Abstract
Introduction
AZURE was a 76-week, randomized, open-label, parallel-group, phase IIIb noninferiority study comparing the efficacy and safety of intravitreal aflibercept (IVT-AFL) in a treat-and-extend (T&E) regimen with fixed dosing in patients with neovascular age-related macular degeneration (nAMD) previously receiving IVT-AFL for ≥ 1 year.
Methods
Patients were aged ≥ 51 years and had completed ≥ 1 year of IVT-AFL treatment prior to enrollment (IVT-AFL once per month [– 1 or + 2 weeks] for 3 months followed by IVT-AFL every 2 months [6–12 weeks]). Patients were randomly assigned (1:1) to receive IVT-AFL 2 mg in either a T&E (minimum treatment interval of 8 weeks with no upper limit, adjusted according to functional and anatomic outcomes, as assessed by the investigator; n = 168), or a fixed dosing regimen (treatment every 8 weeks [± 3 days]; n = 168). The primary endpoint was best-corrected visual acuity (BCVA) change from baseline to week (W) 52. The key secondary endpoint was the proportion of patients maintaining vision (< 15-letter loss) at W52.
Results
The full analysis set comprised 332 patients (T&E: n = 165; fixed dosing: n = 167). Mean BCVA change (baseline to W52) was − 0.3 ± 7.5 vs. − 0.5 ± 8.4 letters (T&E vs. fixed dosing; least-squares mean difference [95% CI]: 0.22 [− 1.51 to 1.96] letters; P < 0.0001 for noninferiority test [5-letter margin]). From baseline to W52, 95.2% (T&E) and 94.0% (fixed dosing) of patients maintained vision. Mean central subfield thickness change from baseline to W52 was − 24 ± 55 (T&E) and − 33 ± 47 (fixed dosing) µm. Last treatment interval to W76 was ≥ 12 weeks for 37.0% of T&E patients. No new safety signals were identified.
Conclusion
IVT-AFL T&E can achieve similar functional and anatomic outcomes to fixed dosing every 8 weeks over 52 weeks in patients with nAMD who have completed ≥ 1 year of treatment, while reducing treatment burden.
Trial Registration
ClinicalTrials.gov Identifier: NCT02540954.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
At the time of study design, there was a lack of data regarding the best intravitreal aflibercept (IVT-AFL) regimen to optimize functional and anatomic outcomes while minimizing treatment burden in patients with neovascular age-related macular degeneration (nAMD). |
AZURE was a phase IIIb, 76-week, randomized, noninferiority study to investigate whether, after the first year of fixed-dosing treatment, a change to flexible treat-and-extend (T&E) IVT-AFL regimen (adjusted according to investigator-assessed functional and anatomic outcomes) was comparable to continued fixed dosing every 8 weeks (q8w). |
What was learned from the study? |
The T&E IVT-AFL regimen achieved similar functional and anatomic outcomes compared to fixed dosing (IVT-AFL q8w) in patients with neovascular age-related macular degeneration (nAMD) who previously completed ≥ 1 year of treatment with a fixed dosing regimen, with noninferiority achieved for the primary endpoint of best-corrected visual acuity change from baseline to week 52. |
The safety profile of IVT-AFL was consistent with previous studies, and no new safety signals were identified. |
T&E regimens can be personalized to the needs of patients with nAMD, enabling maintenance of functional and anatomic outcomes while reducing treatment burden. |
Introduction
The efficacy of intravitreal aflibercept (IVT-AFL) has been demonstrated using a range of treatment regimens for patients with neovascular age-related macular degeneration (nAMD) [1]. Treat-and-extend (T&E) is a proactive, individualized dosing strategy, where treatment intervals are decided at every visit and gradually extended based on functional and anatomic outcomes. The goal of T&E is to reduce treatment burden while maintaining the gains in visual acuity (VA) associated with intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy [2, 3].
