Abstract
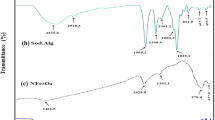
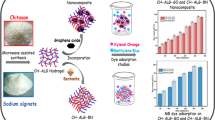
Herein, we have reported the synthesis of a potential bentonite/silver nanoparticles-Alginate (Bent/AgNPs-Alg) bio-nanocomposite and its possible application to adsorb methylene blue (MB) dye from an aqueous solution. Different analytical methods, such as SEM–EDX, TEM, FTIR, and XRD, were used to analyze the surface morphology of the bio-nanocomposite. The specific surface area of (Bent/Ag-Alg) bio-nanocomposite based on monolayer coverage was found to be 562.66 m2.g−1. The maximum adsorption was observed at the optimum condition (equilibrium time 180 min, adsorbent dose 0.5 g.L−1, pH 8 and initial concentration 100 mg.L−1). The Langmuir isotherm best fits the experimental data, with the highest correlation coefficient constant (R2) 0.986, 0.989 and 0.973 at 303, 313 and 323 K, respectively. The maximum monolayer adsorption capacity (qmax) was recorded as 242.56 mg.g−1. Based on the highest correlation coefficient (R2) 0.961, 0.943, and 0.975 at 50, 100, and 150 mg.L−1, respectively, pseudo-second-order kinetics best obeyed the experimental data. The positive value of ΔHº (54.828 kJ.mol−1) and ΔSº (0.215 kJ.mol−1.K−1) confirmed that the adsorption was endothermic and spontaneous. The breakthrough and exhaustion capacity was observed at 200.00 and 680.00 mg.g−1, respectively. 0.1 N HCl solution was used for desorption of the adsorbed MB dye. The spent adsorbent can be regenerated successfully up to the 6th cycle. Therefore, Bent/AgNPs-Alg bio-nanocomposite could be harnessed as a potential adsorbent to remove hazardous MB dye from the wastewater.










Similar content being viewed by others
Data availability
The data supporting this study’s findings are available on request from the corresponding author (Dr. Rais Ahmad). The data is not publicly available due to (state restrictions, e.g., “them containing information that could compromise research participant's privacy/consent”).
References
Choudhry A, Sharma A, Khan TA, Chaudhry SA (2021) Flax seeds based magnetic hybrid nanocomposite: an advance and sustainable material for water cleansing. J Water Process Eng 42:102150. https://doi.org/10.1016/J.JWPE.2021.102150
Somsesta N, Sricharoenchaikul V, Aht-Ong D (2020) Adsorption removal of methylene blue onto activated carbon/cellulose biocomposite films: equilibrium and kinetic studies. Mater Chem Phys 240:122221. https://doi.org/10.1016/j.matchemphys.2019.122221
Sharma A, Mangla D, Choudhry A et al (2022) Facile synthesis, physico-chemical studies of Ocimum sanctum magnetic nanocomposite and its adsorptive application against Methylene blue. J Mol Liq 362:119752. https://doi.org/10.1016/J.MOLLIQ.2022.119752
Abdelrahman EA, Hegazey RM, El-Azabawy RE (2019) Efficient removal of methylene blue dye from aqueous media using Fe/Si, Cr/Si, Ni/Si, and Zn/Si amorphous novel adsorbents. J Mater Res Technol 8:5301–5313. https://doi.org/10.1016/j.jmrt.2019.08.051
Green and Ecofriendly Biochar Preparation from Pumpkin Peel and Its Usage as an Adsorbent for Methylene Blue Removal from Aqueous Solutions. - Document - Gale Academic OneFile. https://go.gale.com/ps/i.do?id=GALE%7CA681124648&sid=googleScholar&v=2.1&it=r&linkaccess=abs&issn=00496979&p=AONE&sw=w&userGroupName=anon~9901b3c. Accessed 12 Aug 2022
