartz, where a characteristic peak at 1090 cm−1 peak may interfere with the position of the main phosphate peak (Snoeck et al. 2014). However, we observed neither on the wildfire specimen.
Spectral analysis
In order to assess the degree of heating using the IR spectra, we compared the results of the IR analyses on the tortoises from the heating experiment to a reference collection of heated bones housed at the University of Tübingen. The spectral database used here is composed of spectra collected from bone elements of medium and large ungulates that were heated in a muffle oven for at least 45 min per bone (SI 7). One set of juvenile domestic sheep (Ovis aries) ribs included in the database was prepared during an exact replication of a heating experiment published by Thompson et al. (2009). With the exception of the spectra collected from the juvenile sheep, the reference spectra were collected from all types of bone tissue (e.g., spongy, cortical, and flat) and from both the exterior surfaces and interior portions. The aim of using the reference materials was to encompass a wide range of variability in bone response to heating. The reference spectra were collected at the University of Tubingen using the same type of FTIR instrument (Cary 660, benchtop mid-infrared Fourier transform infrared spectrometer; Agilent Technologies) and diamond ATR (GladiATR Vision single bounce diamond ATR; Pike Technologies) as the tortoise bones.
We analyzed the spectral data in two complementary approaches. First, we used visual inspection of the spectra by the analysts (visual IR peak analysis) to classify the samples by heating temperature based on previous studies and the reference collections at the University of Tübingen using characteristic peaks: shift of the main phosphate peak to lower wave numbers (Weiner 2010), the appearance of a shoulder and peak at 1088 cm−1 (Mentzer 2014), and the appearance of the PHT peak at 630 cm−1 (Weiner 2010; Thompson et al. 2013) (Figs. 2, 3, and SI 8). Second, we chose modelling as a mode of comparison using index calculations and whole spectrum analysis (Fig. 6). Regarding the former, we applied the following ratios: CI (Thompson et al. 2009), C/P (Olsen et al 2008), CO/CO3, and FWHM (Thompson et al. 2009). The CI, C/P, and CO/CO3 indices were calculated from peak heights measured using the Agilent Resolutions Pro software while we calculated FWHM for the main phosphate peak using Essential FTIR. Finally, for whole spectrum analyses, a variety of pre-processing steps were tested (calculation of the 2nd derivative, baseline correction, normalization, and mean centering), and these were conducted using both Essential FTIR and PLS Toolbox. For our classification models, PLS-DA analyses were conducted using the PLS-Toolbox (version 90; Eigenvector Research Inc.) for Matlab (version R2022a). The spectral files were loaded directly in JCAMP format into PLS Toolbox for model building. The parameters used in the peak measurements, such as the positions of the baseline endpoints, are listed in the Supplemental Online Materials.
IR index plots for spectral data of the reference collection and the tortoise materials, from the wildfire and the experiment. Note that the experimental tortoise data show the same trends as the mammalian data, but are almost always off-set from the rest. (A) PHT/P. (B) Crystallinity Index plotted by temperature. (C) C/P plotted by temperature. (D) CO/CO3 plotted by temperature. (E) FWHM plotted by temperature. (F) P center point position plotted by temperature
Our initial assessments of the results revealed differences in the response to heating between the tortoise shell bones and the mammalian bones (see further details below). Therefore, we focussed on developing a classification model that could be used for both. The linear discriminant analysis model employed by Thompson et al. (2009) used CI, C/P, CO/CO3, and FWHM as the input variables, but our initial observations indicated that several of these indices could be less effective for predicting temperature changes in tortoise bone. In addition, our small set of training spectra risked overfitting a linear discriminant function model. Instead, we tested and applied a partial least squares discriminant analysis (PLS-DA), which is a well-established approach in the food science and pharmaceutical industries for classifying mid-infrared and near-infrared spectral data that have many variables relative to the size of the training set (Botelho et al. 2015; Pereira et al. 2018; Sinelli et al. 2007). We developed models using four approaches: (A) the four indices as input variables, with 9 temperature classes in 100 degree bins; (B) the four indices as input variables, with three temperature classes in 300 degree bins; (C) whole spectra as input variables, with 9 temperature classes in 100 degree bins; and (D) whole spectra as input variables, with three temperature classes in 300 degree bins. We divided the 240 input spectra into a training set (n = 140) and a validation set (n = 100), and define success as classification to the correct temperature or specified range.
Results
Zooarchaeological data
Table 1 summarizes the position, age, sex, degree of heat-alteration, and fuel association of 47 of the collected tortoises. Close examination confirmed that all specimens in the two collection areas were consistent with Chersina angulata, the angulate or bowsprit tortoise. Based on what has been documented in the park and other biogeography reviews, the only other candidate would be the parrot-beaked tortoise (Homopus areolatus), but they are distinctive with smaller body sizes and lower-profile carapaces that have scutes with deeply furrowed margins and depressed centers (South African National Park 2022; Branch 2008).
The tortoises in the two collection regions were impacted differently by the fire. The Gifkommetijie collection area is dominated by thicket vegetation (Fig. 1A), providing the densest cover on the peninsula; more tortoises were collected over a small area, and they show evidence of more intense heat damage. In the Circular Drive collection area (Fig. 1B), the tortoises were found mainly in association with low shrub and open bush vegetation (Fig. 1A). While this vegetation provides less protection (Manning 2018), it may also provide less fuel for the fire. Fewer tortoises were found here and they were less heat affected than in Gifkommetijie: the one extremely heated specimen from the Circular Drive collection area was associated with thickets. Furthermore, we found three surviving tortoises here, two of which showed evidence of heating on their carapaces (Fig. 4E). Vegetation differences are also apparent in the fuel association of the individual specimen observed in the field. The majority of specimens showing only none, light, or moderate heating were found associated with burnt fine organics. Only a few specimens were found associated with burnt branches or shrubs and only few show high or extreme burning, showing a similar trend to the overall pattern of the two collection areas (Table 1).
Missing data pattern
We see little relationship between the qualitative level of heating, based on an initial, subjective assessment of the whole specimen shortly after field collection, and the percentage of recovered elements (Fig. 7, left panel). In contrast, we do see a relationship between the initial heating assessment and the percentage of recovered elements that were heat-altered; the specimens that were only lightly heated overall had low percentages of heated elements, while those that were more heavily heated had higher percentages of heated elements (Fig. 7, right panel). Taken together, the two panels of Fig. 7 suggest that—insofar as the initial heating assessment predicted heating at the element level—the failure to recover an element depended little on whether or not it was heated.
Initial heating assessment for each of N = 47 tortoises, by percentage of elements recovered (left panel) and percentage of recovered elements heated (right panel). Tortoises are ordered in the left panel according to their percentages of elements recovered, out of the total number of elements in a complete specimen. The relatively even distribution of colors along the curve suggests that element recovery was not closely associated with the initial, qualitative, specimen-level heating assessment. Tortoises are ordered in the right panel according to their percentages of elements burned, out of those recovered from each tortoise. The qualitative change along the curve, from open and light pink circles near the top, to hotter colors (magenta and red) near the bottom, suggests that the initial heating assessment was consistent with element-level heating observations used for statistical modelling
We show analogous graphs, using the tortoise’s position at the time of collection instead of the initial heating assessment, in Fig. 8. Element recovery was relatively reduced for the landscape specimens (Fig. 8, left panel), which were already deceased at the time of the fire, and the elements that were recovered from them were more likely to be heated (Fig. 8, right panel). In contrast, element recovery was increased for specimens in the upside down position, and their elements were less likely to be heated. Taken together, the two panels of Fig. 8 suggest that the variable “position” is a predictor of both element missingness and element heating.
Tortoise’s position at the time of collection for each of N = 47 tortoises, by percentage of elements recovered (left panel) and percentage of recovered elements heated (right panel). The ordering of tortoises from top to bottom in the two panels is as in Fig. 7. In the left panel, the clustering of pink and dark blue colors near the top of the curve suggests that tortoises which died before the brushfire and remained on the “Landscape,” or which were found in the “Upright” position, had more missing elements, while those found in the “Upside down” position (light blue color, clustering near the bottom) were more complete specimens. In the right panel, the clustering of light blue circles near the top of the curve suggests that tortoises found in the “Upside down” position tended to have fewer elements heated, while those which had remained on the “Landscape” (pink color, clustering near the bottom) tended to have more elements heated
We then examined patterns of element missingness for the four planned body-region comparisons (Table 4 and Fig. 9). As noted, landscape tortoises were more likely to have missing elements, and furthermore, their carapaces were more likely to have missing elements than their plastrons. Missing carapaces were also more likely for upright and sideways specimens, but not for upside down specimens, suggesting that this inverted position provided some protection for element recovery. Central and marginal carapace elements were equally likely to be missing. Limb elements were missing more frequently than shell elements across all positions.