The VIEW 1 and 2 studies showed that treatment with IVT-AFL q8 (treatment every 8 weeks) following three initial monthly doses resulted in similar VA outcomes compared with treatment every 4 weeks in the first year of treatment. When administered with pro re nata treatment (capped at 12 weeks) during the second year, approximately 50% of patients received only mandatory injections (treatment every 12 weeks) [4, 5]. Based on this, in most countries, prescribing recommendations for IVT-AFL in patients with nAMD were dosing q8 for the first year after three initial monthly doses, followed by T&E, known as flexible dosing at the time of the study [6]. Other than the VIEW trials, no prospective clinical trial had compared the use of alternative IVT-AFL treatment regimens. Considering this, additional studies were conceived to refine individualized IVT-AFL treatment in nAMD in an attempt to optimize patient outcomes and treatment burden. ARIES compared early-start T&E (starting in the first year of treatment, following three initial monthly doses) with late-start T&E (starting in the second year of treatment, after 1 year of fixed dosing) [7]. ARIES showed noninferior best-corrected visual acuity (BCVA) outcomes at week 104 of the early T&E arm compared to the late start arm, with 47.2% and 53.8% of patients having a last injection interval of ≥ 12 weeks and 30.2% and 26.9% of ≥ 16 weeks. ALTAIR investigated the efficacy and safety of two different T&E regimens, with treatment intervals adjusted in 2-week (IVT-AFL-2W) or 4-week (IVT-AFL-4W) increments after three initial monthly doses. ALTAIR demonstrated noninferior outcomes at week 52, with more than half of patients having a last injection interval of ≥ 12 weeks, and at week 96, with the majority having a last injection interval of ≥ 12 weeks [8]. Both studies had a maximum treatment interval of 16 weeks [7, 8]. However, neither study directly established whether T&E would provide similar efficacy to fixed dosing every 8 weeks during the chronic phase of treatment following the first year of fixed-dosing IVT-AFL treatment.
The aim of AZURE was to compare the efficacy and safety of IVT-AFL 2 mg administered using a proactive, individualized T&E regimen with fixed dosing (q8) in patients with nAMD who had completed ≥ 1 year of fixed-dosing IVT-AFL treatment. AZURE was also conducted to assess treatment interval extension with no maximum limit. At the time that AZURE was designed, there were limited data on IVT-AFL T&E. Therefore, the objectives included evaluating the performance of T&E according to investigator-driven assessments, in line with the approved treatment label in the European Union (EU). These data represent the study sponsor’s commitment to publish clinical trial data and are part of a previously published parallel study taking the same approach for patients with diabetic macular edema [9].
Methods
Study Design
AZURE (NCT02540954) was a 76-week, randomized, parallel-group, open-label, multicenter, phase IIIb study conducted to assess the noninferiority of IVT-AFL T&E compared with fixed dosing in patients with nAMD who had previously completed ≥ 1 year of fixed-dosing IVT-AFL treatment (from first treatment to randomization). The study was conducted at 100 study centers in 14 different countries in Europe and Canada from September 2015 to June 2020, in accordance with the Declaration of Helsinki and the International Council for Harmonisation guideline E6: Good Clinical Practice. An independent Central Reading Center evaluated the ophthalmic images obtained by optical coherence tomography (OCT) and fundus photography/fluorescein angiography. The protocol and any amendments were reviewed and approved by each study site’s independent ethics committee or institutional review board before the study started. All enrolled patients provided written informed consent. The study protocol and statistical analysis plan can be accessed at ClinicalTrials.gov Identifier: NCT02540954 (https://clinicaltrials.gov/ct2/show/NCT02540954).
All patients were aged ≥ 51 years at time of study enrollment and had completed ≥ 1 year of fixed-dosing IVT-AFL treatment prior to enrollment. To be eligible, prior treatment had to have been initiated with IVT-AFL once per month (– 1 or + 2 weeks) for 3 months followed by IVT-AFL every 6–12 weeks.
At the start of initial IVT-AFL treatment (i.e., ≥ 1 year prior to this study), patients had active primary subfoveal choroidal neovascularization (CNV) lesions secondary to nAMD, including juxtafoveal lesions that affected the fovea, with the area of CNV occupying ≥ 50% of the total lesion within 4 weeks before initial treatment initiation and BCVA of 73–25 Early Treatment Diabetic Retinopathy Study letters (Snellen equivalent 20/40 to 20/320) in the study eye. For patients who met the eligibility criteria in both eyes during screening for this study, the eye with the worse VA was the study eye; only one eye was selected as the study eye. Full inclusion and exclusion criteria are listed in the Supplemental materials.
