Abstract
Introduction
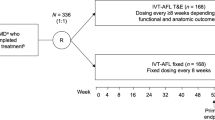
The purpose was to compare two flexible regimens of intravitreal aflibercept (IVT-AFL) with fixed dosing every 8 weeks, beyond the first year of treatment, in patients with diabetic macular edema (DME). VIOLET was a 100-week, randomized, Phase IIIb, non-inferiority study in patients with center-involving DME previously treated with IVT-AFL for ≥ 1 year according to the European label.
Methods
Patients received an initial dose of IVT-AFL at study baseline and were randomly assigned (1:1:1) to treat-and-extend (T&E), pro re nata (PRN), or fixed regimens. The primary endpoint was mean change in best-corrected visual acuity (BCVA) from baseline (randomization) to Week 52.
Results
Full analysis set comprised 458 patients (baseline mean BCVA: 72.5, 71.0, and 72.7 letters in the T&E, PRN, and fixed-dose groups, respectively). Patients received a mean (min–max) of 10.0 (2–14; T&E), 11.5 (1–25; PRN), and 12.3 (3–13; fixed) injections over 100 weeks, with 13.3 (4–23), 25.0 (3–29), and 16.1 (5–25) clinic visits, respectively. At Week 52, mean (± standard deviation) BCVA changes from baseline were + 0.5 ± 6.7 (T&E), + 1.7 ± 6.8 (PRN), and + 0.4 ± 6.7 (fixed-dosing) letters (least squares mean difference [95% confidence interval]: T&E 0.01 [− 1.46, 1.47] and PRN 0.95 (− 0.52, 2.42) letters versus fixed dosing; p < 0.0001 for both non-inferiority tests [4-letter margin]). The IVT-AFL safety profile was consistent with previous studies.
Conclusion
The treatment burden associated with intravitreal injections for DME is lowest with T&E regimens, but there are a range of flexible IVT-AFL dosing regimens, allowing physicians to adopt an individualized treatment plan.
Trial Registration
ClinicalTrials.gov identifier: NCT02818998.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
There is a lack of data regarding the best regimen to optimize functional and anatomical outcomes with long-term intravitreal aflibercept (IVT-AFL) treatment in patients with diabetic macular edema |
What did the study ask? / What was the hypothesis of the study? |
In the phase 3b, randomized, non-inferiority VIOLET study, we investigated whether flexible IVT-AFL dosing (treat and-extend [T&E] or pro re nata [PRN]) was comparable to fixed dosing every 8 weeks (q8w), beyond the first year of treatment in patients with DME |
What was learned from the study? |
In patients who previously received ≥ 1 year of IVT-AFL for DME, both flexible IVT-AFL treatment regimens (T&E and PRN) achieved similar functional outcomes to fixed dosing (IVT-AFL q8w) at Week 52 (primary endpoint) and Week 100. The safety profile was consistent with that known for IVT AFL |
A range of IVT-AFL dosing regimens allow selection of individualized treatment plans |
Introduction
Diabetic macular edema (DME) is a manifestation of diabetic retinopathy and the leading cause of visual impairment in people with diabetes [1]. DME prevalence is predicted to increase over time due to rising rates of diabetes, an aging population and the increased life expectancy of those with diabetes [2]. First-line treatment for the management of DME is anti-vascular endothelial growth factor (anti-VEGF) agents, such as intravitreal aflibercept (IVT-AFL) and ranibizumab [3].
The VIVID-DME (Intravitreal Aflibercept Injection in Vision Impairment Due to DME) and VISTA-DME (Study of Intravitreal Aflibercept Injection in Patients With Diabetic Macular Edema) Phase III studies showed that treatment with IVT-AFL fixed dosing every 4 or 8 weeks, following five initial monthly doses, was associated with significant improvements in functional outcomes compared with laser treatment, and outcomes were maintained over 3 years in patients with DME [4,5,6]. As an alternative to fixed dosing, two flexible treatment strategies are available for the administration of IVT-AFL: treat-and-extend (T&E) and as-needed pro re nata (PRN) regimens [7]. The aim of flexible management of patients with DME is to reduce the treatment burden associated with anti-VEGF therapy, while maintaining visual acuity gains [8]. T&E involves gradual extension of the treatment interval based on maintenance of functional and anatomical stability, and shortening of the treatment interval if deterioration is observed. This approach reduces the frequency of clinic visits and removes the requirement for monitoring between injections, making the disease more manageable for the patient and physician. PRN involves treatment on an as-needed basis, with regular monthly visits being required for monitoring [8].