Missingness of elements in the sample by element group and tortoise position. Center dots show the empirical odds that an element of a given element group and tortoise position was missing, and colored segments indicate the precision of the empirical odds via two-standard-error intervals, based on the formula in Gart (1966, Section 3). The horizontal axes are on a logarithmic scale, though the numerical annotations refer to the odds in their original scaling. An odds of 1.0 implies a 50% probability that an element was missing, and an odds of 2.0, for example, implies a (2/(2 + 1)) × 100% = 67% probability of missingness. Within each element group, elements of tortoises that died before the brushfire (“Landscape” position) were missing more frequently. Limb elements were missing more frequently than shell elements across all positions, as were carapace elements compared to plastron elements
These results suggested that element recovery depended on the element’s body region and the specimen’s position at the time of collection, but not on element heating, supporting an assumption of covariate-dependent missingness. This missing data pattern makes subsequent data analysis relatively easy, as correct inferences can be made from the complete records alone (Little 1992; Little and Rubin 2002), Section 1.3).
Modelling heating scores
We quantified the presence or absence of heating by element group and tortoise position by fitting binomial regression models to the complete records, under a missing-at-random assumption. A graphical display depicting model estimates, along with empirical odds of heating, is shown in Fig. 10, and results for five planned comparisons are reported in Table 2.
Heating of elements in the sample by element group and tortoise position, along with estimates from a multi-level binomial model. Gray dots show the empirical odds that an element of a given element group and tortoise position was heated. Colored vertical bars show the estimated odds from a multi-level binomial model, and horizontal segments indicate model uncertainty via two-standard-error (95% confidence) intervals for the odds. The confidence intervals are not corrected for multiple comparisons and are used here only for model display. Results for planned comparisons are reported in Table 2. The axis scalings and annotations are as in Fig. 10
In Fig. 10, the high degree of overlap between blue and tan intervals implies that pairwise element groups, such as plastron versus carapace, tended to share similar levels of heating, and moreover that heating tended to be similar across tortoise positions. The closeness overall of the estimated odds (vertical lines at interval mid-points) to the empirical odds (gray dots) suggests that the models fit the data well. A tendency for model estimates to lie slightly above the empirical odds can be explained by multi-level adjustment of the estimates to a common, tortoise-level, baseline heating proportion.
Given that a brushfire is appreciably larger than a tortoise (the largest tortoises in our sample were ~ 230 mm), we predicted that the plastron and carapace elements, and the central and marginal carapace elements, would be equally likely to be heated across all positions (fourth and fifth comparisons in Table 2, respectively). The observations are consistent with these predictions.
We predicted that upside down, upright, and sideways tortoises would have more heating on the exterior of their carapaces because the interior would be more protected and have tissue present (first comparison in Table 2), while the carapaces of landscape tortoises would be equally heated on the interior and exterior because decomposition would have already occurred, exposing more surfaces (second comparison). Surprisingly, for the fire-inflicted specimens, our findings support the converse of our prediction: there is statistical evidence for more heating on the interior carapace than the exterior. Although the interior and exterior confidence intervals overlap for each of the three fire-inflicted positions, the contrasts point in a consistent direction and statistical significance is a consequence of the combined evidence. The landscape specimens also showed more heating on the interior than exterior, although here equal levels of heating cannot be ruled out.
We predicted equal heating of limb and shell elements in the landscape position (third comparison in Table 2) because two countervailing mechanisms seemed equally plausible: limbs could be protected from heating because they were retracted toward the shell before death, or limbs could be particularly exposed in the landscape position and more vulnerable to heating. The data are consistent with protection of the limbs from fire in the landscape position. We explored this result with a post hoc check, reasoning that if retraction of the limbs was protective, proximal limb elements (humerus, femur, shoulder, and pelvic girdles) would be less heated than distal limb elements (radius and ulna) (Fig. 11, right panel). This reasoning appears to hold for landscape tortoises, though a formal statistical comparison would return a null result. Distal limb elements were more likely to be missing than proximal limb elements across all positions (Fig. 11, left panel), though the only consequence of this under the missing-at-random assumption is reduced precision of distal heating rates.
Missingness and heating of proximal and distal limbs by tortoise position. Center dots show the empirical odds that an element from a proximal or distal limb was missing (left panel) or heated (right panel) by tortoise position, and colored segments indicate the precision of the empirical odds via two-standard-error intervals, based on the formula in Gart (1966, Sect. 3). The axis scalings and annotations are as in Fig. 9 Distal limbs were more frequently missing than proximal limbs for all tortoise positions, though heating of distal and proximal limbs was comparable
FTIR data
The spectral data and their evaluation provided expected and unexpected results with the tortoise shell bones showing several differences compared to mammalian bones. Our visual IR peak analysis showed no clear spectral changes below 600 °C, namely the appearance of a slight shoulder at ~ 1088 cm−1 (Fig. 2). At 700 °C and 800 °C, this shoulder is very clear and the PHT at 631 cm−1 is also present. There are no clear changes visible at 500 °C and below contrary to studies on mammalian bones and the reference collection. Regarding the modelling, Fig. 6 illustrates the differences in the peak height indices and other measurements such as the FWHM and phosphate peak position in the tortoise bones compared to the juvenile sheep ribs and other mammal bones, also here the tortoise bones stand out. In the plot of the CI, at low temperatures the tortoise bones exhibit low values, while at 600 °C, the spectra from the tortoise bones group towards the center of the distribution, and at the highest temperatures, the tortoise bones have the most elevated values compared to the juvenile sheep ribs and broader collection of ungulates. At low temperatures, the tortoise bones exhibit lower values for the C/P index than those of the mammals, with bones heated to 200 °C falling completely outside of the range for the mammals. At high temperatures, the bones of all animals cluster together. Overall, the tortoise bones show a dampened response in the C/P with temperature. A similar pattern can be observed for the CO/CO3 ratio. At low temperatures, the tortoise bones exhibit lower CO/CO3 values than those of the mammals, with bones heated to 300 and 400 °C falling completely outside of the range for the mammals. At high temperatures, the bones of all animals cluster together. Both the C/P and CO/CO3 indices are impacted by the overall abundance and type of collagen present in the bones. The lower overall values for both indices in tortoise, as well as the rapid decline in values above 200 °C might suggest that the sampled tortoise elements (plastron and carapace) contain less collagen compared to the mammalian bone, and that this collagen combusts more readily. At low temperatures, the tortoise bones have narrower main phosphate peak widths (FWHM) compared to the mammals. At intermediate temperatures, the tortoise bones have wider peaks than the mammals, and at the highest temperatures, the tortoise bones cluster with the mammals. At 800 °C, the spectra form two groups: one with wide main phosphate peaks, and one with narrow main phosphate peaks. Tortoise and mammals are both present in these groups. Overall, the tortoise bone spectra exhibit a much narrower range of peak widths, and this index would not seem to be a strong predictor of temperature in the same way that it is for the mammals. Another consistent marker of heating in mammalian bone is a shift in the position of the main phosphate peak towards higher wavenumbers. Figure 6E shows that the tortoise bones have peak positions that are uniformly high, no matter the temperature. Therefore, it appears that like the FWHM, this marker is not as strong for predicting temperature in tortoise bone. Finally, when scoring the relative height of the PHT, at 700 °C, the tortoise bones appear to have a more pronounced peak compared to both the juvenile sheep bones and the broader collection of ungulates.
Table 5 reports on the input parameters for the four main tested models using PLS-DA and the success rates for the validation set, which contained 30 tortoise spectra and 70 mammal spectra. As predicted from the distribution of values for the spectra indices from tortoise compared to mammal bones, the two models that utilized spectral indices as variables for discriminant function modelling (A and B) did not produce satisfactory results (Table 5). Similarly, model C, using whole spectra as input variables within 9 temperature classes in 100 degree bins shows a low success rate. The most successful spectral classification model was model D, which also used the entire spectra as input variables but classified to three temperature classes: (1) not heated to 200 °C, (2) 300 to 500 °C, and (3) 600 to 800 °C. These temperature bins fit well with processes occurring to bone with increasing temperatures (Fig. 2). During the validation this model had an overall success rate of 96%. Of the four misclassified spectra, three were from mammal bones, which means that the success rate for the tortoise bones in the validation set was 99%. A version of model D—model Dx—was also tested to determine the overall importance of the region of the spectrum associated with the collagen peaks. For this model, the region of the spectrum above 1200 cm−1 was excluded, which means that loss of collagen did not contribute to the temperature classifications. The overall success rate of this model was 95% with a 90% success rate for the tortoise bones. Model D (with collagen peaks) was therefore selected as the model to use to classify the tortoise bones impacted by the wildfire. The 72 spectra were input into the model and the results are listed in Table 3.