After screening, eligible patients were randomly assigned in a 1:1 ratio by central randomization to one of the two parallel treatment groups (Fig. 1). Patients received IVT-AFL 2 mg in either a T&E (minimum treatment interval of 8 weeks with no upper limit; adjusted according to functional and anatomic outcomes, as assessed by the investigator) or a fixed-dosing regimen (treatment q8 [± 3 days]; modification of the treatment interval was not permitted). Further details are available in the Supplemental materials.
The AZURE study design. aPatients aged ≥ 51 years, with active primary subfoveal CNV secondary to nAMD and BCVA of 73–25 ETDRS letters were eligible. bIVT-AFL 2 mg once per month (– 1 or + 2 weeks) for 3 months followed by IVT-AFL 2 mg every 2 months (6–12 weeks). cAs assessed by the investigator and with no upper limit on treatment intervals. When/if visual and anatomic outcomes indicated that the disease had reactivated, the treatment interval reverted to the last treatment interval in which the disease was inactive (i.e., no signs of exudation observed). BCVA best-corrected visual acuity; CNV choroidal neovascularization; ETDRS Early Treatment Diabetic Retinopathy Study; IVT-AFL intravitreal aflibercept; nAMD neovascular age-related macular degeneration; R randomized; T&E treat-and-extend
Study Endpoints
The primary endpoint was mean change in BCVA from baseline to week 52 in the study eye. The key secondary endpoint was the proportion of patients who maintained vision (< 15-letter loss) at week 52.
Secondary endpoints at week 52 included mean change in central subfield thickness (CST) from baseline, as measured by OCT, the proportion of patients who gained ≥ 5 letters and who lost ≥ 30 letters from baseline in the study eye. Exploratory endpoints included the proportion of patients for whom the treatment interval was extended and the total number of IVT-AFL injections required at weeks 52 and 76. All endpoints were exploratory at week 76. Safety was assessed throughout. Other endpoints are listed in the Supplemental materials.
Statistical Analysis
A sample size of 144 patients per treatment group was estimated to provide a power of 90% for the primary endpoint analysis. The primary method for replacing missing values for all efficacy analyses was the last observation carried forward (LOCF).
The primary endpoint analysis was performed using an analysis of covariance model (LOCF), with the treatment group as a fixed factor and baseline BCVA as a covariate. Statistical testing was conducted to evaluate the noninferiority of T&E compared with fixed dosing. Two-sided 95% confidence intervals (CIs) for the difference in the least-squares (LS) mean (T&E group minus the fixed dosing group) of the BCVA change from study baseline to week 52 were calculated. The T&E regimen was considered to be noninferior to fixed dosing if the 95% CI of the difference lay entirely above – 5 letters. If T&E was statistically proven to be noninferior to fixed dosing in the primary efficacy analysis, confirmatory testing was conducted to prove noninferiority regarding the key secondary efficacy variable in the full analysis set (FAS; prespecified margin of – 7%).
In addition, all variables were analyzed by descriptive statistical methods. The statistical evaluation was performed using Statistical Analysis Software v9.4 (SAS Institute Inc., Cary, NC, USA). For full details, see Supplemental materials.
Results
Patients
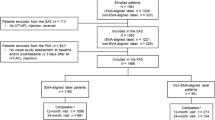
Of the 470 patients screened in AZURE, 336 were randomized (n = 168, T&E; n = 168, fixed dosing) (Fig. 2). Of the 134 patients who were not randomized, 116 failed at screening, 2 had adverse events, and 16 withdrew from the study before randomization. One randomized patient in the T&E group did not receive IVT-AFL study treatment (the eligibility criteria had not been fully met; the patient was withdrawn and excluded from the safety analysis set [SAF]). All patients from the SAF were included in the FAS, except for one patient without a baseline BCVA assessment and two patients without postbaseline BCVA assessments. The FAS comprised 332 patients (n = 165, T&E; n = 167, fixed dosing).
