Abstract
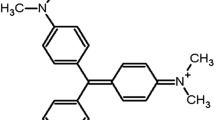
Malachite green (MG) is extensively used in various industries including the food sector, despite having carcinogenic, mutagenic, and teratogenic effects. In the present study, sodium alginate/bentonite (SA/Bnt) nanocomposite was synthesized and characterized to evaluate its potential as an adsorbent to remove MG dye from aqueous solution. The response surface method (RSM) was utilized to optimize the process, and the kinetics of adsorption were evaluated based on experiments carried out thereon. The first-order model was shown to have a decent connection between some parameters and the outcome in this experimental situation. ANOVA results showed that only three of the five parameters (MG dye concentration, contact time, and adsorbent dosage) were important in the removal of the dye. The process parameters such as dye concentration (10–50 mg L−1), contact time (10–60 min), adsorbent dosage (0.5–1.5 g L−1), pH (3–8), and temperature (25–55 °C) were optimized to achieve maximum dye removal, which was around 96%. The correlation of pseudo-second-order kinetic was found to be best fitted with adsorption data with an R2 value of 1.00. The synthesized nanocomposites were found to be effective as bio-adsorbent agents.







Similar content being viewed by others
Data availability
Data available at reasonable request.
References
Al-Tohamy R, Ali SS, Li F, Okasha KM, Mahmoud YAG, Elsamahy T, Sun J (2022) A critical review on the treatment of dye-containing wastewater: ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol Environ Saf 231:113160. https://doi.org/10.1016/j.ecoenv.2021.113160
Antonopoulou M, Konstantinou I (2015) Photocatalytic degradation of pentachlorophenol by visible light Ν-F–TiO 2 in the presence of oxalate ions: optimization, modeling, and scavenging studies. Environ Sci Pollut Res 22:9438–9448. https://doi.org/10.1007/s11356-014-4053-7
Asadi S, Eris S, Azizian S (2018) Alginate-based hydrogel beads as a biocompatible and efficient adsorbent for dye removal from aqueous solutions. ACS Omega 3(11):15140–15148. https://doi.org/10.1021/acsomega.8b02498
Chaker H, Attar AE, Djennas M, Fourmentin S (2021) A statistical modeling-optimization approach for efficiency photocatalytic degradation of textile azo dye using cerium-doped mesoporous ZnO: a central composite design in response surface methodology. Chem Eng Res Des 171:198–212. https://doi.org/10.1016/j.cherd.2021.05.008
Das L, Das P, Bhowal A, Bhattachariee C (2020) Treatment of malachite green dye containing solution using bio-degradable sodium alginate/NaOH treated activated sugarcane bagasse charcoal beads: batch, optimization using response surface methodology and continuous fixed bed column study. J Environ Manage 276:111272. https://doi.org/10.1016/j.jenvman.2020.111272
Fabryanty R, Valencia C, Soetaredjo FE, Putro JN, Santoso SP, Kurniawan A, Ismadji S (2017) Removal of crystal violet dye by adsorption using bentonite–alginate composite. J Environ Chem Eng 5(6):5677–5687. https://doi.org/10.1016/j.jece.2017.10.057
Hassani A, Soltani RDC, Karaca S, Khataee A (2015) Preparation of montmorillonite–alginate nanobiocomposite for adsorption of a textile dye in aqueous phase: isotherm, kinetic and experimental design approaches. J Ind Eng Chem 21:1197–1207. https://doi.org/10.1016/j.jiec.2014.05.034
Ibrahim M, Siddique A, Verma L, Singh J, Koduru JR (2019) Adsorptive removal of fluoride from aqueous solution by biogenic iron permeated activated carbon derived from sweet lime waste. Acta Chim Slov 66(1):123–136. https://doi.org/10.17344/acsi.2018.4717