Availability of a choice of dosing regimens means that the physician and patient can select the treatment option that best suits individual needs and addresses the burden of treatment. However, there is a lack of data regarding the best regimen to optimize functional and anatomical outcomes with long-term IVT-AFL treatment in patients with DME. The aim of the VIOLET study was to assess whether IVT-AFL administered according to two different flexible dosing regimens provided similar efficacy and safety to fixed dosing every 8 weeks (q8w) in patients with DME who had already completed 1 year of IVT-AFL treatment. Here, we report the 52- and 100-week outcomes from the VIOLET study.
Methods
VIOLET was a 100-week, multicenter, randomized, open-label, active-controlled, parallel-group, Phase IIIb, non-inferiority study that investigated whether IVT-AFL administered according to two different flexible-dosing regimens (T&E and PRN) provided similar efficacy and safety to fixed-dosing q8w in patients with DME who had already completed ≥ 1 year of IVT-AFL treatment.
The study was conducted at 64 sites in Europe and Canada from November 2016 to September 2019 and in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines E6: Good Clinical Practice. The study protocol and statistical analysis plan can be accessed at ClinicalTrials.gov (identifier: NCT02818998). The protocol was approved by the independent ethics committee or institutional review board at each study site (see Supplementary Material for full details). All patients provided written informed consent.
Study Design
Patients enrolled in the VIOLET study had received treatment with IVT-AFL 2 mg for at least 1 year, initiated with five monthly injections, followed by treatment q8w until enrollment (one missed or delayed q8w dose was permissible) (Fig. S1). Patients were enrolled from either the AQUA study (NCT02581995; an open-label, single-arm study designed to evaluate vision-related quality of life in patients who received five initial monthly injections of IVT-AFL 2 mg, followed by treatment q8w until Week 52) or from outside the AQUA study, if they fulfilled the eligibility criteria. Details of the AQUA study are published elsewhere [9].
In the VIOLET study, if all data needed for enrollment were available, the screening and baseline visits could take place on the same day. In such cases, procedures scheduled for both visits were conducted only once. Randomization (1:1:1) was conducted at Week 0 (baseline) and was stratified according to whether patients had achieved a ≥ 10-letter gain in best-corrected visual acuity (BCVA; Early Treatment Diabetic Retinopathy Study [ETDRS]) from the start of IVT-AFL treatment (≥ 1 year before baseline).
Patients were randomly assigned to treatment with IVT-AFL according to a fixed-dose, PRN, or T&E regimen, as detailed in Table 1. Further details of the methods are available in the Supplementary Material.
Patients
Patients who had received prior treatment with IVT-AFL for ≥ 1 year under the guidance of the European label – five initial monthly IVT-AFL 2 mg injections, followed by an IVT-AFL injection q8w (for patients enrolled from the AQUA study [9]) or treatment within allowed time windows for interval deviations (patients enrolled outside the AQUA study) – were eligible for inclusion. Patients were aged ≥ 18 years, with type 1 or type 2 diabetes mellitus and DME with central macular involvement (defined as the area of the center subfield on spectral domain optical coherence tomography [OCT]), BCVA 73–24 ETDRS letters (20/40–20/320 Snellen equivalent) in the study eye at pre-study treatment initiation. Only one eye was designated as the study eye. See Supplementary Material for full details.
Endpoints
The primary endpoint was assessed at Week 52, the end of the first year of treatment under the protocol. Thus, patients who completed 1 year in the study had completed 2 years of treatment with IVT-AFL in total. The primary endpoint was the mean change in BCVA (ETDRS letters) from baseline to Week 52 with IVT-AFL 2 mg (after 2 years of treatment). Secondary endpoints were the mean change in BCVA from baseline to Week 100, mean change in central retinal thickness (CRT) from baseline to Weeks 52 and 100, and the proportion of patients with BCVA gains of ≥ 10 or ≥ 15 ETDRS letters (two or three Snellen lines, respectively) and losses of ≥ 30 ETDRS letters (six Snellen lines) from baseline to Weeks 52 and 100. See Supplementary Material for other endpoints.