Table 3 summarizes the heating temperature interpretation for the selected wildfire material, by macroscopic heating code, visual IR peak analysis of the IR spectra and PLS-DA modelling. According to this of the 72 wildfire samples analyzed with FTIR, only five were classified to the lowest temperature bin, which could include bones that are not heated along with those heated up to 200 °C. The vast majority (n = 54) classify to between 300 and 500 °C. A small number (n = 13) are classified to the highest temperature range of 600–800 °C. Using only visual IR peak analysis, the reconstructed heating signal was much lower. For the majority of the spectra, we detected no heating signal (n = 62). For a small number, we observed an often very weakly expressed shoulder of the main phosphate peak indicating heating temperature of 300 to 650 °C (n = 5) or a peak at 630 cm−1 indicating temperatures > 650 °C (n = 5). Bones that were clearly heated macroscopically often did not show obvious changes in the spectral data in our visual IR peak analysis approach. However, the macroscopic heating codes correspond well with predicted temperatures (Table 3). Only in one instance was the predicted temperature lower than the macroscopic interpretation. Sample #21.5 was interpreted as calcined with gray color, but did not show calcination. The gray color may here be the result of a longer duration as Gallo et al. (this issue) suggest for bovid bones. Combined these two approaches applied to the most severely heat-affected tortoise skeletons show that the temperatures sustained by these bones mainly fell into a middle range of 300 to 500 °C. The small number of bones that classify to above 600 °C would also indicate that higher temperatures were reached within the wildfire. Individual discrepancies between the macroscopic and whole spectral analysis approach most likely result from the superficial and uneven heat alteration of the bones.
Discussion
Wildfire impact on angulate tortoises in the Cape Point Peninsula
Angulate tortoises are distributed widely throughout southwestern South Africa inhabiting a variety of habitats, and they are quite common in the sandveld and coastal fynbos typical of Cape Point. They prefer ecotones where they can use vegetation for cover and open areas for basking, nesting, and opportunistically feeding on a variety of grasses, shrubs, herbs, succulents, and herbaceous plants (Branch 2008; Joshua et al. 2010). They are most active at moderate temperatures; when they live in places with hot springs and summers, they shift their activity to the cooler mornings and evenings and become quite active when moisture is available through rain or fog (Branch 1984; Ramsay et al. 2002). Angulate tortoises are well adapted to the fires that previously regularly occurred in their preferred habitats. It is possible to see tortoises with fire damage to their shells, but that nonetheless survived (this study, Branch 1984; Stuart and Meakin 1983; Wright 1988). The sparse data available indicate that animals are more likely to die in open landscapes where shelter, such as rocks, is limited (Wright 1988). In our study area, we observed that heating damage was more severe in the Fynbos: thicket compared to the Fynbos: open bush or Fynbos: low shrubs. The plants in these latter two areas would provide cover for tortoises and other small animals in high winds, but would provide less fuel for brushfire compared to the fynbos thicket and open bush. We see a similar trend in the fuel association, where tortoise directly associated with burnt branches or shrubs show more intense heating alterations compared to those in association with just grasses; it appears that spatial patterning of the wildfire intensity was controlled by natural vegetation cover.
Heating temperature reconstructions for tortoise bones
Heating temperature recorded in bones is a common proxy used to reconstruct the characteristics of past fire events. However, such temperature interpretations from archaeological faunal assemblages are not straight forward with other natural post-depositional processes such as weathering and mineral or organic staining potentially mimicking macroscopic heating traces such as color change and surface fissuring. In our wildfire case study, this did not present a concern and we were able to use our macroscopic observation for rough estimates of heat-alterations. For heating temperature estimates, we relied on more detailed analysis that are based on bones reacting to heat exposure in specific ways on a crystalline and compositional level controlled by temperature. We used FTIR analysis to reconstruct heating temperature as is commonly done for early evidence of fire (Berna et al. 2012; Stepka et al. 2022).
There are three different approaches to analyzing spectral data, which we also used here and compared for their suitability to reconstruct wildfire impacts and to analyze Pleistocene faunal assemblages: visual IR peak observations on the spectral data, indices calculations comparing different peak heights and whole spectra analysis (Table 3). Our visual IR peak analysis severely underestimated the temperature signal of the wildfire with the majority of the spectra not showing clear changes. This is especially true for the low temperature range, but even some bones heated to high temperatures were not detected in this approach. At the same time, our experimentally heated tortoise sample did not show clear changes in the peak configuration until 600 °C showing that the response of bone to heating below the calcination threshold can vary by species. Consequently, this approach will only provide an incomplete picture of past fire events if the animal species is unknown. Similarly, comparison of FTIR indices between the tortoise bones and bones of medium and large mammals suggests that there may be some differences in the way bones of different types of animals respond to heating. Fire-affected faunal assemblages often contain a high proportion of unidentifiable material and in the case of Middle and Later Stone Age often tortoise remains. Furthermore, these indices are susceptible to weathering and may not be apt for Pleistocene materials. In this case study, we were able to generate a classification model that worked well for both mammals and tortoises, using whole spectra analysis, but at the expense of greater resolution (e.g., smaller temperature bins). When dealing with fragmentary, not identifiable bones, we advise the use of such broad classification systems. However, our results also suggest that when bones are identifiable, it may be possible to develop a better classification model specific to that particular species or broad type of animal. The classification model that we used—as well as other models previously published (e.g., Thompson et al. 2013)—rely heavily on the collagen content to identify molecular changes in the range of 200–500 °C. This model was appropriate for the test set in this study, as the recently deceased tortoise bones were not impacted by weathering processes that could contribute to post-depositional collagen loss. For fragmentary, not identifiable Pleistocene material, we suggest the use of classification models that exclude wavelength regions susceptible to weathering. Our tests of a version of model D that excluded the collagen peaks, model Dx, was slightly less successful, with a general success rate of 95% in both cross-validation and also using the independent validation set. This outcome is nevertheless promising for the future development of classification models that can be used for archaeological bone.
Heating signature of wildfires
Our macroscopic color descriptions, the modelled heating scores, and FTIR analysis all indicate that the 2-day wildfire in Cape Point materialized as a quick wildfire event in the study area that lasted only a few minutes with temperatures rarely exceeding 500 °C. In fact, the majority of skeletal remains signal limited to no heating alteration, which indicates that such wildfire events would have a low visibility in the Pleistocene faunal record. Nevertheless, temperatures above calcination were reached in a few instances and we found some distinctions from reported evidence of cooking on the tortoise skeletal remains.
Several wildfire studies (e.g., Buenger 2003; David 1990; Gowlett et al. 2017; Scott 2000) have shown that high temperatures, above the calcination threshold, can be reached in wildfires and that high temperatures in themselves do not provide a robust characteristic for distinguishing wildfire from controlled use of fire. Our study adds to this body of evidence by showing that skeletal remains can record such high temperatures even in wildfire of a generally low intensity. Exposure time has been suggested as a further distinguishing mark (Gowlett et al. 2017), and Gallo et al. (this issue) show that bones can record long duration heating events based on the combined evidence of a white gray color indicative of calcination, but a lack of calcination revealed in spectroscopic analysis. However, we were not able to test this in the current wildfire and similar studies on wildfire events of longer duration and intensity are needed. Buenger (2003) and Gowlett et al. (2017) observed logs and dung respectively burning over several hours at low temperature after a wildfire. These observations suggest the occurrence of local hot spots in wildfire controlled by landscape fuel sources and their density, not only regarding the duration of the fire but also its intensity (Buenger 2003). Our study supports these observations with naturally occurring fuel sources as one controlling factor of the intensity of the heating impact on the tortoise remains. The fynbos vegetation is adapted to regular burning events and easily ignites. Tortoise remains associated with shrubs show a higher heating score while those associated only with fine charred organics show more minor heating alterations. The natural distribution of fuel source thus also results in burning pattern in the landscape. Raptors scavenging the wildfire remains and live tortoise with charred shells will result in further distribution of burnt remains in the landscape. However, we need to add a caveat to our interpretation here. While the study area is located in a national park, this still presents an anthropogenic landscape and thus a complicated analogue to past environments.
