Abstract
Introduction
The aim of this post hoc analysis of the ARIES study is to explore the requirement for intravitreal aflibercept (IVT-AFL) treatment intervals of < 8 weeks (w) in patients with neovascular age-related macular degeneration (nAMD), and to assess vision and anatomic outcomes in such patients who require more intensive treatment.
Methods
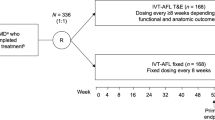
ARIES was a multicenter, randomized, phase 3b/4 study that investigated the efficacy of two IVT-AFL proactive, individualized, treat-and-extend regimens over 2 years in treatment-naïve patients with nAMD. Patients were determined as injection-intensive if the study investigator identified that a treatment interval of < 8 w was needed and if they had ≥ 1 interval of < 8 w after three initial monthly doses. Treatment intervals could be extended subsequently if extension criteria were met. This is a post hoc analysis of patients enrolled in ARIES and statistical analysis is descriptive.
Results
Of 269 patients in the combined treatment arms, 23.0% (n = 62) were injection-intensive (Year 1: 13.8% [n = 37]; Year 2: 9.3% [n = 25]). Time from IVT-AFL initiation to injection-intensive determination varied (range, 16–100 w; median: 43.2 w). Mean treatment interval was 8.4 w before and 6.1 w after injection-intensive determination. Overall, 59.7% achieved treatment intervals of ≥ 8 w following injection-intensive determination. Vision improvements from baseline to Week 104 were smaller for injection-intensive patients than non–injection-intensive patients (mean [SD] best-corrected visual acuity change: + 2.3 [15.6] vs. + 5.9 [12.3] letters). Anatomic outcomes were similar between injection-intensive and non–injection-intensive patients (central retinal thickness change from baseline to Week 104: − 160 [154] vs. − 167 [136] µm).
Conclusions
In ARIES, 23% of treatment-naïve patients with nAMD experienced at least one treatment interval of < 8 w. Injection-intensive patients showed improved vision and anatomic outcomes. For most, treatment intervals could be extended to ≥ 8 w following injection-intensive determination. ClinicalTrials.gov Identifier: NCT02581891.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
It is important that the outcomes of patients with neovascular age-related macular degeneration (nAMD) who require intravitreal aflibercept (IVT-AFL) administered with a treatment interval of < 8 weeks at least once are better understood, and to recognize what proportion of patients could benefit from more frequent dosing than every 8 weeks during the first year of IVT-AFL treatment. |
This post hoc analysis of the ARIES study explored the need for IVT-AFL treatment intervals of < 8 weeks in patients with nAMD, and assessed vision and anatomic outcomes in patients determined as injection-intensive. |
What was learned from the study? |
In ARIES, 23% of treatment-naïve patients with nAMD experienced at least one treatment interval of < 8 weeks; injection-intensive patients showed improved vision and anatomic outcomes, and treatment intervals could subsequently be extended to ≥ 8 weeks following injection-intensive determination for most patients. |
This post hoc analysis suggests that, at times, more frequent than every-8-week IVT-AFL treatment may be deemed necessary; however, this does not restrict a patient’s potential to subsequently extend treatment intervals, and the requirement for injection-intensive treatment intervals is transient for the majority. |
Injection-intensive treatment intervals may be included as part of a proactive, individualized, treat-and-extend IVT-AFL regimen when treating patients with nAMD in routine clinical practice to enable them to achieve improved outcomes. |
Introduction
The duration of intraocular vascular endothelial growth factor (VEGF) inhibition achieved with anti-VEGF therapy varies from individual to individual [1]. Previous studies have determined an average duration of approximately 10 weeks for inhibition of intraocular VEGF in eyes with neovascular age-related macular degeneration (nAMD) treated with intravitreal aflibercept (IVT-AFL), thus supporting durable VEGF inhibition in most patients treated with IVT-AFL every 8 weeks, as per prescribing recommendations [1]. However, post hoc analyses of the pivotal IVT-AFL VIEW 1 and VIEW 2 trials and clinical experience have suggested that some patients may benefit from IVT-AFL treatment administered more frequently than every 8 weeks, given that approximately 20% of eyes initially treated with IVT-AFL had early persistent fluid present after three initial monthly doses [2].