Statistical Analysis
A sample size of 135 evaluable patients per treatment group was planned. Assuming an equal mean change in BCVA from baseline to Week 52 in the treatment groups, a standard deviation (SD) of 9, 11, and 11 ETDRS letters for the mean BCVA change from baseline to Week 52 in the fixed, T&E, and PRN groups, respectively, and a family-wise error rate alpha of 2.5% (one-sided tests), this sample size provided power of 90% to demonstrate non-inferiority at a non-inferiority margin of 4 letters for either T&E versus fixed regimens, or for the comparison of the PRN versus fixed regimens at a significance level of 2.5% (one-sided test). The expected dropout rate was estimated to be 17% [5]; thus, approximately 490 patients were planned to be randomized (the final number of patients was lower; see Supplementary Material for details). The primary analysis was based on an analysis of covariance (ANCOVA) model with the baseline BCVA measurement as covariate and the treatment regimen and stratum (10-letter gain from start of IVT-AFL treatment [yes/no]) as fixed factors. The Hochberg procedure was used to control the overall type-1 error of 2.5% (one-sided tests) for the two non-inferiority tests. The full analysis set (FAS) included all randomized patients who received the study drug and had a baseline BCVA assessment and at least one post-baseline BCVA assessment; the primary statistical analysis was performed on the FAS. The primary method for replacing missing values for all efficacy analyses was last observation carried forward. Secondary endpoints were analyzed descriptively, including 95% confidence intervals (CIs) where appropriate. Statistical evaluation was performed using Statistical Analysis Software v9.4 (SAS Institute Inc., Cary, NC, USA).
Results
In total, 500 patients were enrolled and 463 were randomly assigned to treatment. The FAS was comprised of 458 patients (n = 153 [fixed], n = 152 [T&E], and n = 153 [PRN]). Two patients in each of the fixed and T&E groups and one patient in the PRN group had no post-baseline assessments available and were excluded from the FAS. More than 95% of patients enrolled after completing the AQUA study, in which > 90% of patients received all nine IVT-AFL injections in Year 1 per the protocol (mean number of injections: 8.8 [95% CI 8.7–8.9]) [9]. The Week 100 visit was completed by 88.4% (n = 137), 89.6% (n = 138), and 88.3% (n = 136) of patients in the fixed, T&E, and PRN groups, respectively (Fig. 1).
Patient disposition. aTwo patients in each of the fixed and T&E groups and one patient in the PRN group had no post-baseline assessments available and were excluded from the full analysis set. bReasons for withdrawal in the category “Other” were patient withdrawal by sponsor and lack of efficacy. AE adverse event, IVT-AFL intravitreal aflibercept, PRN pro re nata, T&E treat-and-extend
Patient baseline demographics and disease characteristics were similar between the groups (Table 2). Mean age was 64.4–65.5 years across the groups and 58.2–64.1% of patients were male. Mean BCVA was 72.7, 72.5, and 71.0 letters (approximately 20/32 to 20/40 Snellen equivalent) in the fixed, T&E, and PRN groups, respectively, and mean CRT was 289.9 µm, 285.9 µm, and 294.6 µm, respectively.
Over 52 weeks, patients received a mean (min–max) of 6.7 (1–7; fixed), 5.6 (1–7; T&E), and 6.2 (1–13; PRN) injections. Over 100 weeks, patients received a mean (min–max) of 12.3 (3–13; fixed), 10.0 (2–14; T&E), and 11.5 (1–25; PRN) injections (Figs. S2A and S2B), which occurred during a mean 95.5, 94.8, and 84.9 weeks of exposure to IVT-AFL, respectively. The mean (min–max) number of clinic visits, including Weeks 52 and 100, was 16.1 (5–25; fixed), 13.3 (4–23; T&E), and 25.0 (3–29; PRN). The mean (range) number of visits per patient-year was 8.8 (7–16; fixed), 7.2 (5–12; T&E), and 14.2 (10–31; PRN). The mean (SD) last treatment interval was 8.0 (0.7), 11.5 (4.9), and 11.8 (14.3) weeks for the fixed, T&E, and PRN groups, respectively. Overall, in accordance with the protocol, no patients in the fixed group achieved a last treatment interval up to Week 100 of ≥ 12 weeks; 40.8% (n = 62) and 32.0% (n = 49) of patients in the T&E and PRN groups achieved a last treatment interval of ≥ 12 weeks.
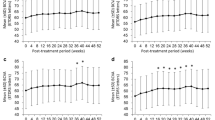
At Week 52, mean (SD) BCVA change from baseline was + 0.4 (6.7; fixed), + 0.5 (6.7; T&E), and + 1.7 (6.8; PRN) (Fig. 2). Least squares mean difference (95% CI) to IVT-AFL fixed dosing was 0.01 (− 1.46, 1.47) letters for the T&E regimen and 0.95 (− 0.52, 2.42) letters for the PRN regimen. Compared with fixed dosing, both flexible regimens achieved a non-inferior outcome in mean BCVA change for the prespecified margin of 4 letters (p < 0.0001 for both comparisons).