Patient disposition. aOne randomized patient in the T&E group did not receive IVT-AFL study treatment (inclusion criteria were not met; therefore, the patient was withdrawn from the study by the investigator and excluded from the SAF). AE adverse event; IVT-AFL intravitreal aflibercept; SAF safety analysis set; T&E treat-and-extend
Baseline demographics and disease characteristics were similar between treatment groups (Table 1). Mean (standard deviation [SD]) age was 76.2 (8.3) and 74.7 (7.0) years, and 64.2% and 64.7% were female in the T&E and fixed-dosing groups, respectively. Mean (SD) BCVA in the study eye was 59.4 (10.5) and 60.9 (9.9) letters at IVT-AFL treatment initiation and 69.0 (12.1) and 70.1 (10.9) letters at study baseline in the T&E and fixed-dosing groups, respectively. Mean change (SD) in BCVA from IVT-AFL treatment initiation to study baseline was + 9.6 (11.6) letters in the T&E group and + 8.9 (11.6) letters in the fixed-dosing group. Mean (SD) CST at study baseline was 257 (68) µm in the T&E group and 264 (60) µm in the fixed-dosing group. The only notable difference was the proportion of patients aged ≥ 85 years (15.2%, T&E; 6.0%, fixed dosing); however, this difference was not statistically significant.
Efficacy
Functional Outcomes
At week 52, mean (SD) BCVA change from baseline was − 0.3 (7.5; T&E) and − 0.5 (8.4; fixed dosing) letters (Fig. 3). The LS mean difference (95% CI) was 0.22 (− 1.51 to 1.96) letters for T&E relative to fixed dosing. Compared with fixed dosing, T&E achieved a noninferior mean change in BCVA at week 52 (prespecified margin of 5 letters; P < 0.0001). Mean (95% CI; SD) BCVA letter change from baseline was − 1.5 (− 3.2, 0.2; 10.9) for T&E and − 0.9 (− 2.5, 0.7; 10.4) for fixed dosing at week 76.
Mean change in BCVA from start of IVT-AFL treatment to week 76. Full analysis set, last observation carried forward. Mean (SD) unless otherwise stated. aAll patients entering the study had completed ≥ 1 year of IVT-AFL treatment prior to enrollment. bMean change in BCVA from study baseline. BCVA best-corrected visual acuity; BL baseline; ETDRS Early Treatment Diabetic Retinopathy Study; IVT-AFL intravitreal aflibercept; SD standard deviation; SEM standard error of the mean; T&E treat-and-extend
From baseline to week 52, 95.2% (n = 157; T&E) and 94.0% (n = 157; fixed dosing) of patients maintained vision (< 15-letter loss) (Fig. 4). The treatment difference (95% CI) was 1.1% (– 3.7 to 6.0%) for T&E relative to fixed dosing. T&E was noninferior to fixed dosing also regarding maintaining vision in the study eye at week 52 (prespecified margin of –7%).
At week 76, 92.1% (n = 152; T&E) and 94.0% (n = 157; fixed dosing) of patients maintained vision (95% CI difference: − 7.4%, 3.6%). At weeks 52 and 76, 73.3% (n = 121) and 73.3% (n = 121) of patients in the T&E group and 78.4% (n = 131) and 73.7% (n = 123) of patients in the fixed-dosing group experienced a high level of vision maintenance (< 5-letter loss), respectively. At week 76, 22.4% (n = 37; T&E) and 25.7% (n = 43; fixed dosing) of patients gained ≥ 5 letters.
Anatomic Outcomes
CST in the study eye remained stable throughout the study in both groups (Fig. 5). From baseline to week 52, mean (SD) change in CST was − 24 (55; T&E) µm and − 33 (47; fixed dosing) µm. Mean (SD) change in CST was − 22 (57; T&E) µm and − 38 (51; fixed dosing) µm from baseline to week 76. The difference between treatment groups was not considered to be clinically relevant.