Our zooarchaeological analysis, namely heating score, supports Thompson (2010) and Thompson and Henshilwood (2014b) assumption that there is a difference in the heating pattern on skeletal tortoise remains between those cooked in a fire upside down and those exposed to a wildfire in an upright position. We generally observed random heating patterns on the skeletal remains, as postulated by Thompson (2010) and Thompson and Henshilwood (2014b), with one deviation, the heating score on the outer shell was smaller compared to the inner shell. This may be the result of ongoing combustion of the organic materials inside the shell in the wildfire. Once ignited, fat will increase the heating process, while the outer shell is protected by a keratin layer. In contrast, during cooking one would want to avoid combustion of the fat. However, if the tortoises were cooked upright this distinction would be less clear. Detailed analytical approaches, as proposed here and used on the archaeological record, are needed to clarify the impact of wildfire and different cooking practices.
Conclusion
Fire use by early hominins is still an elusive subject. This is in part due to the low preservation potential of its evidence but also due to an overt research focus on hearths and controlled fire use. Wildfires presented an environmental entity for early hominins and at some point in the past were recognized and exploited as a food source. Landscapes prone to wildfire presented an attractive niche for early hominins shaping their behavior (Herzog et al 2022). Wildfire scavenging may explain the occurrence of heated faunal materials in early sites. However, the recognition of such wildfire scavenging is hampered by a dearth of systematic wildfire studies, where we use the same tools on both the zooarchaeological record and wildfire remains to clearly distinguish between the two. To this end, this tool kit must include methods to determine if material has been heated beyond macroscopic inferences and under what conditions and behaviors (Stahlschmidt et al 2015; Goldberg et al. 2017).
Here, we present one such example of a wildfire leaving only superficial, but characteristic heating traces on tortoises that got caught in this fire event. We designed an approach that allowed us to capture the temperature variance recorded in the bones as well as their skeletal patterning and that can be easily transferred to the archaeological record. First, we verified our macroscopic heating observations using FTIR spectroscopy and developed a model enabling robust temperature estimations independent of animal species. Many of the collected tortoises from this wildfire show no or only minimal traces of fire impact, but we also observed that temperatures above calcination were reached. The fire impact on the landscape was patterned, local hotspots were associated with thickets while minimal impact was observed in more open locales. The tortoise skeletal patterning of heating was random showing clear differences to those reported for cooking remains (Schneider and Everson 1989) and enabling the distinction between cooking and wildfire remains. While paleo-wildfires are commonly reconstructed using microcharcoal, we here want to highlight that zooarchaeological records present a further archive to reconstruct past wildfire events as may other find categories such as heated lithics.
Data availability
All zooarchaeological data and the R scripts for their analysis are available in the supplementary materials. In the primary data files and R scripts for zooarchaeological analyses, the terms “burn,” “burning,” etc. may occur instead of “heat,” “heating.” Spectral data for the tortoises will be made accessible at www.irug.org and the website of the Geoarchaeology working group of the University of Tübingen.
References
Ahler SA (1983) Heat treatment of knife river flint. Lithic Technol 12:1–8. https://doi.org/10.1080/01977261.1983.11760607
Aldeias V (2017) Experimental approaches to archaeological fire features and their behavioral relevance. Curr Anthropol 58:S191–S205. https://doi.org/10.1086/691210
Aldeias V, Dibble HL, Sandgathe D, Goldberg P, McPherron SJP (2016) How heat alters underlying deposits and implications for archaeological fire features: a controlled experiment. J Archaeol Sci 67:64–79. https://doi.org/10.1016/j.jas.2016.01.016
Álvarez MC, Massigoge A, Scheifler NA, Gonzalez ME, Kaufmann CA, Gutierrez MA, Rafuse DJ (2017) Taphonomic effects of a grassland fire on a modern faunal sample and its implications for the archaeological record. J Taphonomy 15(1–3):77–90
Asscher Y, Boaretto E (2019) Charred micro-particles characterization in archaeological contexts: Identifying mixing between sediments with implications for stratigraphy. J Archaeol Sci 107:32–39. https://doi.org/10.1016/j.jas.2019.04.007
Avery G, Kandel AW, Klein RG, Conard NJ, Cruz-Úribe K (2004) Tortoises as food and taphonomic elements in palaeo « landscapes », in: Brugal, J.P., Desse, J. (Eds.), Petits Animaux et Sociétés Humaines. Du Complement Alimentaire Aux Ressources Utilitaires, XXIV Rencontres Internationales d’Archéologie et d’Histoire d’Antibes. Editions APDCA, Antibes, pp. 147–161
Backhouse PN, Johnson E (2007) Where were the hearths: an experimental investigation of the archaeological signature of prehistoric fire technology in the alluvial gravels of the Southern Plains. J Archaeol Sci 34:1367–1378. https://doi.org/10.1016/j.jas.2006.10.027
Bellomo RV (1993) A methodological approach for identifying archaeological evidence of fire resulting from human activities. J Archaeol Sci 20:525–553. https://doi.org/10.1006/jasc.1993.1033
Bellomo RV (1994) Methods of determining early hominid behavioral activities associated with the controlled use of fire at FxJj 20 Main, Koobi Fora, Kenva. J Hum Evol 27:173–195. https://doi.org/10.1006/jhev.1994.1041
Bellomo RV, Harris JWK (1990) Preliminary reports of actualistic studies of fire within Virunga National Park, Zaire: toward an understanding of archaeological occurrences, in: Evolution of Environments and Hominidae in the African Western Rift Valley, Memoir. Virginia Museum of Natural History, Martinsville, VA, pp. 317–338
Bennett JL (1999) Thermal alteration of buried bone. J Archaeol Sci 26:1–8. https://doi.org/10.1006/jasc.1998.0283
Berna F, Goldberg P, Horwitz LK, Brink J, Holt S, Bamford M, Chazan M (2012) Microstratigraphic evidence of in situ fire in the Acheulean strata of Wonderwerk Cave, Northern Cape province, South Africa. PNAS 109:E1215–E1220. https://doi.org/10.1073/pnas.1117620109
Blasco R (2008) Human consumption of tortoises at Level IV of Bolomor Cave (Valencia, Spain). J Archaeol Sci 35:2839–2848. https://doi.org/10.1016/j.jas.2008.05.013
Blasco R, Blain H-A, Rosell J, Carlos Díez J, Huguet R, Rodríguez J, Arsuaga JL, Bermúdez de Castro JM, Carbonell E (2011) Earliest evidence for human consumption of tortoises in the European Early Pleistocene from Sima del Elefante, Sierra de Atapuerca, Spain. J Hum Evol 61:503–509. https://doi.org/10.1016/j.jhevol.2011.06.002
Blasco R, Rosell J, Smith KT, Maul LC, Sañudo P, Barkai R, Gopher A (2016) Tortoises as a dietary supplement: a view from the Middle Pleistocene site of Qesem Cave, Israel. Quatern Sci Rev 133:165–182. https://doi.org/10.1016/j.quascirev.2015.12.006
Bonta M, Gosford R, Eussen D, Ferguson N, Loveless E, Witwer M (2017) Intentional fire-spreading by “Firehawk” raptors in Northern Australia. J Ethnobiol 37:700–718. https://doi.org/10.2993/0278-0771-37.4.700
Botelho BG, Reis N, Oliveira LS, Sena MM (2015) Development and analytical validation of a screening method for simultaneous detection of five adulterants in raw milk using mid-infrared spectroscopy and PLS-DA. Food Chem 181:31–37. https://doi.org/10.1016/j.foodchem.2015.02.077
Brain CK, Sillen A (1988) Evidence from the Swartkrans cave for the earliest use of fire. Nature 336:464. https://doi.org/10.1038/336464a0