Iqbal N, Al-Hussain SA, Batool F, Mumtaz A, Irfan A, Noreen S, Zaki ME (2023) Alginate-based sustainable green composites of polymer and reusable birm for mitigation of malachite green dye: characterization and application for water decontamination. Sustainability 15(4):3194. https://doi.org/10.3390/su15043194
Kayan B, Kalderis D, Kulaksız E, Gözmen BDWT (2017) Adsorption of malachite green on Fe-modified biochar: influencing factors and process optimization. Desalin Water Treat 74:383–394. https://doi.org/10.5004/dwt.2017.20601
Khaleghi H, Jaafarzadeh N, Esmaeili H, Ramavandi B (2023) Alginate@ Fe3O4@ Bentonite nanocomposite for formaldehyde removal from synthetic and real effluent: optimization by central composite design. Environ Sci Pollut Res 30(11):29566–29580. https://doi.org/10.1007/s11356-022-24189-w
Li H, Zhang Z, Zhao ZZ (2019) Data-mining for processes in chemistry, materials, and engineering. Process 7(3):151. https://doi.org/10.3390/pr7030151
Majdi H, Esfahani JA, Mohebbi M (2019) Optimization of convective drying by response surface methodology. Comput Electron Agric 156:574–584. https://doi.org/10.1016/j.compag.2018.12.021
Mohammadi A, Daemi H, Barikani M (2014) Fast removal of malachite green dye using novel superparamagnetic sodium alginate-coated Fe3O4 nanoparticles. Int J Biol Macromol 69:447–455. https://doi.org/10.1016/j.ijbiomac.2014.05.042
Oussalah A, Boukerroui A, Aichour A, Djellouli B (2019) Cationic and anionic dyes removal by low-cost hybrid alginate/natural bentonite composite beads: adsorption and reusability studies. Int J Biol Macromol 124:854–862. https://doi.org/10.1016/j.ijbiomac.2018.11.197
Öztürk H, Barışçı S, Turkay O, Veli S (2019) Electrocatalytic degradation of phenol by the electrooxidation–electrocoagulation hybrid process: kinetics and identification of degradation intermediates. Environ Eng 145(5):04019014. https://doi.org/10.1061/(ASCE)EE.1943-7870.0001514
Pannakkong W, Thiwa-Anont K, Singthong K, Parthanadee P, Buddhakulsomsiri J (2022) Hyperparameter tuning of machine learning algorithms using response surface methodology: a case study of ANN, SVM, and DBN. Math Probl Eng 2022:1–17. https://doi.org/10.1155/2022/8513719
Putra EK, Pranowo R, Sunarso J, Indraswati N, Ismadji S (2009) Performance of activated carbon and bentonite for adsorption of amoxicillin from wastewater: mechanisms, isotherms and kinetics. Water Res 43(9):2419–2430. https://doi.org/10.1016/j.watres.2009.02.039
Rawat S, Samreen K, Nayak AK, Singh J, Koduru JR (2021) Fabrication of iron nanoparticles using Parthenium: a combinatorial eco-innovative approach to eradicate crystal violet dye and phosphate from the aqueous environment. Environ Nanotechnol Monit Manag 15:100426. https://doi.org/10.1016/j.enmm.2021.100426
Rawat S, Singh J (2022) Synthesis of nZnO from waste batteries by hydrometallurgical method for photocatalytic degradation of organic pollutants under visible light irradiation. J Environ Manage 318:115518. https://doi.org/10.1016/j.jenvman.2022.115518
Sharma AK, Kaith BS, Tanwar V, Bhatia JK, Sharma N, Bajaj S, Panchal S (2019) RSM-CCD optimized sodium alginate/gelatin based ZnS-nanocomposite hydrogel for the effective removal of biebrich scarlet and crystal violet dyes. Int J Biol Macromol 129:214–226. https://doi.org/10.1016/j.ijbiomac.2019.02.034
Shukla B K, Rawat S, Gautam MK, Bhandari H, Garg S, Singh J (2022) Photocatalytic degradation of orange G dye by using bismuth molybdate: photocatalysis optimization and modeling via definitive screening designs. Molecule 27(7):2309. https://doi.org/10.3390/molecules27072309
Singh J, Reddy KJ, Chang YY, Kang SH, Yang JK (2016) A novel reutilization method for automobile shredder residue as an adsorbent for the removal of methylene blue: mechanisms and heavy metal recovery using an ultrasonically assisted acid. Process Saf Environ Prot 99:88–97. https://doi.org/10.1016/j.psep.2015.10.011