Mean absolute CST from study baseline to week 76. Full analysis set, last observation carried forward. Mean (SD) unless otherwise stated. CST as measured by OCT at each visit. BL baseline; CST central subfield thickness; IVT-AFL intravitreal aflibercept; OCT optical coherence tomography; SD standard deviation; T&E treat-and-extend
Treatment Exposure
Mean (SD) number of IVT-AFL injections was 6.0 (1.0) and 6.8 (0.8) at week 52 and 8.0 (1.8) and 9.6 (1.4) at week 76 in the T&E and fixed-dosing groups, respectively. The maximum number of IVT-AFL injections received by any patient was ten injections at week 76, with 24.2% (n = 40; T&E) and 83.8% (n = 140; fixed dosing) of patients receiving 10 injections.
Mean (SD) duration of the last treatment interval up to week 76 was 11.1 (3.6) weeks for the T&E group; as expected, and in line with the protocol, the last treatment interval was 8.2 (1.2) weeks for the fixed-dosing group. In the T&E group, 37.0% of patients (n = 61) achieved a last treatment interval of ≥ 12 weeks, and 9.1% of patients (n = 15) achieved a last treatment interval of > 16 weeks up to week 76 (Fig. 6). In total, 55.4% of study completers (n = 82) in the T&E group had a scheduled extended treatment interval of ≥ 12 weeks (Table 2). In the T&E group, when a treatment interval was extended, the subsequent treatment interval was either maintained or further extended in 63.8% of cases (Table 3).
Safety
The proportion of patients experiencing treatment-emergent adverse events (TEAEs) was similar for the T&E (77.8%) and fixed dosing (73.8%) groups; these were predominantly mild or moderate in severity (Table 4). The incidence of ocular TEAEs in the study eye was comparable in the T&E (45.5%) and fixed dosing (48.8%) groups. The most common ocular TEAEs were cataract (9.3%), subretinal fluid (5.4%) and increased intraocular pressure (5.1%).
Serious TEAEs were reported in 14.6% (n = 49) of patients: 15.6% (n = 26) in the T&E group and 13.7% (n = 23) in the fixed-dosing group (Table 4). Overall, five patients (1.5%) had nine treatment-emergent Antiplatelet Trialists’ Collaboration (APTC) events: 2.4% (n = 4) in the T&E group and 0.6% (n = 1) in the fixed-dosing group. Non-fatal myocardial infarction was reported for four patients (three in the T&E group and one in the fixed-dosing group), and vascular death was reported for one patient in the T&E group. Two patients (0.6%) experienced endophthalmitis; no cases of retinal vasculitis and a single case (0.3%) of intraocular inflammation were reported (uveitis of moderate severity in a patient with active hematologic malignancy that resolved after 3 days of treatment with topical corticosteroids and was not associated with vasculitis). Three deaths were reported, all in the fixed-dosing group. Two of the deaths were due to TEAEs (one lung adenocarcinoma and one acute myeloid leukemia), a further death due to lung adenocarcinoma not due to TEAEs was reported, and none were considered to be related to IVT-AFL.
Discussion
The results of AZURE demonstrate the efficacy and safety of proactive IVT-AFL treatment in patients with nAMD who had previously received ≥ 1 year of fixed-dosing IVT-AFL therapy prior to either changing to T&E or continuing on a fixed dosing regimen, with maintenance of BCVA gains beyond 2 years from the start of treatment being observed. Furthermore, safety outcomes were comparable for the two regimens, and no new safety signals were identified; five patients had nine treatment-emergent APTC events, of which three were adjudicated as being related to IVT-AFL.
As patients had received ≥ 1 year of fixed-dosing IVT-AFL treatment prior to enrollment and already achieved good outcomes, considerable improvements in functional and anatomic outcomes were not expected. Importantly, reported BCVA gains/losses are from the score at study baseline, not the start of IVT-AFL treatment. However, improvements were maintained through the 76-week study period in both groups, and the proportion of patients in the T&E group who maintained vision at week 52 (95%) was comparable to that reported in other IVT-AFL T&E studies [7, 8]. The stable CST observed throughout the study was as expected for a maintenance regimen, and the small decreases in mean CST observed at weeks 52 and 76 in both groups can be explained by the schedule of assessments: all CST measurements were taken at injection visits at the end of treatment intervals, except for those at weeks 52 and 76, when measurements were taken 4 weeks after the last injection in the fixed-dosing group and at the middle of injection intervals for many patients in the T&E group.