Brain CK (1981) The hunters or the hunted? An introduction to African cave taphonomy. University of Chicago Press, Chicago
Branch WR (1984) Preliminary observations on the ecology of the angulate tortoise (Chersina angulata) in the Eastern Cape Province, South Africa. Amphibia-Reptilia 5:43–55. https://doi.org/10.1163/156853884X00084
Branch B (2008) Tortoises, terrapins and turtles of Africa. Struik Publishers, Cape Town
Buenger BA (2003) The impact of wildland and prescribed fire on archaeological resources (PhD). University of Kansas, Lawrence, USA
Butler DH, Shahack-Gross R (2017) Formation of biphasic hydroxylapatite-beta magnesium tricalcium phosphate in heat treated salmonid vertebrae. Sci Rep 7:3610. https://doi.org/10.1038/s41598-017-03737-2
Carmody RN, Weintraub GS, Wrangham RW (2011) Energetic consequences of thermal and nonthermal food processing. Proc Natl Acad Sci USA 201112128. https://doi.org/10.1073/pnas.1112128108
Chazan M (2017) Toward a long prehistory of fire. Curr Anthropol 58:S351–S359. https://doi.org/10.1086/691988
Collins B, Steele TE (2017) An often overlooked resource: Ostrich (Struthio spp.) eggshell in the archaeological record. J Archaeol Sci Rep 13:121–131. https://doi.org/10.1016/j.jasrep.2017.03.036
Cruz-Uribe K, Schrire C (1991) Analysis of faunal remains from Oudepost I, an early outpost of the Dutch East India Company, Cape Province. S Afr Archaeo Bull 46:92–106. https://doi.org/10.2307/3889088
David B (1990) How Was This Bone Burnt? In: Solomon S, Davidson I, Watson D (eds) Problem solving in taphonomy: archaeology and palaeontological studies from Europe, Africa, and Oceania, Archaeology and Material Culture Studies in Anthropology. University of Queensland Anthropology Museum, St. Lucia, Tempus, pp 65–79
Davies B, Power MJ, Braun DR, Douglass MJ, Mosher SG, Quick LJ, Esteban I, Sealy J, Parkington J, Faith JT (2022) Fire and human management of late Holocene ecosystems in southern Africa. Quatern Sci Rev 289:107600. https://doi.org/10.1016/j.quascirev.2022.107600
Davis KP (1959) Forest fire-control and use. McGraw-Hill, New York
De Graaff G (1961) Gross effects of a primitive hearth on bones. S Afr Archaeol Bull 16:25–26. https://doi.org/10.2307/3887419
DeBano LF, Dunn PH, Conrad CE, U.S. Forest Service (1977) Fire’s effect on physical and chemical properties of chaparral soils., in: Mooney, H.A., Conrad, C.E. (Eds.), Symposium on the Environmental Consequences of Fire and Fuel Management in Mediterranean Ecosystems, General Technical Report. Palo Alto, CA. USDA Forest Service, Washington, DC, pp. 65–74
del Papa LM (2016) Opportunistic use of tortoises (Chelonoidis chilensis) in a site of the Chaco-Santiagueña region (Province of Santiago del Estero, Argentina). Quat Int South Am Zooarchaeol 391:74–81. https://doi.org/10.1016/j.quaint.2015.08.046
Dubay S (2018) Behavioural and physiological responses of chacma baboons (Papio ursinus) to wildfire in the Cape Peninsula of South Africa (Master thesis). University of Cape Town
Ellingham STD, Thompson TJU, Islam M (2016) The effect of soft tissue on temperature estimation from burnt bone using Fourier transform infrared spectroscopy. J Forensic Sci 61:153–159. https://doi.org/10.1111/1556-4029.12855
Esque TC, Schwalbe CR, Defalco LA, Duncan RB, Hughes TJ (2003) Effects of desert wildfires on desert tortoise (Gopherus agassizii) and other small vertebrates. Swna 48:103–111. https://doi.org/10.1894/0038-4909(2003)048%3c0103:EODWOD%3e2.0.CO;2
Etok SE, Valsami-Jones E, Wess TJ, Hiller JC, Maxwell CA, Rogers KD, Manning DAC, White ML, Lopez-Capel E, Collins MJ, Buckley M, Penkman KEH, Woodgate SL (2007) Structural and chemical changes of thermally treated bone apatite. J Mater Sci 42:9807–9816. https://doi.org/10.1007/s10853-007-1993-z
Felger RS, Beck Moser M, Moser EW (1981) The desert tortoise in Seri Indian culture, in: Hashagen, K.A. (Ed.), Proceedings of the 1981 Desert Tortoise Council Symposium. Desert Tortoise Council, Long Beach, CA, pp. 113–120
Gallo G, Fyhrie M, Paine C, Ushakov SV, Izuho M, Gunchinsuren B, Zwyns N, Navrotsky A (2021) Characterization of structural changes in modern and archaeological burnt bone: Implications for differential preservation bias. PLOS ONE 16:e0254529. https://doi.org/10.1371/journal.pone.0254529
Gallo G, Ushakov SV, Navrotsky A, Stahlschmidt MC (this issue) Detection of prolonged heating in experimentally burnt bone. Archaeol Anthropol Sci
Gart JJ (1966) Alternative analyses of contingency tables. J Roy Stat Soc: Ser B (Methodol) 28:164–179
Geist V (1978) Life strategies, human evolution, environmental design - toward a biological theory of health. Springer, New York
Gelman A, Hill J (2006) data analysis using regression and multilevel/hierarchical models. Cambridge University Press, Cambridge
Gillson L, MacPherson AJ, Hoffman MT (2020) Contrasting mechanisms of resilience at mesic and semi-arid boundaries of fynbos, a mega-diverse heathland of South Africa. Ecol Complex 42:100827. https://doi.org/10.1016/j.ecocom.2020.100827
Goldberg P, Miller CE, Schiegl S, Ligouis B, Berna F, Conard NJ, Wadley L (2009) Bedding, hearths, and site maintenance in the Middle Stone Age of Sibudu Cave, KwaZulu-Natal, South Africa. Archaeol Anthropol Sci 1:95–122. https://doi.org/10.1007/s12520-009-0008-1
Goldberg P, Miller CE, Mentzer SM (2017) Recognizing fire in the Paleolithic archaeological record. Curr Anthropol 58:S175–S190. https://doi.org/10.1086/692729
Goudsblom J (1986) The human monopoly on the use of fire: its origins and conditions. Hum Evol 1:517–523. https://doi.org/10.1007/BF02437468
Gowlett JAJ (2016) The discovery of fire by humans: a long and convoluted process. Phil Trans R Soc B Biol Sci 371:20150164
Gowlett JaJ, Harris JWK, Walton D, Wood BA (1981) Early archaeological sites, hominid remains and traces of fire from Chesowanja, Kenya. Nature 294:125–129. https://doi.org/10.1038/294125a0
Gowlett JAJ, Brink JS, Caris A, Hoare S, Rucina SM (2017) Evidence of burning from bushfires in Southern and East Africa and its relevance to hominin evolution. Curr Anthropol 58:S206–S216. https://doi.org/10.1086/692249
Henry AG, Brooks AS, Piperno DR (2011) Microfossils in calculus demonstrate consumption of plants and cooked foods in Neanderthal diets (Shanidar III, Iraq; Spy I and II, Belgium). PNAS 108:486–491. https://doi.org/10.1073/pnas.1016868108
Herzog NM, Pruetz JD, Hawkes K (2022) Investigating foundations for hominin fire exploitation: Savanna-dwelling chimpanzees (Pan troglodytes verus) in fire-altered landscapes. J Human Evol 167:103193. https://doi.org/10.1016/j.jhevol.2022.103193
Hlubik S, Cutts R, Braun DR, Berna F, Feibel CS, Harris JWK (2019) Hominin fire use in the Okote member at Koobi Fora, Kenya: new evidence for the old debate. J Hum Evol 133:214–229. https://doi.org/10.1016/j.jhevol.2019.01.010
Hoare S (2019) The possible role of predator–prey dynamics as an influence on early hominin use of burned landscapes. Evol Anthropol 28(6):287–332. https://doi.org/10.1002/evan.21807
Howard WE, Fenner HE, Childe HE (1959) Wildlife survival in brush burns. Rangel Ecol Manag/J Range Manag Arch 12:230–234
Isaac G (1982) Early hominids and fire at Chesowanja, Kenya. Nature 296:870–870. https://doi.org/10.1038/296870a0
James SR, Dennell RW, Gilbert AS, Lewis HT, Gowlett JAJ, Lynch TF, McGrew WC, Peters CR, Pope GG, Stahl AB, James SR (1989) Hominid use of fire in the Lower and Middle Pleistocene: a review of the evidence [and comments and replies]. Curr Anthropol 30:1–26. https://doi.org/10.1086/203705
Joshua QI, Hofmeyr MD, Henen BT (2010) Seasonal and site variation in angulate tortoise diet and activity. J Herpetol 44:124–134. https://doi.org/10.1670/08-306R1.1
Karkanas P (2021) All about wood ash: Long term fire experiments reveal unknown aspects of the formation and preservation of ash with critical implications on the emergence and use of fire in the past. J Archaeol Sci 135:105476. https://doi.org/10.1016/j.jas.2021.105476