In the VIEW studies, IVT-AFL was administered in a fixed dosing regimen in Year 1 and capped pro re nata (PRN) dosing was used in year 2; however, proactive, individualized treat-and-extend (T&E) regimens with anti-VEGF agents, such as IVT-AFL and ranibizumab, are becoming increasingly popular for the management of nAMD [3]. Regardless of the treatment regimen used, effective inhibition of VEGF is key to optimizing treatment outcomes in patients with nAMD. Although the principle of a T&E regimen is to extend treatment intervals while maintaining best-corrected visual acuity (BCVA) and central retinal thickness (CRT) outcomes, an advantage of such a flexible regimen is that it permits the shortening of treatment intervals, when necessary, to maintain outcomes.
The recent 2-year ARIES study was, to our best knowledge, the first randomized clinical trial of IVT-AFL administered using a T&E regimen that allowed the requirement for injection-intensive treatment (treatment intervals < 8 weeks) to be evaluated during the first 2 years of treatment [4]. ARIES demonstrated similar clinical outcomes between treatment-naïve patients with nAMD treated with an IVT-AFL early start (from week 16) or late start (from week 48) IVT-AFL T&E regimen following initial dosing. Approximately half of patients achieved a last treatment interval of ≥ 12 weeks at Week 104. The study protocol stipulated that treatment intervals should not be < 8 weeks unless patients were considered by the investigator to require injections more frequently. If the study investigator identified the need for a treatment interval of < 8 weeks at least once after randomization into the trial, and if the patients had at least one treatment interval of < 8 weeks, the patients were considered to be injection-intensive and continued in the study, although they were excluded from the per-protocol analyses. Injection-intensive patients could be extended again to longer treatment intervals, based on investigator judgment and, in that respect, the regimen is reflective of routine clinical practice and clinical guidelines for IVT-AFL T&E [5].
It is important that the outcomes of such injection-intensive patients with nAMD are better understood, and to recognize what proportion of patients could benefit from more frequent IVT-AFL dosing than every 8 weeks during the first year of treatment. This post hoc analysis of the ARIES study explores the requirement, as per investigator decision, for IVT-AFL to be administered more frequently than every 8 weeks, at least once during the ARIES study duration. The frequency, point in time, and reasons for an IVT-AFL treatment interval of < 8 weeks were evaluated, and BCVA (vision) and CRT (anatomic) outcomes in such injection-intensive patients with nAMD were assessed.
Methods
The ARIES Study
ARIES (NCT02581891) was a multicenter, randomized, phase 3b/4 study that compared the efficacy of two different IVT-AFL T&E dosing regimens over 2 years in treatment-naïve patients with nAMD, published previously [4]. Briefly, patients received 2 mg IVT-AFL at Week 0, Week 4, Week 8, and Week 16. At Week 16, patients were stratified based on BCVA outcomes (< 8 or ≥ 8 letters gained in BCVA) and randomized 1:1 to early-start T&E or late-start T&E arms. Treatment intervals were extended by 2 weeks each time, up to a maximum of 16 weeks if the criteria were met. The predefined extension, maintenance, or shortening criteria are listed in the Supplementary Material.
The ARIES study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines E6: Good Clinical Practice. The protocol and any amendments were approved by the independent ethics committee/institutional review board at each site. All patients provided written informed consent to participate in the study.