Ozer C, Imamoglu M, Turhan Y, Boysan F (2012) Removal of methylene blue from aqueous solutions using phosphoric acid activated carbon produced from hazelnut husks. 101080/027722482012707656 94:1283–1293. https://doi.org/10.1080/02772248.2012.707656
Ravi PLM (2019) Enhanced adsorption capacity of designed bentonite and alginate beads for the effective removal of methylene blue. Appl Clay Sci 169:102–111. https://doi.org/10.1016/j.clay.2018.12.019
Isik B, Ugraskan V, Cakar F, Yazici O (2022) A comparative study on the adsorption of toxic cationic dyes by Judas tree (Cercis siliquastrum) seeds. Biomass Convers Biorefinery. https://doi.org/10.1007/S13399-022-02679-8
Bergaoui M, Nakhli A, Benguerba Y et al (2018) Novel insights into the adsorption mechanism of methylene blue onto organo-bentonite: Adsorption isotherms modeling and molecular simulation. J Mol Liq 272:697–707. https://doi.org/10.1016/j.molliq.2018.10.001
Ozer C, Imamoglu M (2017) Adsorptive transfer of methylene blue from aqueous solutions to hazelnut husk carbon activated with potassium carbonate. https://doi.org/10.5004/dwt.2017.21582
Aichour A, Zaghouane-Boudiaf H (2019) Highly brilliant green removal from wastewater by mesoporous adsorbents: kinetics, thermodynamics and equilibrium isotherm studies. Microchem J 146:1255–1262. https://doi.org/10.1016/j.microc.2019.02.040
Gupta VK, Gupta B, Rastogi A et al (2011) A comparative investigation on adsorption performances of mesoporous activated carbon prepared from waste rubber tire and activated carbon for a hazardous azo dye-Acid Blue 113. J Hazard Mater 186:891–901. https://doi.org/10.1016/j.jhazmat.2010.11.091
Saleh TA, Gupta VK (2012) Synthesis and characterization of alumina nano-particles polyamide membrane with enhanced flux rejection performance. Sep Purif Technol 89:245–251. https://doi.org/10.1016/j.seppur.2012.01.039
Ghimire U, Jang M, Jung SP et al (2019) Electrochemical removal of ammonium nitrogen and cod of domestic wastewater using platinum coated titanium as an anode electrode. Energies 12.https://doi.org/10.3390/en12050883
Klauck C, Rodrigues M, Silva L (2015) Evaluation of phytotoxicity of municipal landfill leachate before and after biological treatment. Braz J Biol 75:57–62. https://doi.org/10.1590/1519-6984.1813
Ali I, Asim M, Khan TA (2013) Arsenite removal from water by electro-coagulation on zinc-zinc and copper-copper electrodes. Int J Environ Sci Technol 10:377–384. https://doi.org/10.1007/s13762-012-0113-z
Sharma A, Mangla D, Shehnaz CSA (2022) Recent advances in magnetic composites as adsorbents for wastewater remediation. J Environ Manage 306:114483. https://doi.org/10.1016/J.JENVMAN.2022.114483
Hasan I, Ahamd R (2019) A facile synthesis of poly (methyl methacrylate) grafted alginate@Cys-bentonite copolymer hybrid nanocomposite for sequestration of heavy metals. Groundw Sustain Dev 8:82–92. https://doi.org/10.1016/j.gsd.2018.09.003
Ahmad R, Mirza A (2015) Sequestration of heavy metal ions by Methionine modified bentonite/Alginate (Meth-bent/Alg): a bionanocomposite. Groundw Sustain Dev 1:50–58. https://doi.org/10.1016/j.gsd.2015.11.003
Tiwari S, Hasan A, Pandey LM (2017) A novel bio-sorbent comprising encapsulated Agrobacterium fabrum (SLAJ731) and iron oxide nanoparticles for removal of crude oil co-contaminant, lead Pb(II). Elsevier B.V.