In routine clinical practice, fixed dosing is associated with a high burden and loss of efficiency for patients and physicians since it can lead to overtreatment or undertreatment if the fixed intervals between treatments are too short or long [2]. With T&E, the need for interim monitoring is minimized, and the predictable timing of the next injection benefits patient and clinic. Consequently, adopting an individualized treatment approach can lead to improvements in patient compliance and quality of life. AZURE demonstrates that a substantial proportion of patients (37%) could be maintained on treatment intervals of ≥ 12 weeks, and when treatment intervals are not capped at a maximum of 16 weeks, such as in ARIES and ALTAIR [7, 8].
In line with other studies, fewer injections were required with T&E, saving approximately 0.8 injections (6.0 vs. 6.8) up to week 52 and 1.6 mean injections (8.0 vs. 9.6) up to week 76 versus fixed dosing. In the ALTAIR study, the first year of treatment required a mean of 7.2 and 6.9 injections for the 2- and 4-week adjustment groups, respectively, and of 10.4 injections for both groups up to week 96. The ARIES study showed mean numbers of injections of 7.1 vs. 8.0 for early T&E vs. late T&E after 52 weeks, increasing to 12.0 vs. 13.0 at 104 weeks. For reference, the pooled 2q8 (2 mg every 2 months after 3 initial monthly doses) groups of the VIEW studies received a mean of 7.5 injections up to week 52 (fixed dosing) and of 11.2 injections up to week 96 (PRN capped to maximum intervals of 12 weeks) [5, 10]. While the differences in numbers of injections between T&E and fixed dosing in this study are clinically meaningful, they are modest. This represents a limitation of the T&E regimen, being unable to immediately reduce the number of injections because of a very gradual incremental extension of treatment intervals. In AZURE, it also reflects the conservative approach taken by investigators when applying the T&E regimen according to their assessment of visual and anatomic outcomes. Previous studies have demonstrated the noninferiority of ranibizumab T&E compared with fixed dosing regarding visual outcomes, with fewer injections administered in the T&E group [11, 12]. In CANTREAT, during the first year, patients treated with a T&E regimen received 9.4 injections while patients on fixed dosing received 11.8 injections, increasing to 17.6 vs. 23.5, respectively, during the second year [11]. The TREND study reported a mean of 8.7 injections for T&E compared to 11.1 for fixed dosing during the first year of treatment [12]. AZURE, with a mean of 6.0 injections over 52 weeks, showed that it is possible to treat patients with nAMD using an IVT-AFL regimen with fewer injections than with fixed dosing, resulting in reduced treatment burden. Importantly, AZURE demonstrates that receiving fewer IVT-AFL injections does not put patients at risk of undertreatment or compromise treatment outcomes compared with fixed dosing.
Based on the results from randomized controlled trials, T&E has become an option in routine clinical practice. However, there is currently no formal consensus on the optimal criteria for interval adjustment in T&E regimens. The implementation of T&E varies among physicians globally, and criteria vary across studies. In ARIES, treatment interval extension was based on anatomic criteria [7], whereas in ALTAIR, if none of the shortening criteria were met and there was no fluid on OCT, the treatment interval could be extended [8]. Comparatively, an investigator-driven decision-making approach was adopted in AZURE, with treatment intervals extended, according to assessment of visual and anatomic outcomes by the investigator, with no maximum limit. This approach reflected standard practices when the study was conducted, and it generates important understanding of how ophthalmologists treated patients within the scope of the EU label. While it observed smaller proportions of patients achieving extended treatment intervals than studies where such criteria were clearly defined, it led to comparable reductions in number of injections. Retreatment criteria influence the proportion of patients achieving different treatment intervals and require further investigation to establish specific clinical guidance for IVT-AFL T&E regimens. In terms of limitations, AZURE, like other similar studies [11], was an open-label study, since the flexibility of adjustment treatment intervals makes is impractical to mask patients and physicians to treatment assignments through sham injections. The number of patients included was relatively small compared with pivotal trials, and many patients (n = 116) failed screening for various protocol-specified reasons. Inclusion only of patients with at least 1 year of previous treatment may have led to some selection bias in the patient population enrolled; however, these inclusion criteria were selected to ensure reasonable compliance with the approved IVT-AFL label and enrollment of a patient population most relevant to answering the research question and for whom efficacy has previously been demonstrated [5, 10]. While other studies take stricter approaches in setting extension criteria, the investigator-driven decision-making approach adopted in AZURE aimed to ascertain and evaluate how investigators use T&E in routine clinical practice.