The Injection-Intensive Analysis
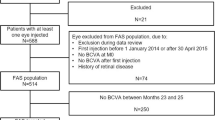
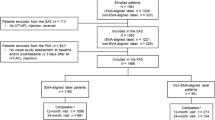
This post hoc analysis was performed on the full analysis set (FAS), as the per-protocol population (on which the ARIES primary endpoint was based) excluded injection-intensive patients. The FAS was defined as all randomized patients who received IVT-AFL and had a BCVA assessment at Week 16 and at least one additional post-Week 16 BCVA assessment; both treatment arms were combined. Patients were determined by the study investigator to be injection-intensive (injection-intensive determination) if they identified the need for a treatment interval of < 8 weeks at least once after randomization into the trial, and if the patients had at least one treatment interval of < 8 weeks, even if they were subsequently extended beyond 8-week treatment intervals. The first visit when a patient was determined as injection-intensive is subsequently described as “the injection-intensive visit.”
The injection-intensive visit (a treatment interval of < 49 days since the previous injection) could occur at any time from randomization (Week 16) to Week 104. The first shortened interval could be 4 weeks (21–35 days) or 6 weeks (36–48 days), with patients categorized as receiving an 8-week interval if treatment was given after 49–63 days. Treatment intervals were not permitted to drop below 4 weeks. In the early-start T&E arm, patients with an injection-intensive visit could be subsequently returned to a T&E regimen with treatment intervals of > 8 weeks, where appropriate, following an injection-intensive interval in Year 1. In the late-start T&E arm, patients with an injection-intensive visit could be subsequently extended back to 8-week treatment intervals after an injection-intensive interval in Year 1, but treatment intervals could not be extended further until after Week 48. In this analysis, the percentage of injection-intensive patients in Year 1 and 2 was evaluated, as well as the timing of the injection-intensive requirement; BCVA and CRT outcomes were also evaluated in injection-intensive patients, including the proportion of patients with visual gains and losses at end of study (Week 104) overall and per baseline BCVA subgroup level (high, ≥ 70 letters; intermediate, 55–69 letters; low, < 55 letters). Due to the exploratory nature of these analyses, data are reported descriptively.
Results
Patients
The overall patient disposition for ARIES, as well as their baseline demographics and disease characteristics, have been published previously [4]. Of the 269 patients included in the ARIES FAS, 23.0% (n = 62) of patients were determined to be injection-intensive at some point during the ARIES study. A total of 16.1% (n = 10) of patients had a single injection-intensive visit (a treatment interval of < 49 days since the previous visit), 8.1% (n = 5) had two injection-intensive visits, and 75.8% (n = 47) had more than two injection-intensive visits. In Year 1 of ARIES, overall, 13.8% (n = 37) of patients were determined to be injection-intensive and a further 9.3% (n = 25) of patients in Year 2 received this determination.
There were no relevant differences in the key baseline characteristics between injection-intensive and non–injection-intensive patients (see Table S1).
Treatment Interval and Injection-Intensive Status
The time from IVT-AFL initiation to the visit where patients were determined as injection-intensive varied considerably (range, 16–100 weeks; median of 43.2 weeks) (Fig. 1).
The mean last treatment interval immediately prior to the timepoint at which patients were determined to be injection-intensive was 8.4 weeks (Table 1). Most patients (90.3%; n = 56) had a last treatment interval before the injection-intensive visit of 8 weeks; 4.8% (n = 3) of patients became injection-intensive after a previous treatment interval of 10 weeks, and 3.2% (n = 2) of patients became injection-intensive after a previous treatment interval of 12 weeks. One patient had a serious adverse event (atrioventricular block) that led to the interruption of IVT-AFL, and consequently had an interval of 20 weeks before being determined to be injection-intensive; subsequently, the treatment interval was extended, and the patient completed the study with a 12-week extension interval.