Saxena V, Hasan A, Sharma S, Pandey LM (2018) Edible oil nanoemulsion: An organic nanoantibiotic as a potential biomolecule delivery vehicle. Int J Polym Mater Polym Biomater 67:410–419. https://doi.org/10.1080/00914037.2017.1332625
Tang J, Wang R, Liu M et al (2018) Construction of novel Z-scheme Ag/FeTiO3/Ag/BiFeO3 photocatalyst with enhanced visible-light-driven photocatalytic performance for degradation of norfloxacin. Chem Eng J 351:1056–1066. https://doi.org/10.1016/j.cej.2018.06.171
Baranyaiová T, Bujdák J (2016) Reaction kinetics of molecular aggregation of rhodamine 123 in colloids with synthetic saponite nanoparticles. Appl Clay Sci 134:103–109. https://doi.org/10.1016/j.clay.2016.01.036
Heybet EN, Ugraskan V, Isik B, Yazici O (2021) Adsorption of methylene blue dye on sodium alginate/polypyrrole nanotube composites. Int J Biol Macromol 193:88–99. https://doi.org/10.1016/J.IJBIOMAC.2021.10.084
Ahmad R, Mirza A (2017) Adsorption of Pb(II) and Cu(II) by Alginate-Au-Mica bionanocompositeKinetic, isotherm and thermodynamic studies. Process Saf Environ Prot 109:1–10. https://doi.org/10.1016/j.psep.2017.03.020
Liu F, Li W, Zhou Y (2021) Preparation and characterization of magnetic sodium alginate-modified zeolite for the efficient removal of methylene blue. Colloids Surf A Physicochem Eng Asp 629:127403. https://doi.org/10.1016/J.COLSURFA.2021.127403
Abdulla NK, Siddiqui SI, Fatima B et al (2021) Silver based hybrid nanocomposite: A novel antibacterial material for water cleansing. J Clean Prod 284:124746. https://doi.org/10.1016/J.JCLEPRO.2020.124746
Gurunathan S, Park JH, Han JW, Kim JH (2015) Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 human breast cancer cells: Targeting p53 for anticancer therapy. Int J Nanomedicine 10:4203–4223. https://doi.org/10.2147/IJN.S83953
Ahmed S, Ahmad M, Swami BL, Ikram S (2016) A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J Adv Res 7:17–28. https://doi.org/10.1016/j.jare.2015.02.007
Ahmad R, Mirza A (2018) Synthesis of Guar gum/bentonite a novel bionanocomposite: Isotherms, kinetics and thermodynamic studies for the removal of Pb (II) and crystal violet dye. J Mol Liq 249:805–814. https://doi.org/10.1016/j.molliq.2017.11.082
Ahmad R, Ansari K (2020) Chemically treated Lawsonia inermis seeds powder (CTLISP): An eco-friendly adsorbent for the removal of brilliant green dye from aqueous solution. Groundw Sustain Dev 11:100417. https://doi.org/10.1016/j.gsd.2020.100417
Sukla Baidya K, Kumar U (2021) Adsorption of brilliant green dye from aqueous solution onto chemically modified areca nut husk. S Afr J Chem Eng 35:33–43. https://doi.org/10.1016/j.sajce.2020.11.001
Devi N, Dutta J (2017) Preparation and characterization of chitosan-bentonite nanocomposite films for wound healing application. Int J Biol Macromol 104:1897–1904. https://doi.org/10.1016/j.ijbiomac.2017.02.080
Ahmad R, Ansari K (2022) Fabrication of alginate@silver nanoparticles (Alg@AgNPs) bionanocomposite for the sequestration of crystal violet dye from aqueous solution. Int J Biol Macromol 218:157–167. https://doi.org/10.1016/J.IJBIOMAC.2022.07.092
Martins LR, Rodrigues JAV, Adarme OFH et al (2017) Optimization of cellulose and sugarcane bagasse oxidation: Application for adsorptive removal of crystal violet and auramine-O from aqueous solution. J Colloid Interface Sci 494:223–241. https://doi.org/10.1016/j.jcis.2017.01.085
Zhang F, Ma B, Jiang X, Ji Y (2016) Dual function magnetic hydroxyapatite nanopowder for removal of malachite green and Congo red from aqueous solution. Powder Technol 302:207–214. https://doi.org/10.1016/j.powtec.2016.08.044
Simsek G, Imamoglu M (2014) Investigation of equilibrium, kinetic and thermodynamic of methylene blue adsorption onto dehydrated hazelnut husk carbon. New Pub Balaban 54:1747–1753. https://doi.org/10.1080/19443994.2014.892838
SK Lagergren - Sven. Vetenskapsakad. Handingarl (1898) About the theory of so-called adsorption of soluble substances. ci.nii.ac.jp