Thakur S, Verma A, Raizada P, Gunduz O, Janas D, Alsanie WF, Thakur VK (2022) Bentonite-based sodium alginate/dextrin cross-linked poly (acrylic acid) hydrogel nanohybrids for facile removal of paraquat herbicide from aqueous solutions. Chemosphere 291:133002. https://doi.org/10.1016/j.chemosphere.2021.133002
Ünügül T, Nigiz FU (2022) Optimization of sodium alginate-graphene nanoplate-kaolin bio-composite adsorbents in heavy metal adsorption by response surface methodology (rsm). Arab J Sci Eng 47(5):6001–6012. https://doi.org/10.1007/s13369-021-05905-z
Wang BH, Zhang Q, Hong JM, Li L (2018) Highly effective iron–carbon–bentonite–alginate beads (Fe/C-BABs) as catalyst to treat benzalkonium chloride in fixed-bed column systems. Process Saf Environ Prot 119:75–86. https://doi.org/10.1016/j.psep.2018.07.018
Wu Y, Qi H, Shi C, Ma R, Liu S, Huang Z (2017) Preparation and adsorption behaviors of sodium alginate-based adsorbent-immobilized β-cyclodextrin and graphene oxide. RSC Adv 7(50):31549–31557. https://doi.org/10.1039/C7RA02313H
Xi H, Jiang H, Zhao D, Zhang AH, Fan B, Yang Y, Zhang J (2021) Highly selective adsorption of phosphate from high-salinity water environment using MgO-loaded and sodium alginate-immobilized bentonite beads. J Clean Prod 313:127773. https://doi.org/10.1016/j.jclepro.2021.127773
Xie Y, Gao P, He F, Zhang C (2022) Application of alginate-based hydrogels in hemostasis. Gels 8(2):109. https://doi.org/10.3390/gels8020109
Zafari P, Ghaemi A (2023) Modeling and optimization of CO2 capture into mixed MEA-PZ amine solutions using machine learning based on ANN and RSM models. Results Eng 19:101279. https://doi.org/10.1016/j.rineng.2023.101279
Zheng X, Zheng H, Xiong Z, Zhao R, Liu Y, Zhao C, Zheng C (2020) Novel anionic polyacrylamide-modify-chitosan magnetic composite nanoparticles with excellent adsorption capacity for cationic dyes and pH-independent adsorption capability for metal ions. J Chem Eng 392:123706. https://doi.org/10.1016/j.cej.2019.123706
Zhou J, Sun Q (2022) Sodium alginate/modified bentonite composite bead adsorptive removal of norfloxacin: static and dynamic adsorption. Polymers 14(19):3984. https://doi.org/10.3390/polym14193984
Acknowledgements
The authors of this study gratefully acknowledge Stat-Ease, Inc., Minneapolis, USA, for providing design expert trial version 13 and University Sophisticated Instrumentation Center of Babasaheb Bhimrao Ambedkar University (Lucknow, India) for providing the facility for sample characterizations. One of us (Devesh Vishwakarma) also thanks the University Grant Commission (UGC), New Delhi, Government of India, for providing a UGC-Non-NET fellowship.
Author information
Authors and Affiliations
Contributions
Devesh Vishwakarma (Ph.D. student): conceptualization, methodology, formal analysis, writing—original draft, investigation, and visualization. Shalu Rawat (Ph.D. student): data curation and writing—review and editing. Deepa Kannaujiya (Ph.D. student): writing—review and editing. Shikha (Professor): writing—review and editing and supervision. Brijesh Shukla (Ph.D.): data curation and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Broder J. Merkel
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vishwakarma, D., Rawat, S., Kannaujiya, D. et al. Adsorption of malachite green onto sodium alginate/bentonite (SA/Bnt) nanocomposite: optimization and modeling via RSM-DSD and insight kinetics and mechanism of adsorption. Arab J Geosci 17, 152 (2024). https://doi.org/10.1007/s12517-024-11964-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-024-11964-x