The first injection-intensive interval was 4 weeks for 43.5% of patients; for the remaining patients (56.5%), the first shortened interval was 6 weeks (Table 2). The mean treatment interval following injection-intensive determination was 6.1 weeks. Following injection-intensive determination, 59.7% (n = 37) of patients achieved at least one treatment interval of ≥ 8 weeks (Fig. 2). The mean (standard deviation [SD]) length of the longest treatment interval following the injection-intensive visit was 9.2 (4.2) weeks for patients determined to be injection-intensive in the first year of treatment, and 7.0 (1.6) weeks for patients determined to be injection-intensive in the second year, who did not have as long to extend treatment intervals following the injection-intensive visit before the last study visit at Week 104. Consistent with this observation, in patients determined to be injection-intensive in the first year, 40.5% (n = 15) of patients achieved a treatment interval of ≥ 10 weeks, and 21.6% (n = 8) achieved a treatment interval of ≥ 12 weeks after the injection-intensive visit; the longest observed treatment interval after the injection-intensive visit was 10 weeks for patients determined to be injection-intensive in the second year (8.0% [n = 2] of second-year injection-intensive patients).
BCVA and CRT Outcomes
In injection-intensive patients, mean (SD) BCVA at the injection-intensive visit was 61.9 (16.7) letters compared with 65.7 (12.6) letters at Week 16; mean CRT was 411 (112) µm and 379 (113) µm, respectively (Fig. 3). At the end of the study (Week 104), mean (SD) BCVA was 62.6 (18.7) letters and CRT was 337 (101) µm in injection-intensive patients. Mean (SD) BCVA change from baseline to Week 104 was + 2.3 (15.6) letters in injection-intensive patients and + 5.9 (12.3) letters in non–injection-intensive patients. CRT outcomes at Week 104 were similar between injection-intensive and non–injection-intensive patients, with mean (SD) CRT decreases from baseline of 160 (154) µm and 167 (136) µm, respectively.
a BCVA and b CRT outcomes by injection-intensive status. Full analysis set. Last observation carried forward. Injection-intensive visit could occur at any time from randomization (Week 16) to Week 104. BCVA best-corrected visual acuity, BL baseline, CRT central retinal thickness, SEM standard error of the mean
Overall, more than half of the injection-intensive patients (51.6%; 32/62) gained at least five letters by the end of the study (see Fig. S1). At least five letters were gained by 41% (7/17) of the injection-intensive patients with high baseline visual acuity (≥ 70 letters), 65% (17/26) of those with intermediate baseline visual acuity (55–69 letters), and approximately 42% (8/19) of those with low baseline visual acuity (< 55 letters).
Safety
The overall safety of patients in the ARIES study has been previously reported [4], with no cases of endophthalmitis or retinal vasculitis observed. There were no identified differences observed in terms of treatment-emergent adverse events between injection-intensive and non–injection-intensive patient groups (see Table S2).
Discussion
This post hoc analysis of the ARIES study provides valuable insights into the proportion and treatment outcomes of patients with nAMD who require IVT-AFL administered in a treatment interval of less than 8 weeks. Our first observation was that 23% of treatment-naïve patients with nAMD required IVT-AFL more frequently than every 8 weeks at some point during treatment; there was a relatively linear relationship between duration of IVT-AFL and the proportion of patients requiring an injection-intensive interval, indicating that this need may arise at any point during the first 2 years of treatment. The larger numbers of patients with such intervals observed at Week 16, Week 20, and Week 24 (as displayed in Fig. 1), are largely driven by the synchronicity of the 8-weekly treatment intervals in the late-start T&E arm in Year 1. Secondly, although 23% of patients were determined to be injection-intensive at some point over the 2 years of the ARIES study, more than half (60%) achieved at least one treatment interval of ≥ 8 weeks, after their injection-intensive visit, and two patients achieved a maximum treatment interval of ≥ 16 weeks. Finally, improvements in both BCVA and CRT outcomes were observed at the end of study, both from the injection-intensive visit and from baseline, indicating that an injection-intensive episode does not imply that such patients are necessarily poor responders; in fact, more than half of the injection-intensive patients gained at least five letters by the end of the study, while BCVA remained stable in another 26% of patients.