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465. https://doi.org/10.1016/S0032-9592(98)00112-5
Jaseela PK, Garvasis J, Joseph A (2019) Selective adsorption of methylene blue (MB) dye from aqueous mixture of MB and methyl orange (MO) using mesoporous titania (TiO2) – poly vinyl alcohol (PVA) nanocomposite. J Mol Liq 286:110908. https://doi.org/10.1016/j.molliq.2019.110908
Ugraskan V, Isik B, Yazici O (2021) Adsorptive removal of methylene blue from aqueous solutions by porous boron carbide: isotherm, kinetic and thermodynamic studies. 101080/0098644520211948406 209:1111–1129. https://doi.org/10.1080/00986445.2021.1948406
Zhang Y, Liu J, Du X, Shao W (2019) Preparation of reusable glass hollow fiber membranes and methylene blue adsorption. J Eur Ceram Soc 39:4891–4900. https://doi.org/10.1016/j.jeurceramsoc.2019.06.038
Dali Youcef L, Belaroui LS, López-Galindo A (2019) Adsorption of a cationic methylene blue dye on an Algerian palygorskite. Appl Clay Sci 179:105145. https://doi.org/10.1016/j.clay.2019.105145
Jawad AH, Rashid RA, Ismail K, Sabar S (2017) High surface area mesoporous activated carbon developed from coconut leaf by chemical activation with H3PO4 for adsorption of methylene blue. Desalin Water Treat 74:326–335. https://doi.org/10.5004/DWT.2017.20571
Tang Y, Zhao Y, Lin T et al (2019) Adsorption performance and mechanism of methylene blue by H3PO4- modified corn stalks. J Environ Chem Eng 7:103398. https://doi.org/10.1016/j.jece.2019.103398
Jawad AH, Abdulhameed AS, Hanafiah MAKM et al (2021) Numerical desirability function for adsorption of methylene blue dye by sulfonated pomegranate peel biochar: Modeling, kinetic, isotherm, thermodynamic, and mechanism study. Korean J Chem Eng 38:1499–1509. https://doi.org/10.1007/S11814-021-0801-9
Jawad AH, Kadhum AM, Ngoh YS (2018) Applicability of dragon fruit (Hylocereus polyrhizus) peels as low-cost biosorbent for adsorption of methylene blue from aqueous solution: Kinetics, equilibrium and thermodynamics studies. Desalin Water Treat 109:231–240. https://doi.org/10.5004/DWT.2018.21976
Acknowledgements
The authors are grateful for the research facilities provided by the Chairman, Department of Applied Chemistry, AMU, Aligarh, India. We also thank USIF, AMU, for the SEM/EDX and TEM facilities and the Department of Physics, AMU, for the XRD facility.
Funding
We express our sincere gratitude to UGC, New Delhi, for giving financial support to Mr. Mohammad Osama Ejaz.
Author information
Authors and Affiliations
Contributions
Rais Ahmad: conceptualization, supervision, visualization, funding acquisition, data curation, writing—review and editing. Mohammad Osama Ejaz: methodology, formal analysis, software, validation, writing—original draft. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• A novel and potential (Bent/AgNPs-Alg) bio-nanocomposite has been synthesized.
• The maximum monolayer adsorption capacity (qmax) was recorded as 242.56 mg.g−1.
• The Langmuir isotherm and pseudo second order kinetic model obeyed the experimental data.
• The Bent/AgNPs-Alg adsorbent can be regenerated successfully up to the 6th cycle.
Novelty statement
A novel and potential (Bent/AgNPs-Alg) bio-nanocomposite has been synthesized by the green method for the first time and has not been reported in literature till now. The bio-nanocomposite showed an excellent adsorption capacity of 242.56 mg.g−1 for the removal of MB dye. The spent adsorbent was successfully regenerated up to the 6th cycle, making the treatment process economical and eco-friendly in developing countries.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmad, R., Ejaz, M.O. Adsorption of methylene blue dye from aqueous solution onto synthesized bentonite/silvernanoparticles-alginate (Bent/AgNPs-Alg) bio-nanocomposite. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-03350-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03350-y