Mean change in BCVA from baseline to Week 100a. Full analysis set; last observation carried forward. The apparent spike at Week 52 and Week 100 is because this was a mandatory visit for all patients. aPatients had previously received 1 year of IVT-AFL treatment prior to the VIOLET study baseline. bAt Week 52 (primary endpoint), compared with IVT-AFL fixed, IVT-AFL T&E, and IVT-AFL PRN groups achieved a non-inferior outcome in mean BCVA change for the prespecified margin of 4 letters (p < 0.0001 for both comparisons). BCVA best-corrected visual acuity, ETDRS Early Treatment Diabetic Retinopathy Study, IVT-AFL intravitreal aflibercept, PRN pro re nata; SD standard deviation, T&E treat-and-extend
Mean (SD) BCVA change from baseline was + 0.1 (7.2; fixed), − 0.1 (9.1; T&E), and + 1.8 (9.0; PRN) at Week 104 (Fig. 2). Least squares mean difference (95% CI) to IVT-AFL fixed dosing was − 0.30 (− 2.13, 1.52) letters for the T&E regimen and 1.39 (− 0.40, 3.19) letters for the PRN regimen. Findings at Week 100 confirmed results from Week 52 and achieved nominal significance for non-inferiority (p < 0.0001). Absolute BCVA values at baseline, Week 52, and Week 100 are shown in Fig. 2. The within-group changes observed over the 100-week study period were similar across groups. Mean change in BCVA from the start of the previous IVT-AFL treatment to Week 100 of the VIOLET study is presented in Fig. S3. Both Fig. 2 and Fig. S3 present data according to a last observation carried forward approach. A supporting observed cases analysis (data not shown) indicated that the results and their interpretation were not affected by missing data.
At Week 52, gains of ≥ 10 and ≥ 15 letters (two to three Snellen lines) respectively, were observed in 6.5% and 2.6% (fixed), 9.2% and 3.3% (T&E), and 8.5% and 2.6% (PRN) of patients (Fig. S4). A loss of ≥ 30 letters (or six Snellen lines) was observed in one patient (0.7%) in the fixed group, and no patients in either of the T&E or PRN groups. At Week 100, gains of ≥ 10 and ≥ 15 letters, respectively, were observed in 6.5% and 2.0% (fixed), 11.2% and 2.6% (T&E), and 14.4% and 3.9% (PRN) of patients. A loss of ≥ 30 letters was observed in 1/153 (0.7%), 2/152 (1.3%), and 2/153 (1.3%) of patients in the fixed, T&E, and PRN groups, respectively.
Except for baseline, Week 52, and Week 100, CRT measurements were only mandatory for patients in the PRN arm and were conducted in the fixed-dose and T&E arms when clinically indicated per investigator judgment. Mean (SD) CRT change (µm) from baseline was − 18.8 (45.5; fixed), − 2.1 (56.2; T&E), and + 2.2 (77.8; PRN) at Week 52. Least squares mean difference (95% CI) to IVT-AFL fixed dosing was 14.38 (3.39, 25.37) µm for the T&E regimen and 21.22 (7.65, 34.80) µm for the PRN regimen. At Week 100, mean (SD) CRT change (µm) from baseline was − 15.5 (64.3; fixed), + 2.3 (81.8; T&E), and − 13.9 (74.4; PRN). Least squares mean difference (95% CI) to IVT-AFL fixed dosing was 16.14 (0.62, 31.66) µm for the T&E regimen and 4.12 (− 9.52, 17.77) µm for the PRN regimen.
At Week 52 (in the second year of treatment), 4.4% (fixed), 5.0% (T&E), and 3.8% (PRN) of patients achieved a ≥ 2-step improvement in the Diabetic Retinopathy Severity Score (DRSS) and 2.2% (n/N = 3/135) of patients in the fixed group achieved a ≥ 3-step improvement in DRSS. No patients in the T&E and PRN groups achieved a ≥ 3-step improvement in DRSS at Week 52. At Week 100 (in the third year of treatment), overall, 4.9% of patients experienced either a two-step improvement or a two-step worsening in DRSS during the study (Table 3).
The incidence of ocular treatment-emergent adverse events was comparable in the fixed (52.9%), T&E (52.6%), and PRN (55.2%) groups. The safety profile was consistent with results from prior studies of IVT-AFL [4,5,6] and only 2 patients experienced intraocular inflammation events, which were mild and transient in nature (Table 4). The overall incidence of arterial thromboembolic events defined by the Anti-Platelet Trialists’ Collaboration criteria was 3.2% (fixed), 1.9% (T&E), and 3.9% (PRN) of patients. Overall, 17 deaths were reported: three (1.9%) fixed, six (3.9%) T&E, and eight (5.2%) PRN (Table S1). The incidence of treatment-emergent deaths in the fixed, T&E, and PRN groups was one (0.6%), three (1.9%), and four (2.6%), respectively (Table S2).