The results demonstrate that baseline visual acuity does not provide an indication of whether patients will be more or less likely to respond to treatment (see Fig. S1). In fact, the results suggest that the requirement for an injection-intensive interval cannot be predicted from patient baseline characteristics, as there were no key differences between injection-intensive and non–injection-intensive groups. A difference was observed in the length of the longest treatment interval following the injection-intensive visit between patients who were determined to be injection-intensive in the first and second years of treatment. Patients determined as injection-intensive in the first year achieved longer treatment intervals following the injection-intensive visit, a fact that is likely due, at least in part, to the insufficient time before the final study visit at Week 104 to extend treatment intervals after an injection-intensive visit occurring later in the course of the study.
Notably, the determination of injection-intensive status (i.e., if the study investigator identified a need for, and the patient had, a treatment interval of < 49 days since the previous visit) used in this analysis is likely to be different, and more conservative, compared with how anti-VEGF treatment would be considered intensive in routine clinical practice. Over a 2-year treatment period, the need for more intensive treatment would be more likely to be considered clinically relevant if patients required at least 2 consecutive shorter treatment intervals (< 8 weeks) and could not subsequently be extended beyond 8-week treatment intervals.
In the VIEW studies, which compared fixed dosing every 4 weeks (q4w) and every 8 weeks in Year 1, both IVT-AFL treatment regimens were considered to be equivalent to ranibizumab 0.5 mg q4w and additional efficacy with more frequent dosing was not demonstrated in most patients [6]. By the end of Week 96 in VIEW (capped PRN), 2–4% of patients required all injections on a monthly basis, and a third of patients required at least one injection with a treatment interval of only one month. As previously noted, post hoc analysis of the VIEW trials and clinical experience have suggested that some patients may benefit from dosing of IVT-AFL more frequently than every 8 weeks, given that approximately 20% of eyes initially treated with IVT-AFL had early persistent fluid present after the three initial monthly doses [2]. There are some regional variations in IVT-AFL prescribing recommendations for nAMD based on these data, with those in the USA, Canada, and Australia acknowledging that some patients may require every-4-week (monthly) dosing after the first 12 weeks (3 months) [7]. In Europe, IVT-AFL prescribing recommendations have been recently revised based on the data presented here, to recognize that treatment intervals shorter than every 8 weeks may be needed in some patients during the course of nAMD management [8]. In contrast, prescribing recommendations in some Latin American countries (e.g., Argentina, Colombia), and some Asian countries (e.g., China, South Korea) currently have a recommendation of dosing every 8 weeks or more.
The duration of intraocular VEGF inhibition achieved with IVT-AFL treatment varies in patients with nAMD [1]; therefore, there is a clear need for a truly individualized treatment regimen, where treatment intervals can be adjusted upwards and downwards to optimize VEGF inhibition and preserve vision outcomes. The ARIES and ALTAIR studies, which both compared different T&E approaches, supported the efficacy and safety of IVT-AFL treatment in a T&E regimen as an alternative to fixed dosing in the first year [4, 9]. The ALTAIR study was conducted in Japanese patients with treatment-naïve nAMD, and demonstrated similar outcomes to those achieved in the VIEW studies; in ALTAIR, three initial monthly IVT-AFL injections were administered, followed by one injection after a further 2 months, and subsequently the adoption of a T&E regimen with treatment intervals altered in 2- or 4-week increments up to a maximum treatment interval of 16 weeks (according to prespecified criteria). The injection-intensive requirement could not be investigated in ALTAIR, as the protocol stipulated that the minimum treatment interval must not be < 8 weeks during the entire study period [9]. This ARIES post hoc analysis further substantiates the benefits of a proactive, individualized T&E regimen, as it means that injection-intensive patients can be identified, and treatment tailored to the needs of each patient throughout the course of their disease management. As an alternative to decreasing treatment intervals to optimize outcomes, the principle of increasing the administered dose to 8 mg has been investigated in the phase 2 CANDELA study [10], with results of phase 3 studies expected to be reported later this year.
