Abstract
Introduction
Asian patients with type 2 diabetes (T2D) are younger, leaner, and more likely to develop renal dysfunction than White populations. In this multiethnic analysis of data from phase 3 trials, we investigated the efficacy and safety of the dipeptidyl peptidase-4 inhibitor linagliptin in Asians stratified by these subphenotypes.
Methods
Data from randomized, double-blind, placebo-controlled trials evaluating linagliptin (as monotherapy, add-on therapy to metformin ± sulfonylurea, combined with pioglitazone or added to insulin) were pooled with efficacy data from 11 randomized trials of at least 24 weeks and safety data from 15 trials of various durations.
Results
In the efficacy set, 1404 Asian patients received linagliptin [mean (standard deviation) age 54.5 (10.1) years; body mass index (BMI) 26.0 (3.9) kg/m2] and 661 received placebo [age 55.0 (9.7) years; BMI 26.1 (3.9) kg/m2] with the same glycated hemoglobin (HbA1c): 8.2 (0.9)% in both groups. At 24 weeks, the placebo-corrected adjusted mean ± standard error change from baseline in HbA1c with linagliptin was −0.73 ± 0.04% (95% confidence interval −0.81, −0.65; P < 0.0001). Reductions in HbA1c were similar upon stratification by age [<65 years, −0.71 ± 0.05% (−0.80, −0.62; P < 0.0001); ≥65 years, −0.81 ± 0.10% (−1.01, −0.60; P < 0.0001)], BMI (<25 kg/m2, −0.82 ± 0.06% [−0.94, −0.70; P < 0.0001]; ≥25 kg/m2, −0.65 ± 0.06% [−0.76, −0.54; P < 0.0001]) and estimated glomerular filtration rate [<90 mL/min/1.73 m2, −0.71 ± 0.06% (−0.82, −0.60; P < 0.0001); ≥90 mL/min/1.73 m2, −0.75 ± 0.06% (−0.87, −0.64; P < 0.0001)]. In the safety set (linagliptin, n = 1842; placebo, n = 839), 52.2% and 54.6% of patients, respectively, experienced adverse events. The rates of drug-related adverse events were 10.9% in the linagliptin group and 10.4% in the placebo group. The respective rates of hypoglycemia were 8.3% and 9.5%, mainly among patients treated with sulfonylurea or insulin. Severe hypoglycemia was rare (<1.0% in either group).
Conclusion
Linagliptin effectively reduced hyperglycemia in Asian patients with uncontrolled T2D, irrespective of age, BMI, renal function, or ethnic subgroups, and was well tolerated.
Funding
Boehringer Ingelheim, Eli Lilly and Company, and the Diabetes Alliance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asia has experienced a marked rise in the prevalence of type 2 diabetes (T2D) in recent years [1, 2]. Countries such as India and China have undergone rapid economic development with dietary and lifestyle changes resulting in obesity which has unmasked a genetic predisposition for T2D [3]. If left unchecked, the diabetic population is predicted to rise from 72.1 million in 2013 to 123 million by 2035 in Southeast Asia alone with major socioeconomic and healthcare implications [1]. Asian patients exhibit phenotypic profiles different from Western populations including young age of diagnosis and propensity for renal disease [3,4,5,6]. Given the long disease duration, high risk for renal disease, and importance of glucose control for renoprotection, early intervention with new glucose-lowering therapies with few side effects (e.g., hypoglycemia, weight gain) may be particularly relevant to Asian patients with T2D [3]. With increasing numbers of Asian patients participating in clinical trials, pooled analysis of these data may inform clinical practice [7].
Dipeptidyl peptidase-4 (DPP-4) inhibitors prolong the half-life of the incretin hormone, glucagon-like peptide-1 (GLP1), which augments prandial insulin secretion and suppresses glucagon secretion [8]. Linagliptin is a potent and selective DPP-4 inhibitor with a predominately non-renal route of elimination and does not require dose adjustment [9]. In multinational phase 3 trials, linagliptin 5 mg once daily compared with placebo improved glycemic control without weight gain or an increased risk for hypoglycemia [10,11,12,13]. Dedicated trials in different ethnic populations confirmed the efficacy and safety of linagliptin in Japanese [14], Chinese [15], and Asian [16, 17] patients with T2D. In a pooled data analysis from phase 3 clinical trials, linagliptin improved glycemic control in Asian patients with T2D (4 trials; n = 1029), which was well tolerated (10 trials; n = 1477) [18].