Discussion
The VIOLET study showed that, in patients who had already received at least 1 year of IVT-AFL treatment for DME under the guidance of the European label, both flexible IVT-AFL treatment regimens (T&E and PRN) achieved a non-inferior outcome in mean BCVA change compared with fixed dosing (IVT-AFL 2 mg q8w) at Week 52 (primary endpoint) and Week 100. No clinically relevant differences were observed between the IVT-AFL fixed and T&E groups, or between the fixed and PRN groups, for the changes in BCVA letter score at Week 52 and Week 100. Differences in CRT changes between treatment groups were not clinically meaningful. Of the three regimens investigated, a reduced treatment burden was observed in the T&E group, which received the lowest number of injections. Furthermore, T&E dosing was associated with a reduced mean number of clinic visits.
Most patients in the VIOLET study had participated in the AQUA study and, in that study, achieved a mean gain in BCVA of + 10 letters after 1 year of treatment [9]; therefore, no substantial improvements were expected during the VIOLET study. Improvements in BCVA and CRT achieved in the year prior to enrollment in VIOLET were maintained over the second and third years of treatment with either a fixed, T&E, or PRN dosing regimen. Furthermore, late visual gain was observed in 2–3% of patients who gained ≥ 15 letters in the second and third years of treatment in this study. Thus, unlike neovascular age-related macular degeneration (nAMD), where treatment delays of as little as 6 weeks impact functional outcome [10, 11], the exact timing of treatment is less critical in DME. Indeed, functionally driven treatment is likely to work well, and this helps to explain why, in contrast to nAMD, PRN and T&E work similarly well regarding functional outcomes. In this study, in addition to a mean 10-letter gain with IVT-AFL treatment prior to randomization, more than 20% of patients with DME gained ≥ 5 letters in the second year of treatment with IVT-AFL. Most longer-term studies of anti-VEGF agents report change from baseline rather than change beyond Year 1; however, fluid persisting over 2 years is not a rare or atypical finding [12]. Even if a complete response to treatment is not achieved in the first year, treatment continuation will not only prevent further visual deterioration, but may result in a valuable late functional gain in a relevant proportion of patients. Identification of baseline factors associated with outcomes in DME may help to inform physicians regarding treatment expectations and support identification of patients who may be late responders. This has previously been investigated at 12 months in patients treated with intravitreal ranibizumab [13], and requires further research in patients treated with IVT-AFL.
Although little is known regarding the dynamics of DRSS changes due to anti-VEGF therapy in the long term, most of the improvement in DRSS was achieved prior to the study during the first 12 months of treatment (during which 20.5% and 3.4% of patients had a ≥ 2- and ≥ 3-step DRSS improvement, respectively [9]), which is consistent with previous findings [4]. The present study provides evidence that proportions of change in DRSS remain stable for a further 2 years following the first year of treatment with IVT-AFL.
As patients with DME are usually younger than those with nAMD [14], long-term treatment is often required. Thus, the availability of easy-to-manage and flexible treatment strategies that meet individual patient needs are important. The Diabetic Retinopathy Clinical Research Network (DRCR.net) has contributed to substantial advances in the relative benefits of anti-VEGF agents as the first-line therapy for eyes with visual impairment due to DME. Protocol I, the first study of an anti-VEGF agent, ranibizumab PRN (compared with non-anti-VEGF treatment), demonstrated 8–9 letter gains after 1 year of treatment, with improvements being maintained up to 5 years [15, 16]. It became apparent that fewer injections were needed after the first year of treatment, and the majority of patients did not receive anti-VEGF injections during the fifth year of treatment [16]. Protocol T, which compared ranibizumab 0.3 mg with IVT-AFL and bevacizumab (using the same regimen, with a modified PRN protocol based on vision and OCT findings), demonstrated that IVT-AFL was more effective at improving vision in patients with worse levels of visual acuity (≤ 65 letters) after 1 and 2 years of treatment [17, 18]. Beyond Year 2 to the end of Year 5, two-thirds of study eyes received at least one anti-VEGF injection (median four injections in 3 years; interquartile range 0–12); visual acuity was improved from baseline after 5 years of treatment, driven by gains in Year 1 and 2, as visual acuity decreased in Years 3–5 compared with the end of Year 2 [19]. Both Protocol T and Protocol I assessed the role of deferred laser photocoagulation within the management of patients with DME, but neither were designed to compare different anti-VEGF treatment regimens. Thus, VIOLET adds to this evidence base by evaluating three different treatment regimens with a single anti-VEGF agent.