Klein RG, Cruz-Uribe K (1983) Stone Age population numbers and average tortoise size at Byneskranskop Cave 1 and Die Kelders Cave 1, Southern Cape Province, South Africa. S Afr Archaeol Bull 38:26–30. https://doi.org/10.2307/3888212
Koon HEC (2012) Identification of cooked bone using TEM imaging of bone collagen. Methods Mol Biol 915:249–261. https://doi.org/10.1007/978-1-61779-977-8_15
Koon HEC, Nicholson RA, Collins MJ (2003) A practical approach to the identification of low temperature heated bone using TEM. J Archaeol Sci 30:1393–1399. https://doi.org/10.1016/S0305-4403(03)00034-7
Kruger FJ (1977) Ecology of Cape Fynbos in relation to fire, in: Proceedings of the Symposium on the Environmental Consequences of Fire and Fuel Management in Mediterranean Ecosystems., United States Forest Service General Technical Report WQ-3. Presented at the Symposiumon the Environmental Consequences of Fire and Fuel Management in Mediterranean Ecosystems
Larbey C, Mentzer SM, Ligouis B, Wurz S, Jones MK (2019) Cooked starchy food in hearths ca. 120 kya and 65 kya (MIS 5e and MIS 4) from Klasies River Cave, South Africa. J Hum Evol 131:210–227. https://doi.org/10.1016/j.jhevol.2019.03.015
Little RJA (1992) Regression With Missing X’s: A Review. J Am Stat Assoc 87:1227–1237. https://doi.org/10.2307/2290664
Little RJA, Rubin DB (2002) Statistical Analysis with Missing Data. Wiley & Sons, Hoboken, New Jersey
Lloveras L, Moreno-García M, Nadal J (2009) Butchery, cooking and human consumption marks on rabbit (Oryctolagus cuniculus) bones: an experimental study. J Taphonomy 7(2&3):179–201
Lupo KD, Schmitt DN (2005) Small prey hunting technology and zooarchaeological measures of taxonomic diversity and abundance: ethnoarchaeological evidence from Central African forest foragers. J Anthropol Archaeol 24:335–353. https://doi.org/10.1016/j.jaa.2005.02.002
Mackay A, Armitage SJ, Niespolo EM, Sharp WD, Stahlschmidt MC, Blackwood AF, Boyd KC, Chase BM, Lagle SE, Kaplan CF, Low MA, Martisius NL, McNeill PJ, Moffat I, O’Driscoll CA, Rudd R, Orton J, Steele TE (2022) Environmental influences on human innovation and behavioural diversity in southern Africa 92–80 thousand years ago. Nat Ecol Evol 6:361–369. https://doi.org/10.1038/s41559-022-01667-5
Mallol C, Marlowe FW, Wood BM, Porter CC (2007) Earth, wind, and fire: ethnoarchaeological signals of Hadza fires. J Archaeol Sci 34:2035–2052. https://doi.org/10.1016/j.jas.2007.02.002
Mallol C, Hernández CM, Cabanes D, Sistiaga A, Machado J, Rodríguez Á, Pérez L, Galván B (2013) The black layer of Middle Palaeolithic combustion structures. Interpretation and archaeostratigraphic implications. J Archaeol Sci 40:2515–2537. https://doi.org/10.1016/j.jas.2012.09.017
Manning J (2018) Field guide to fynbos, 2nd edition. Random House South Africa, Cape Twon
Marchenko DV, Zhilich SV, Rybin EP, Nokhrina TI, Bazargur D, Gunchinsuren B, Olsen JW, Khatsenovich AM (2022) Evidence of wildfire versus anthropogenic combustion features: Spatial and macro-charcoal analyses of the final middle Paleolithic horizon at Orkhon 7, Central Mongolia. Archaeol Res Asia 32:100409. https://doi.org/10.1016/j.ara.2022.100409
Marques MPM, Mamede AP, Vassaolo AR, Makhoul C, Cunha E, Gonçalves D, Parker SF, Batista de Carvalho LAE (2018) Burned bones tell their own stories: A review of methodological approaches to assess heat-induced diagenesis. Appl Spectrosc Rev 8:15935. https://doi.org/10.1038/s41598-018-34376-w
Medina ME, Teta P, Rivero YD (2012) Burning damage and small-mammal human consumption in Quebrada del Real 1 (Cordoba, Argentina): an experimental approach. J Archaeol Sci 39:737–743. https://doi.org/10.1016/j.jas.2011.11.006
Mentzer SM (2014) Microarchaeological Approaches to the Identification and Interpretation of Combustion Features in Prehistoric Archaeological Sites. J Archaeol Method Theory 21:616–668. https://doi.org/10.1007/s10816-012-9163-2
Möllhausen B (1958) Diary of a Journey from the Mississippi to the Coasts of the Pacific with a United States Government Expedition. Longman, Brown, Green, Longmans, & Roberts, London
Mucina L, Rutherford M (2006) The vegetation of South Africa, Lesotho and Swaziland. Strelitzia (19). Pretoria: South African National Biodiversity Institute
Munro LE, Longstaffe FJ, White CD (2007) Burning and boiling of modern deer bone: Effects on crystallinity and oxygen isotope composition of bioapatite phosphate. Palaeogeogr Palaeoclimatol Palaeoecol 249:90–102. https://doi.org/10.1016/j.palaeo.2007.01.011
Nicholson RA (1993) A morphological investigation of burnt animal bone and an evalutation of its utility in archaeology. J Archaeol Sci 20:411–428
Oakley K (1956) Fire as Palaeolithic tool and weapon. Proc Prehist Soc 21:36–48. https://doi.org/10.1017/S0079497X00017382
Olsen J, Heinemeier J, Bennike P, Krause C, Margrethe Hornstrup K, Thrane H (2008) Characterisation and blind testing of radiocarbon dating of cremated bone. J Archaeol Sci 35:791–800. https://doi.org/10.1016/j.jas.2007.06.011
Orton J (2012) Tortoise burials in Namaqualand: uncovering ritual behaviour on South Africa’s west coast. Azania Archaeol Res Africa 47(1):99–114. https://doi.org/10.1080/0067270X.2011.647950
Parker CH, Keefe ER, Herzog NM, O’connell JF, Hawkes K (2016) The Pyrophilic Primate Hypothesis. Evol Anthropol 25:54–63. https://doi.org/10.1002/evan.21475
Pasteris JD, Wopenka B, Freeman JJ, Rogers K, Valsami-Jones E, van der Houwen JAM, Silva MJ (2004) Lack of OH in nanocrystalline apatite as a function of degree of atomic order: implications for bone and biomaterials. Biomaterials 25:229–238. https://doi.org/10.1016/S0142-9612(03)00487-3
Pate FD, Hutton JT (1988) The use of soil chemistry data to address post-mortem diagenesis in bone mineral. J Archaeol Sci 15:729–739. https://doi.org/10.1016/0305-4403(88)90062-3
Pereira LSA, Lisboa FLC, Coelho Neto J, Valladão FN, Sena MM (2018) Screening method for rapid classification of psychoactive substances in illicit tablets using mid infrared spectroscopy and PLS-DA. Forensic Sci Int 288:227–235. https://doi.org/10.1016/j.forsciint.2018.05.001
Pérez L, Sanchis A, Hernández CM, Galván B, Sala R, Mallol C (2017) Hearths and bones: An experimental study to explore temporality in archaeological contexts based on taphonomical changes in burnt bones. J Archaeol Sci Rep 11:287–309. https://doi.org/10.1016/j.jasrep.2016.11.036
Pickering TR, Heaton JL, Throckmorton ZJ, Prang TC, Brain CK (2017) A burned primate cuboid from Swartkrans Cave, South Africa. Annals of the Ditsong National Museum of Natural History 7:1–7
Pop E, Kuijper W, van Hees E, Smith G, García-Moreno A, Kindler L, Gaudzinski-Windheuser S, Roebroeks W (2016) Fires at Neumark-Nord 2, Germany: An analysis of fire proxies from a Last Interglacial Middle Palaeolithic basin site. J Field Archaeol 41:603–617. https://doi.org/10.1080/00934690.2016.1208518
Pothier-Bouchard G, Mentzer SM, Riel-Salvatore J, Hodgkins J, Miller CE, Negrino F, Wogelius R, Buckley M (2019) Portable FTIR for on-site screening of archaeological bone intended for ZooMS collagen fingerprint analysis. J Archaeol Sci Rep 26:101862. https://doi.org/10.1016/j.jasrep.2019.05.027
Potter RM, Rossman GR (1979) The tetravalent manganese oxides: identification, hydration, and structural relationships by infrared spectroscopy. Am Miner 64:1199–1218
Preece RC, Gowlett JaJ, Parfitt SA, Bridgland DR, Lewis SG (2006) Humans in the Hoxnian: habitat, context and fire use at Beeches Pit, West Stow, Suffolk, UK. J Quat Sci 21:485–496. https://doi.org/10.1002/jqs.1043
Pruetz JD, Herzog NM (2017) Savanna Chimpanzees at Fongoli, Senegal, Navigate a Fire Landscape. Curr Anthropol 58:S337–S350. https://doi.org/10.1086/692112
Pruetz JD, LaDuke TC (2010) Brief communication: reaction to fire by savanna chimpanzees (pan troglodytes verus) at Fongoli, Senegal: conceptualization of “fire behavior” and the case for a chimpanzee model. Am J Phys Anthropol 141:646–650. https://doi.org/10.1002/ajpa.21245