We must acknowledge that this was a post hoc analysis, and the ARIES study was not designed to compare outcomes in injection-intensive and non–injection-intensive populations. Additionally, as discussed above, the protocol-specified definition of treatment intensiveness (requirement of one treatment interval of < 8 weeks only) is rather conservative compared to what might be considered intensive in the clinical setting, where a single treatment interval < 8 weeks can also be needed for logistical reasons. It should also be noted that the determination of injection-intensive status used in the ARIES study was not based on a predefined set of criteria but left at the discretion of the investigator, which aligns with routine clinical practice. Furthermore, some apparent observations, specifically the shorter treatment intervals subsequently achieved in patients determined to require injection-intensive treatment in Year 2, are impacted by when the injection-intensive visit occurred, as there is a shorter timeframe for these patients to extend their treatment intervals again before the end of the study.
Conclusions
This post hoc analysis is the first known analysis to evaluate injection-intensive patients within an IVT-AFL T&E clinical trial setting. The results suggest that, at times, IVT-AFL treatment more frequent than every 8 weeks may be deemed necessary for some patients with active nAMD. However, this does not restrict a patient’s potential to subsequently extend treatment intervals, as the requirement for injection-intensive treatment intervals is transient for the majority of patients. Thus, injection-intensive treatment intervals may be included as part of a proactive, individualized T&E IVT-AFL regimen when treating patients with nAMD in routine clinical practice to enable them to achieve improved vision and anatomic outcomes.
References
Fauser S, Schwabecker V, Muether PS. Suppression of intraocular vascular endothelial growth factor during aflibercept treatment of age-related macular degeneration. Am J Ophthalmol. 2014;158:532–6.
Jaffe GJ, Kaiser PK, Thompson D, et al. Differential response to anti-VEGF regimens in age-related macular degeneration patients with early persistent retinal fluid. Ophthalmology. 2016;123:1856–64.
Lanzetta P, Loewenstein A, Vision Academy Steering Committee. Fundamental principles of an anti-VEGF treatment regimen: optimal application of intravitreal anti-vascular endothelial growth factor therapy of macular diseases. Graefes Arch Clin Exp Ophthalmol. 2017;255:1259–73.
Mitchell P, Holz GF, Hykin P, et al. Efficacy and safety of intravitreal aflibercept using a treat-and-extend regimen for neovascular age-related macular degeneration: the ARIES study. Retina. 2021;41:1911–20.
Ross AH, Downey L, Devonport H. Recommendations by a UK expert panel on an aflibercept treat-and-extend pathway for the treatment of neovascular age-related macular degeneration. Eye (Lond). 2020;34:1825–34.
Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121:193–201.
Eylea [prescribing information]. In. Tarrytown, NY: Regeneron Pharmaceuticals, Inc. 2021.
Aflibercept (Eylea) summary of product characteristics, Nov 2021. https://www.ema.europa.eu/en/documents/product-information/eylea-epar-product-information_en.pdf. Accessed June 2022.
Ohji M, Takahashi K, Okada AA, et al. Efficacy and safety of intravitreal aflibercept treat-and-extend regimens in exudative age-related macular degeneration: 52- and 96-week findings from ALTAIR. Adv Ther. 2020;37:1173–87.
Brown DM. Evaluation of 8 mg intravitreal aflibercept injection for neovascular age-related macular degeneration: results from the phase 2 CANDELA study. Association for Research in Vision and Ophthalmology (ARVO) 2022.
Acknowledgements
Funding
The ARIES study was sponsored by Bayer AG, Leverkusen, Germany. This post hoc analysis and the journal’s Rapid Service Fees were funded by Bayer Consumer Care AG, Basel, Switzerland.