The VIOLET study explored the use of flexible-dosing IVT-AFL regimens, allowing physicians to adopt the optimal treatment plan based on individual patient needs and clinical practice requirements [7, 8]. The PRN regimen included monthly visits, which may have an impact on adherence in real-world clinical practice due to the frequency of visits and increased burden for patients and physicians due to clinic constraints. Under T&E, treatment intensity is adapted to individual needs, which is more readily accepted and more likely to be adhered to by patients. Treatment is administered at every visit under a T&E protocol, which helps to plan clinic capacity. Furthermore, as the patient knows that they will be treated at every visit, they are prepared to receive an injection, and any uncertainty about treatment is eliminated [8]. In this clinical trial setting, the number of injections and subsequent functional outcomes were similar with PRN and T&E dosing. However, the burden of treatment in terms of clinic visits differed, with a notably higher number of visits in the PRN group (25.0 compared with 16.1 [fixed] and 13.3 [T&E] visits). This was due to the nature of the PRN regimen, whereby patients were required to attend the clinic monthly, but did not receive an injection at every visit. Given the flexible nature of the proactive, individualized T&E regimen, 40.8% of patients achieved a treatment interval of ≥ 12 weeks, compared with 32.0% of patients receiving PRN IVT-AFL (and no patients receiving fixed dosing). In real-world clinical practice, it is likely that reduced clinic visits may have a positive impact on treatment adherence [8].
Regarding study strengths, we investigated the direct comparison of three IVT-AFL regimens, including T&E dosing, from the second year of treatment when physicians are often facing decisions regarding choice of management strategy. Furthermore, this was a long-term study with a large sample size. The safety profile of IVT-AFL for the treatment of DME in this study was consistent with results from prior studies [4,5,6]. Limitations include that the results refer to a largely White population and that two-thirds of patients had a glycated hemoglobin value below 8% at baseline, indicating a potential selection bias toward diabetes treatment-compliant patients. In addition, at baseline, most patients had mild-to-moderate diabetic retinopathy, thus limiting the interpretation of DRSS responses.
Conclusions
Results from the VIOLET study demonstrate that, in patients who received at least 1 year of IVT-AFL treatment for DME under the guidance of the European label, both flexible IVT-AFL treatment regimens (T&E and PRN) resulted in similar functional outcomes to fixed dosing (IVT-AFL 2 mg q8w) in the second and third years of treatment, with the T&E group requiring the lowest number of injections and clinic visits. Findings highlight the range of flexible-dosing regimens available with IVT-AFL, allowing physicians to adopt the optimal treatment plan based on individual patient needs and clinical practice requirements.
References
Mathew C, Yunirakasiwi A, Sanjay S. Updates in the management of diabetic macular edema. J Diabetes Res. 2015;2015:794036.
Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015. https://doi.org/10.1186/s40662-015-0026-2.
Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, et al. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2017;237:185–222.
Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247–54.
Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology. 2015;122:2044–52.
Heier JS, Korobelnik JF, Brown DM, et al. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology. 2016;123:2376–85.
Freund KB, Korobelnik JF, Devenyi R, et al. Treat-and-extend regimens with anti-VEGF agents in retinal diseases: a literature review and consensus recommendations. Retina. 2015;35:1489–506.
Lanzetta P, Loewenstein A, Vision Academy Steering Committee. Fundamental principles of an anti-VEGF treatment regimen: optimal application of intravitreal anti-vascular endothelial growth factor therapy of macular diseases. Graefes Arch Clin Exp Ophthalmol. 2017;255:1259–73.
Garweg JG, Stefanickova J, Hoyng C, et al. Vision-related quality of life in patients with diabetic macular edema treated with intravitreal aflibercept: the AQUA study. Ophthalmol Retin. 2019;3:567–75.
Canan H, Sizmaz S, Altan-Yaycioglu R, Sariturk C, Yilmaz G. Visual outcome of intravitreal ranibizumab for exudative age-related macular degeneration: timing and prognosis. Clin Interv Aging. 2014;9:141–5.
Rauch R, Weingessel B, Maca SM, Vecsei-Marlovits PV. Time to first treatment: the significance of early treatment of exudative age-related macular degeneration. Retina. 2012;32:1260–4.
Bressler NM, Beaulieu WT, Glassman AR, et al. Persistent macular thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for central-involved diabetic macular edema with vision impairment: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2018;136:257–69.
Bressler SB, Qin H, Beck RW, et al. Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch Ophthalmol. 2012;130:1153–61.