Conclusions
The AZURE study results demonstrate noninferiority of IVT-AFL T&E compared to fixed dosing, covering a period up to 2.5 years of treatment through enrollment of patients with nAMD who had previously received ≥ 1 year of fixed-dosing IVT-AFL treatment. It supports the results from ARIES and ALTAIR by formally confirming for the first time the noninferiority of changing to IVT-AFL T&E compared to continuous fixed dosing. AZURE shows that T&E dosing regimens can be personalized to the needs of each patient and enables them to achieve maintenance of BCVA gains comparable to those achieved with fixed dosing, with comparable safety and while reducing treatment burden. Thus, AZURE substantiates proactive, individualized T&E as a preferred regimen to reduce the treatment burden associated with anti-VEGF injections while maintaining improvements in functional and anatomic outcomes in patients with nAMD.
Data Availability
Professor Laurent Kodjikian and Sergio Leal had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Thomas Schmelter conducted and is responsible for the data analysis. Availability of the data underlying this publication will be determined later according to Bayer’s commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing”. This pertains to scope, time point and process of data access. As such, Bayer commits to sharing upon request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the United States (US) and European Union (EU) as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after January 1, 2014. Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the Study sponsors section of the portal. Data access will be granted to anonymized patient-level data, protocols and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
References
Gale RP, Mahmood S, Devonport H, Patel PJ, Ross AH, Walters G, et al. Action on neovascular age-related macular degeneration (nAMD): recommendations for management and service provision in the UK hospital eye service. Eye (Lond). 2019;33(Suppl 1):1–21.
Lanzetta P, Loewenstein A, Vision ASC. Fundamental principles of an anti-VEGF treatment regimen: optimal application of intravitreal anti-vascular endothelial growth factor therapy of macular diseases. Graefes Arch Clin Exp Ophthalmol. 2017;255(7):1259–73.
Patel PJ, Devonport H, Sivaprasad S, Ross AH, Walters G, Gale RP, et al. Aflibercept treatment for neovascular AMD beyond the first year: consensus recommendations by a UK expert roundtable panel, 2017 update. Clin Ophthalmol. 2017;11:1957–66.
Khurana RN, Rahimy E, Joseph WA, Saroj N, Gibson A, Vitti R, et al. Extended (every 12 weeks or longer) dosing interval with intravitreal aflibercept and ranibizumab in neovascular age-related macular degeneration: post hoc analysis of VIEW trials. Am J Ophthalmol. 2019;200:161–8.
Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, Nguyen QD, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121(1):193–201.
Bayer. Eylea (aflibrecept) Summary of Product Characteristics 2018. https://www.ema.europa.eu/documents/product-information/eylea-epar-product-information_en.pdf. Accessed Oct 2023.
Paul M, Frank GH, Philip H, Edoardo M, Eric S, Helmut A, et al. Efficacy and safety of intravitreal aflibercept using a treat-and-extend regimen for neovascular age-related macular degeneration: the ARIES study. Retina. 2021;41:1911–20.
Ohji M, Takahashi K, Okada AA, Kobayashi M, Matsuda Y, Terano Y, et al. Efficacy and safety of intravitreal aflibercept treat-and-extend regimens in exudative age-related macular degeneration: 52- and 96-week findings from ALTAIR. Adv Ther. 2020;37(3):1173–87.
Garweg JG, Stefanickova J, Hoyng C, Niesen T, Schmelter T, Leal S, et al. Dosing regimens of intravitreal aflibercept for diabetic macular edema beyond the first year: VIOLET, a prospective randomized trial. Adv Ther. 2022;39(6):2701–16.
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–48.