Pyne SJ, Andrews PL, Laven RD (1996) Introduction of Wildland Fire, 2nd edn. Weiley, New York
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed date 9 Dec 2022
Ramsay SL, Hofmeyr MD, Joshua QI (2002) Activity Patterns of the Angulate Tortoise (Chersina angulata) on Dassen Island, South Africa. J Herpetol 36:161–169. https://doi.org/10.2307/1565987
Reidsma FH (2022) Laboratory-based experimental research into the effect of diagenesis on heated bone: implications and improved tools for the characterisation of ancient fire. Sci Rep 12:17544. https://doi.org/10.1038/s41598-022-21622-5
Reidsma FH, van Hoesel A, van Os BJH, Megens L, Braadbaart F (2016) Charred bone: Physical and chemical changes during laboratory simulated heating under reducing conditions and its relevance for the study of fire use in archaeology. J Archaeol Sci Rep 10:282–292. https://doi.org/10.1016/j.jasrep.2016.10.001
Roebroeks W, Villa P (2011) On the earliest evidence for habitual use of fire in Europe. PNAS 108:5209–5214. https://doi.org/10.1073/pnas.1018116108
Sampson CG (2000) Taphonomy of tortoises deposited by birds and bushmen. J Archaeol Sci 27:779–788. https://doi.org/10.1006/jasc.1999.0500
Sandgathe DM (2017) Identifying and describing pattern and process in the evolution of hominin use of fire. Curr Anthropol 58:S360–S370. https://doi.org/10.1086/691459
Sandgathe D, Dibble HL, Goldberg P, McPherron S, Turq A, Niven L, Hodgkins J (2011) On the role of fire in Neandertal adaptations in Western Europe: evidence from Pech de l’Azé and Roc de Marsal, France. Paleo Anthropology 2011:216–242. https://doi.org/10.4207/PA.2011.ART54
Sanz-Aguilar A, Anadón JD, Giménez A, Ballestar R, Graciá E, Oro D (2011) Coexisting with fire: The case of the terrestrial tortoise Testudo graeca in mediterranean shrublands. Biological Conservation, The New Conservation Debate: Beyond Parks vs. People 144:1040–1049. https://doi.org/10.1016/j.biocon.2010.12.023
Schiegl S, Goldberg P, Pfretzschner H-U, Conard NJ (2003) Paleolithic burnt bone horizons from the Swabian Jura: Distinguishing between in situ fireplaces and dumping areas. Geoarchaeology 18:541–565. https://doi.org/10.1002/gea.10080
Schneider JS, Everson GD (1989) The Desert Tortoise (Xerobates agassizii) in the Prehistory of the Southwestern Great Basin and Adjacent Areas. J Calif Gt Basin Anthropol 11:175–202
Scott AC (1989) Observations on the nature and origin of fusain. Int J Coal Geol 12:443–475. https://doi.org/10.1016/0166-5162(89)90061-X
Scott AC (2000) The Pre-Quaternary history of fire. Palaeogeography, Palaeoclimatology, Palaeoecology, Fire and the Palaeoenvironment 164:281–329. https://doi.org/10.1016/S0031-0182(00)00192-9
Sergant J, Crombé P, Perdaen Y (2006) The ‘invisible’ hearths: a contribution to the discernment of Mesolithic non-structured surface hearths. J Archaeol Sci 33:999–1007. https://doi.org/10.1016/j.jas.2005.11.011
Shahack-Gross R, Bar-Yosef O, Weiner S (1997) Black-coloured bones in Hayonim Cave, Israel: differentiating between burning and oxide staining. J Archaeol Sci 24:439–446. https://doi.org/10.1006/jasc.1996.0128
Shahack-Gross R, Berna F, Karkanas P, Lemorini C, Gopher A, Barkai R (2014) Evidence for the repeated use of a central hearth at Middle Pleistocene (300 ky ago) Qesem Cave, Israel. J Archaeol Sci 44:12–21. https://doi.org/10.1016/j.jas.2013.11.015
Shimelmitz R, Kuhn SL, Jelinek AJ, Ronen A, Clark AE, Weinstein-Evron M (2014) ‘Fire at will’: The emergence of habitual fire use 350,000 years ago. J Hum Evol 77:196–203. https://doi.org/10.1016/j.jhevol.2014.07.005
Shipman P, Foster G, Schoeninger M (1984) Burnt bones and teeth: an experimental study of color, morphology, crystal structure and shrinkage. J Archaeol Sci 11:307–325. https://doi.org/10.1016/0305-4403(84)90013-X
Sinelli N, Cosio MS, Gigliotti C, Casiraghi E (2007) Preliminary study on application of mid infrared spectroscopy for the evaluation of the virgin olive oil “freshness.” Anal Chim Acta 598:128–134. https://doi.org/10.1016/j.aca.2007.07.024
Skaug H, Fournier D, Bolker B, Magnusson A, Nielsen A (2016) generalized linear mixed models using 'AD Model Builder'. R package version 0.8.3.3
Smith LJ, Holycross AT, Painter CW, Douglas ME (2001) Montane rattlesnakes and prescribed fire. Southwest Nat 46:54–61. https://doi.org/10.2307/3672373
Snoeck C, Lee-Thorp JA, Schulting RJ (2014) From bone to ash: Compositional and structural changes in burned modern and archaeological bone. Palaeogeogr Palaeoclimatol Palaeoecol 416:55–68. https://doi.org/10.1016/j.palaeo.2014.08.002
Sorensen A, Roebroeks W, van Gijn A (2014) Fire production in the deep past? The expedient strike-a-light model. J Archaeol Sci 42:476–486. https://doi.org/10.1016/j.jas.2013.11.032
South African National Parks (2022), Fauna – reptiles and amphibians. South African National Parks website https://www.sanparks.org/parks/table_mountain/conservation/fauna.php Accessed 23 Mar 2022
Speth JD, Tchernov E (2002) Middle Paleolithic tortoise use at Kebara Cave (Israel). J Archaeol Sci 29:471–483. https://doi.org/10.1006/jasc.2001.0740
Stahlschmidt MC, Miller CE, Ligouis B, Hambach U, Goldberg P, Berna F, Richter D, Urban B, Serangeli J, Conard NJ (2015) On the evidence for human use and control of fire at Schöningen. J Hum Evol 89:181–201. https://doi.org/10.1016/j.jhevol.2015.04.004
Stapert D, Johansen L (1999) Flint and pyrite: making fire in the Stone Age. Antiquity 73:765–777. https://doi.org/10.1017/S0003598X00065510
Steele TE, Mackay A, Fitzsimmons KE, Igreja M, Marwick B, Orton J, Schwortz S, Stahlschmidt MC (2016) Varsche Rivier 003: a Middle and Later Stone Age Site with Still Bay and Howiesons Poort Assemblages in Southern Namaqualand, South Africa. PaleoAnthropology 2016:100–163. https://doi.org/10.4207/PA.2016.ART101
Steele TE, Klein RG (2013) The Middle and Later Stone Age faunal remains from Diepkloof Rock Shelter, Western Cape, South Africa. J Archaeological Sci 40:3453–3462. https://doi.org/10.1016/j.jas.2013.01.001
Stepka Z, Azuri I, Horwitz LK, Chazan M, Natalio F (2022) Hidden signatures of early fire at Evron Quarry (1.0 to 0.8 Mya). Proc Natl Acad Sci 119:e2123439119. https://doi.org/10.1073/pnas.2123439119
Stiner MC, Kuhn SL, Weiner S, Bar-Yosef O (1995) Differential burning, recrystallization, and fragmentation of archaeological bone. J Archaeol Sci 22:223–237. https://doi.org/10.1006/jasc.1995.0024
Stinson KJ, Wright HA (1969) Temperatures of headfires in the southern mixed prairie of Texas. J Range Manag 22:169–174. https://doi.org/10.2307/3896335
Stuart CL, Meakin PR (1983) A note on the effect of fire on a population of angulate tortoises, Chersina angulata (cryptodira: Testudinidae), with an estimate of biomass. J Herpetol Assoc Africa 29:7–8. https://doi.org/10.1080/04416651.1983.9650124
Surovell TA, Stiner MC (2001) Standardizing infra-red measures of bone mineral crystallinity: an experimental approach. J Archaeol Sci 28:633–642. https://doi.org/10.1006/jasc.2000.0633
Téllez E, Saladié P, Pineda A, Marín J, Vallverdú J, Chacón MG, Carbonell E (2022) Incidental burning on bones by Neanderthals: the role of fire in the Qa level of Abric Romaní rock-shelter (Spain). Archaeol Anthropol Sci 14:119. https://doi.org/10.1007/s12520-022-01577-4
Théry-Parisot I (2002) Fuel Management (Bone and Wood) During the Lower Aurignacian in the Pataud Rock Shelter (Lower Palaeolithic, Les Eyzies de Tayac, Dordogne, France). Contribution of Experimentation. J Archaeol Sci 29:1415–1421. https://doi.org/10.1006/jasc.2001.0781
Thompson JC (2010) Taphonomic analysis of the Middle Stone Age faunal assemblage from Pinnacle Point Cave 13B, Western Cape, South Africa. J Hum Evol 59:321–339. https://doi.org/10.1016/j.jhevol.2010.07.004