Medical Writing and Editorial Assistance
Medical writing and editorial support for the preparation of this manuscript, under the direction of the authors, was provided by Charlotte Head, ApotheCom (London, United Kingdom), and funded by Bayer Consumer Care AG, Basel, Switzerland, in accordance with Good Publication Practice (GPP3) guidance (Ann Intern Med 2015;163:461–464).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception, design, and interpretation of the results. Helmut Allmeier, George Lambrou, and Tobias Machewitz performed the data curation and analyses. All authors provided critical review of the manuscript, and all authors read and approved the final manuscript.
Prior Presentation
The Association for Research in Vision and Ophthalmology (ARVO) Virtual Meeting, May 1–7, 2021; German Retina Society Congress (GRSC) Meeting, Munich, Germany, June 25–26, 2021.
Disclosures
Sebastian Wolf has served as a Steering Committee member for Bayer; consultant for Allergan, Bayer, Boehringer Ingelheim, Chengdu Kanghong Biotech, Heidelberg Engineering, Novartis, Oxurion, Roche, and Zeiss; and has received grant support from Heidelberg Engineering. Frank G. Holz has served as a Steering Committee member for Bayer; has served as a consultant for Acucela, Apellis Pharmaceuticals, Bayer, Bioeq/Formycon AG, Boehringer Ingelheim, Chengdu Kanghong Pharmaceuticals, Geuder AG, Graybug Vision, Heidelberg Engineering, Lin Bioscience, Novartis, Oxurion, Pixium Vision, Roche/Genentech, and Stealth BioTherapeutics; has received research support from Acucela, Apellis Pharmaceuticals, Bayer, Bioeq/Formycon AG, CenterVue, Chengdu Kanghong Pharmaceuticals, Heidelberg Engineering, NightstaRx, Novartis, Optos, Pixium Vision, Roche/Genentech, and Zeiss Pharma; and has received honoraria or travel reimbursement from Acucela, Apellis Pharmaceuticals, Bayer, Ellex, Graybug Vision, Heidelberg Engineering, Lin Bioscience, Novartis, Oxurion, Pixium Vision, Roche/Genentech, Stealth BioTherapeutics, and Zeiss Pharma. Edoardo Midena has served as a Steering Committee member for Bayer. Eric Souied has served as a Steering Committee member for Bayer; an expert consultant for Allergan/AbbVie, Bayer, Novartis, Roche, and Teva. George Lambrou, Tobias Machewitz, and Helmut Allmeier are employees of Bayer. Paul Mitchell has served as a Steering Committee member for Bayer and has received consulting fees from Allergan, Bayer, and Novartis.
Compliance with Ethics Guidelines
This article is based on a previously conducted study (ARIES; NCT02581891) and does not contain any new studies with human participants or animals performed by any of the authors. The ARIES study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines E6: Good Clinical Practice. The protocol and any amendments were approved by the independent ethics committee/institutional review board at each site. All patients provided written informed consent to participate in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available. Availability of the data underlying this publication will be determined according to Bayer’s commitment to the EFPIA/PhRMA, “Principles for responsible clinical trial data sharing.” This pertains to scope, time point, and process of data access. As such, Bayer commits to sharing, upon request from qualified scientific and medical researchers, patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the United States (US) and European Union (EU) as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after January 1, 2014. Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the Study sponsors section of the portal. Data access will be granted to anonymized patient-level data, protocols and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
Patients and Investigators
The authors thank all the patients and investigators who participated in the ARIES study.
Author information
Authors and Affiliations
Consortia
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wolf, S., Holz, F.G., Midena, E. et al. Patients with Neovascular Age-Related Macular Degeneration Requiring Intensive Intravitreal Aflibercept Treatment: An ARIES Post Hoc Analysis. Ophthalmol Ther 11, 1793–1803 (2022). https://doi.org/10.1007/s40123-022-00541-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00541-8