Kertes PJ, Galic IJ, Greve M, Williams G, Baker J, Lahaie M, et al. Efficacy of a treat-and-extend regimen with ranibizumab in patients with neovascular age-related macular disease: a randomized clinical trial. JAMA Ophthalmol. 2020;138(3):244–50.
Silva R, Berta A, Larsen M, Macfadden W, Feller C, Mones J, et al. Treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: results with ranibizumab from the TREND study. Ophthalmology. 2018;125(1):57–65.
Acknowledgements
The authors thank all the patients and investigators who participated in the AZURE study.
Medical Writing and Editorial Assistance.
Medical writing and editorial support for the preparation of this manuscript, under the direction of the authors, was provided by Charlotte Head and Luke Shelton of ApotheCom (London) and funded by Bayer Consumer Care AG, Pharmaceuticals, Switzerland, in accordance with Good Publication Practice guidance (Ann Intern Med 2015;163:461–464).
Funding
The AZURE study, and the journal’s Rapid Service and Open Access fees, was sponsored by Bayer AG, Leverkusen, Germany. The sponsor participated in the design of the study, conducting of the study, data collection, data management, data analysis, data interpretation and preparation, review and approval of the manuscript.
Author information
Authors and Affiliations
Contributions
Laurent Kodjikian, Lluís Arias Barquet, András Papp, Peter J. Kertes, Edoardo Midena, Jan Ernest, Rufino Silva, Thomas Schmelter, Tobias Niesen, and Sergio Leal all contributed to the study conception and design. Data were collected by Laurent Kodjikian, Lluís Arias Barquet, András Papp, Peter J. Kertes, Edoardo Midena, Jan Ernest, and Rufino Silva. Statistical analysis of the data was performed by Thomas Schmelter. All named authors were involved in the interpretation of data and critical review of the manuscript at all stages.
Corresponding author
Ethics declarations
Conflict of Interest
Laurent Kodjikian: Consultant for AbbVie/Allergan, Alimera/Horus, Bayer, Novartis, Roche, and Thea. Lluís Arias Barquet: Consultant for Allergan, Bayer, Novartis, Roche, and Topcon. András Papp: Consultant for Bayer, Novartis, and Roche; received travel grants from Novartis; and his department has been involved in the conduct of several studies sponsored by Novartis, Bayer, Ophthotech/Iveric Bio, Samsung Bioepis, Oculis, Amgen, Qilu, Chengdu Kanghong, Roche, PanOptica, Xbrane, Genentech, Bioeq, Allergan, ThromboGenics, Regeneron, and Clearside Biomedical. Peter J. Kertes: Honoraria: Novartis, Bayer, Roche, Boehringer Ingelheim, Pfizer, and Zeiss; Advisory board: Novartis, Bayer, Roche, and Novelty Nobility; Financial support (to institution): Roche, Novartis; Equity owner: ArcticDx. Edoardo Midena: Served as a steering committee member for Bayer. Jan Ernest: Nothing to disclose. Rufino Silva: Advisory Board: AbbVie, Alimera, Bayer, Novartis, Thea, Roche, and Novo Nordisk. Thomas Schmelter: Employee of Bayer. Tobias Niesen: Employee of Bayer. Sergio Leal: Employee of Bayer. No authors of this manuscript have any proprietary interests.
Ethical Approval
The study was conducted at 64 sites in Europe and Canada from September 2015 to June 2020, in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines E6: Good Clinical Practice. The study protocol and statistical analysis plan can be accessed at ClinicalTrials.gov (identifier: NCT02540954). The protocol was approved by the independent ethics committee or institutional review board at each study site. All patients provided written informed consent.
Additional information
Prior Presentation: The Association for Research in Vision and Ophthalmology (ARVO) Congress; Virtual, May 1–7, 2021. The 21st European Society of Retina Specialists (EURETINA) Congress; Virtual, September 9–12, 2021.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kodjikian, L., Arias Barquet, L., Papp, A. et al. Intravitreal Aflibercept for Neovascular Age-Related Macular Degeneration Beyond One Year of Treatment: AZURE, a Randomized Trial of Treat-and-Extend vs. Fixed Dosing. Adv Ther 41, 1010–1024 (2024). https://doi.org/10.1007/s12325-023-02719-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02719-3