Ziemssen F, Feltgen N, Holz FG, et al. Demographics of patients receiving Intravitreal anti-VEGF treatment in real-world practice: healthcare research data versus randomized controlled trials. BMC Ophthalmol. 2017. https://doi.org/10.1186/s12886-017-0401-y.
Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–77.
Elman MJ, Ayala A, Bressler NM, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology. 2015;122:375–81.
Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–203.
Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123:1351–9.
Glassman AR, Wells JA III, Josic K, et al. Five-year outcomes after initial aflibercept, bevacizumab, or ranibizumab treatment for diabetic macular edema (Protocol T extension Study). Ophthalmology. 2020;127:1201–10.
Acknowledgements
Funding
The VIOLET study was sponsored by Bayer AG, Leverkusen, Germany. In conjunction with the VIOLET steering committee (Professor Justus G. Garweg, Dr Jana Štefanickova, Professor Sobha Sivaprasad, and Professor Carel Hoyng), Bayer participated in the design of the study; analysis and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication. Additionally, Bayer was responsible for the conduct of the study and oversight of the collection and management of data. The journal’s Rapid Service and Open Access Fees were funded by Bayer Consumer Care AG.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
Justus G. Garweg, Jana Štefanickova, Carel Hoyng, Tobias Niesen, Thomas Schmelter, Sergio Leal and Sobha Sivaprasad all contributed to the study conception and design. Data collection was performed by Justus G. Garweg, Jana Štefanickova, Sobha Sivaprasad, and Carel Hoyng. Statistical analysis of the data was performed by Thomas Schmelter. All named authors were involved in the interpretation of data and critical review of the manuscript at all stages.
Medical Writing, Editorial, and Other Assistance
Medical writing and editorial support for the preparation of this manuscript (under the guidance of the authors) was provided by Louise Brady and Sarah Feeny of ApotheCom (UK), in accordance with Good Publication Practice (GPP3) guidance (Ann Intern Med 2015;163:461–464), and was funded by Bayer Consumer Care AG, Basel, Switzerland.
Compliance with Ethics Guidelines
The study was conducted at 64 sites in Europe and Canada from November 2016 to September 2019, in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines E6: Good Clinical Practice. The study protocol and statistical analysis plan can be accessed at ClinicalTrials.gov (identifier: NCT02818998). The protocol was approved by the independent ethics committee or institutional review board at each study site. All patients provided written informed consent.
Disclosures
Justus G. Garweg is a consultant for AbbVie, Allergan, Bayer, Chengdu Khanghong, and Novartis. Jana Štefanickova has received funding/fees from Bayer and Novartis. Carel Hoyng has no conflicts of interest to disclose. Tobias Niesen is an employee of Bayer AG, Leverkusen, Germany. Thomas Schmelter is an employee of Bayer AG, Berlin, Germany. Sergio Leal is an employee of Bayer Consumer Care AG, Basel, Switzerland, and reports a patent pending (WO2018/229034). Sobha Sivaprasad has received funding/fees from Bayer, Novartis, Allergan, Roche, Boehringer Ingelheim, and Heidelberg Engineering.
Data Availability
Availability of the data underlying this publication will be determined later according to Bayer’s commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing”. This pertains to scope, time point, and process of data access. As such, Bayer commits to sharing upon request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the United States (US) and European Union (EU) as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after January 01, 2014. Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the Study sponsors section of the portal. Data access will be granted to anonymized patient-level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded. The study protocol and statistical analysis plan can be accessed at ClinicalTrials.gov (Identifier: NCT02818998).
Patient Involvement
The authors thank all the patients and investigators who contributed to this study.
Prior Presentation
Data from this study were presented at EURETINA 2019 Congress, 5–8 September, Paris, France, and at EURETINA 2020 Congress, 2–4 October, Virtual.