Thompson JC, Henshilwood CS (2014a) Tortoise taphonomy and tortoise butchery patterns at Blombos Cave, South Africa. J Archaeol Sci 41:214–229. https://doi.org/10.1016/j.jas.2013.08.017
Thompson JC, Henshilwood CS (2014b) Nutritional values of tortoises relative to ungulates from the Middle Stone Age levels at Blombos Cave, South Africa: implications for foraging and social behaviour. J Hum Evol 67:33–47. https://doi.org/10.1016/j.jhevol.2013.09.010
Thompson TJU, Gauthier M, Islam M (2009) The application of a new method of Fourier transform infrared spectroscopy to the analysis of burned bone. J Archaeol Sci 36:910–914. https://doi.org/10.1016/j.jas.2008.11.013
Thompson TJU, Islam M, Bonniere M (2013) A new statistical approach for determining the crystallinity of heat-altered bone mineral from FTIR spectra. J Archaeol Sci 40:416–422. https://doi.org/10.1016/j.jas.2012.07.008
Thompson TJU (2004) Recent advances in the study of burned bone and their implications for forensic anthropology. Forensic Sci. Int., Mediterranean Academy of Forensic Sciences 1st Workshop 146, S203–S205. https://doi.org/10.1016/j.forsciint.2004.09.063
Turner E, Hutson J, Villaluenga A, García Moreno A, Gaudzinski-Windheuser S (2018) Bone staining in waterlogged deposits: a preliminary contribution to the interpretation of near-shore find accumulation at the Schöningen 13II-4 ‘Spear-Horizon’ site, Lower Saxony, Germany. Hist Biol 30:767–773. https://doi.org/10.1080/08912963.2017.1334203
van Hoesel A, Reidsma FH, van Os BJH, Megens L, Braadbaart F (2019) Combusted bone: Physical and chemical changes of bone during laboratory simulated heating under oxidising conditions and their relevance for the study of ancient fire use. J Archaeol Sci Rep 28:102033. https://doi.org/10.1016/j.jasrep.2019.102033
Wadley L, Luong S, Sievers C, Prinsloo L (2019) Underground transfer of carbonised organic residues to lithics during preliminary fire experiments: implications for archaeology. Heritage Science 7:59. https://doi.org/10.1186/s40494-019-0301-y
Walker MJ, Anesin D, Angelucci DE, Avilés Fernández A, Berna F, López BTA, Fernández Jalvo Y, Haber Uriarte M, López Jiménez A, López Martínez M, Martín Lerma I, Ortega Rodrigáñez J, Camacho PLJ, Rhodes SE, Richter D, Rodríguez Estrella T, Schwenninger JL, Skinner AR (2016) Combustion at the late Early Pleistocene site of Cueva Negra del Estrecho del Río Quípar (Murcia, Spain). ANTIQUITY 90:571–589. https://doi.org/10.15184/aqy.2016.91
Weiner S (2010) Microarchaeology: Beyond the Visible Archaeological Record. Cambridge University Press, Cambridge
Weiner S, Bar-Yosef O (1990) States of preservation of bones from prehistoric sites in the Near East: A survey. J Archaeol Sci 17:187–196. https://doi.org/10.1016/0305-4403(90)90058-D
Weiner S, Wagner HD (1998) The material bone: structure-mechanical function relations. Annu Rev Mater Sci 28:271–298. https://doi.org/10.1146/annurev.matsci.28.1.271
White DRM, Stevens DW (1980) An overview of desert tortoises, Gopherus agassizi, ethnozoology, in: Hashagen, K.A. (Ed.), Proceedings of the 1980 Desert Tortoise Council Symposium. Desert Tortoise Council, Long Beach, CA, pp. 102–108
Wopenka B, Pasteris JD (2005) A mineralogical perspective on the apatite in bone. Materials Science and Engineering: C, NATO Advanced Study Institute (ASI on Learning from Nature How to design New Implantable Biomaterials: From Biomineralization Fundamentals to Biomimetic Materials and Processing Routes) 25, 131–143. https://doi.org/10.1016/j.msec.2005.01.008
Wrangham RW, Jones JH, Laden G, Pilbeam D, Conklin-Brittain N (1999) The Raw and the Stolen: Cooking and the Ecology of Human. Curr Anthropol 40:567–594. https://doi.org/10.1086/300083
Wrangham R (2009) Catching Fire: How Cooking Made Us Human. Basic Books
Wright MG (1988) A note on the reaction or angulate tortoises to fire in fynbos. South African Journal of Wildlife Research 18. https://doi.org/10.10520/AJA03794369_2294
Zazzo A, Saliège J-F, Person A, Boucher H (2009) Radiocarbon dating of calcined bones: where does the carbon come from? Radiocarbon 51:601–611. https://doi.org/10.1017/S0033822200055958
Zilhão J, Angelucci DE, Igreja MA, Arnold LJ, Badal E, Callapez P, Cardoso JL, d’Errico F, Daura J, Demuro M, Deschamps M, Dupont C, Gabriel S, Hoffmann DL, Legoinha P, Matias H, Monge Soares AM, Nabais M, Portela P, Queffelec A, Rodrigues F, Souto P (2020) Last interglacial iberian neandertals as fisher-hunter-gatherers. Science 367:eaaz7943. https://doi.org/10.1126/science.aaz7943
Acknowledgements
We thank South African National (SAN) Parks for granting us permission to collect these tortoise specimens and for facilitating our research. Carly Cowell (Regional Ecologist) and Deborah Winterton (Science Liaison Officer) facilitated access, and Table Mountain National Park rangers Marisa De Kock and Fagan Goodheart generously participated in the collection of the tortoises, and Chad Cheney (Environmental Specialist) provided details about the wildfire. Alex Mackay (University of Wollongong) and Aara Welz provided invaluable assistance in specimen storage and data collection. At the University of Cape Town, Department of Archaeology, we thank Simon Hall and Judith Sealy for their support of the project and curation of the specimens; Louisa Hutten (Senior Scientist/Collections Manager), Oscar Noëls, and Dolores Jacobs prepared and curated the specimens, where they are now available for perpetuity. At the University of Tübingen, we thank Britt Starkovich and Maximillian Zerrer for obtaining reference mammal bones and Matthias Czechowski for collecting some of the spectra. Funding was provided by the US National Science Foundation (NSF)-Archaeology (Steele) and Research Experiences for Undergraduates (REU) program (Wilder), and the University of California, Davis.
Funding
Open access funding provided by University of Vienna. Partial financial support was received from the National Science Foundation (to Steele and Wilder) and was furthermore supported by the Max Planck Society, the University of Tübingen and the University of California, Davis.
Author information
Authors and Affiliations
Contributions
MCS, TES, and SMM designed this study. SMM, SH, MCS, and AC performed and evaluated the FTIR analysis. TES, MNG, MSC, and JCBW performed zooarchaeological analyses. PJM created and interpreted Fig. 1. MCS, TES, and SMM wrote the paper with contributions from all co-authors.
Corresponding authors
Ethics declarations
Ethics approval
All tortoise specimens were collected with the full permission and support of South African National (SAN) Parks, granted to T.E. Steele. All tortoises were already deceased when collected, and none were sacrificed for this sample. Their conservation status is of “least concern” on International Union for Conservation of Nature (IUCN) Red List of Threatened Species, and therefore, they were available for collection without additional permissions. The mammal bones used in the model were likewise obtained from animals that had died due to other causes, with the majority purchased from local butchers or obtained from game wardens. A smaller number of bones were obtained from roadkill and surface recovery of dead animals in local forests.
Consent to participate and for publication
All authors consented to participate in this study and its publication.
Competing interests
MCS is an associate editor for the journal and is co-organizing this topical collection but was not involved in the editorial process of this article. The authors declare no further competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stahlschmidt, M.C., Mentzer, S.M., Heinrich, S. et al. Impact of a recent wildfire on tortoises at Cape Point, South Africa, and implications for the interpretation of heated bones in the archaeological record. Archaeol Anthropol Sci 15, 126 (2023). https://doi.org/10.1007/s12520-023-01806-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12520-023-01806-4