VIOLET Investigators
Austria: Ursula Schmidt-Erfurth (Medical University of Vienna, Vienna), Andreas Wedrich (Medical University of Graz, Graz); Canada: Fareed Ali (Canadian Centre for Advanced Eye Therapeutics Inc, Mississauga, Ontario), David Chow (Toronto Retina Hospital, Toronto, Ontario), John Dickinson (Nova Scotia Health Authority, Halifax, Nova Scotia), Michel Giunta (Office of Michel Giunta MD, Sherbrooke, Quebec), Jesia Hasan (Eye Health MD, Montreal, Quebec); Czech Republic: Jaroslava Dusova (University Hospital Hradec Kralove, Hradec Kralove), Jan Hamouz (University Hospital Kralovske Vinohrady, Prague); France: Laurent Kodjikian (Hospital la Croix Rousse, Lyon), Eric Souied (Intercommunal Hospital of Creteil, Creteil); Germany: Claudia Dahlke (University of Cologne, Cologne), Karl-Heinz Emmerich (Darmstadt Eye Clinic, Darmstadt-Eberstadt), Nicolas Feltgen (Goettingen University Eye Clinic, Goettingen), Frank Holz (University Clinic of Bonn, Bonn), Frank Koch (University Hospital Frankfurt Ophthalmology Clinic, Frankfurt), Dirk Sandner (Carl Gustav Carus University Clinic, Dresden), Walter Sekundo (University Hospital of Giessen and Marburg, Marburg); Hungary: Agnes Kerenyi (Bajcsy-Zsilinszky Hospital, Budapest), Andras Papp (Semmelweis University, Budapest), Andras Seres (Budapest Retina Associates, Budapest), Attila Vajas (University of Debrecen, Debrecen), Balazs Varsanyi (Ganglion Medical Center, Pécs); Italy: Francesco Bandello (Central Foundation San Raffaele del Monte Tabor, Milan), Francesco Boscia (University Hospital of Sassari, Sassari), Chiara Eandi (Ophthalmic Hospital of Torino, Torino), Edoardo Midena (University of Padova, Padova), Massimo Nicolo (University Hospital of San Martino, Genova), Enrico Peiretti (University of Cagliari, Cagliari), Federico Ricci (Hospital Tor Vergata, Rome), Francesco Viola (Maggiore Policlinico Hospital, Milan), Gianni Virgili (University Hospital of Careggi, Florence); Lithuania: Vilma-Jurate Balciuniene (Hospital of the Lithuanian University of Health Sciences, Kaunas), Andrius Cimbalas (Vilnius University Hospital, Vilnius); Poland: Ewa Graczynska (IBIS Hospital, Warsaw), Andrzej Grzybowski (Poznan City Hospital, Poznan), Jakub Kaluzny (Oftalmika Eye Hospital, Bydgoszcz), Zofia Michalewska (Ophthalmic Clinic Jasne Blonia, Lodz), Dorota Raczynska (Ophthalmology Center, Gdansk), Marek Rekas (Military Medical Institute, Warsaw), Bozena Romanowska-Dixon (University Hospital of Krakow, Krakow), Slawomir Teper (Ophthalmic Office of Prof. Edward Wylęgała, Katowice), Tomasz Zarnowski (Medical University of Lublin, Lublin); Portugal: Miguel Amaro (Hospital Vila Franca de Xira, Vila Franca de Xira), João Castro Sousa (Central Hospital of Leiria, Leiria), Manuel Falcão (São João Central Hospital, Porto), João Pereira Figueira (Association for Innovation and Biomedical Research on Light and Image, Coimbra), Sara Vaz-Pereira (Santa Maria Hospital, Lisbon); Slovakia: Mikulas Alexik (Faculty Hospital Žilina, Žilina), Monika Gajdosova (OFTAL s.r.o., Zvolen), Gabriela Pavlovicova (University Hospital Nitra, Nitra), Jana Štefanickova (University Hospital Bratislava, Bratislava), Katarina Struharova (University Hospital Bratislava, Bratislava); Spain: Alfredo Adan (Barcelona Hospital Clinic, Barcelona), Lluis Arias Barquet (Bellvitge University Hospital, Barcelona), Anniken Bures (Barcelona Institute of Ocular Microsurgery, Barcelona), Carlos Cava Valenciano (Albacete University Hospital Complex, Albacete), Enrique Cervera (Valencia General University Hospital, Valencia), Laura Sararols (Catalunya General Hospital, Barcelona); Switzerland: Justus Garweg (Bern Eye Clinic, Lindenhof Hospital, Bern), Ioannis Petropoulos (Rive Ophthalmological Centre, Geneva); United Kingdom: Andrew Lotery (Southampton General Hospital, Southampton), Martin McKibbin (The Leeds Teaching Hospitals NHS Trust, Leeds), Sobha Sivaprasad (NIHR Moorfields Clinical Research Facility, London), Deepali Varma (Sunderland Eye Infirmary, Sunderland).
Author information
Authors and Affiliations
Consortia
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Garweg, J.G., Štefanickova, J., Hoyng, C. et al. Dosing Regimens of Intravitreal Aflibercept for Diabetic Macular Edema Beyond the First Year: VIOLET, a Prospective Randomized Trial. Adv Ther 39, 2701–2716 (2022). https://doi.org/10.1007/s12325-022-02119-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02119-z