In this study, we expanded the aforementioned pooled analysis using data from 15 phase 3 trials of linagliptin including subanalysis stratified by ethnicity, BMI, age, and renal function.
Methods
Study Design and Patient Population
This was a retrospective analysis of patient-level data that was pooled from randomized, double-blind, placebo-controlled phase 3 clinical trials comparing linagliptin versus placebo as monotherapy or in combination with other glucose-lowering drugs. The analysis included subpopulations of Asian patients from 15 trials lasting for 12–52 weeks (Table 1) recruited from East Asia (China, Korea, Taiwan, Japan); Southeast Asia (Indonesia, Malaysia, Philippines, Singapore, Thailand); South Asia (India).
The study design and enrollment criteria for the 15 trials were similar, with enrollment of treatment-naïve or treatment-exposed patients with T2D. All patients were at least 18 years of age and had a BMI of ≤40 kg/m2. The glycated hemoglobin (HbA1c) levels ranged from 7.0% to 11.0% in treatment-naïve patients or from 6.5% to 10.5% in treatment-exposed patients. Exclusion criteria included impaired hepatic function (serum alanine transaminase, aspartate transaminase, or alkaline phosphatase levels more than three times the upper limit of normal); recent occurrence of myocardial infarction, stroke, or transient ischemic attack; any requirement for hemodialysis and kidney transplantation.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all trial participants.
Efficacy and Safety Assessments
Efficacy data were pooled from 11 randomized trials of at least 24 weeks’ duration (Table 1). The primary efficacy variable was the mean change from baseline at week 24 in HbA1c. Secondary endpoints included the mean change from baseline at week 24 in HbA1c by subgroups of region (East Asia, Southeast Asia, South Asia), BMI (<25 or ≥25 kg/m2), and age (<65 or ≥65 years); the mean change from baseline at week 24 in fasting plasma glucose (FPG); the mean change from baseline in HbA1c and FPG levels over time; the mean change from baseline after 24 weeks in incremental postprandial glucose (iPPG) levels.
Safety data were pooled from 15 trials (including the 11 trials from the efficacy set) of various durations to allow the broadest possible detection of adverse events. Safety assessments included the frequency and intensity of adverse events, including hypoglycemia, as coded by the Medical Dictionary for Regulatory Activities (MedDRA, version 16.0). Investigator-reported hypoglycemic events were defined as a blood glucose level of 3.9 mmol/L or less, with or without symptoms; severe hypoglycemia was defined as needing assistance from another person to administer resuscitative actions, irrespective of blood glucose concentration. The incidence of hypoglycemia was also analyzed according to whether or not patients were receiving sulfonylurea and/or insulin background therapies.
Statistical Analysis
Efficacy assessments were conducted on the full analysis set (FAS), which comprised all randomized patients treated with at least one dose of study drug and who had a baseline and at least one on-treatment HbA1c measurement. The mean change in HbA1c from baseline to week 24 was compared between the linagliptin and placebo groups in the pooled population using an analysis of covariance (ANCOVA). The model included the terms “treatment”, “baseline HbA1c”, “prior oral antidiabetes drugs (OADs; yes/no)”, and “individual study”. A last observation carried forward (LOCF) approach was used to replace missing data.
Secondary efficacy endpoints were analyzed using the FAS. Mean change in HbA1c from baseline after 24 weeks by Asian regional subpopulations and by ≥65 years age group were analyzed using ANCOVA similar to the primary analysis. Mean change in HbA1c from baseline after 24 weeks by BMI category (<25 kg/m2, ≥25 mg/m2) was analyzed using ANCOVA similar to the primary analysis, and with the same terms as the primary analysis model plus the term “BMI category (<25, ≥25 kg/m2)”. The mean change in FPG from baseline to week 24 was compared between the linagliptin and placebo groups using an ANCOVA model similar to the primary analysis with a LOCF approach, and with the same terms as the primary analysis model plus “baseline FPG”. Mean changes in HbA1c and FPG levels over time were analyzed using descriptive statistics with missing data replaced using a LOCF approach. The mean change in iPPG from baseline after 24 weeks was compared between the linagliptin and placebo groups in a subset of patients who had undergone a meal tolerance test and who had available data (observed cases, OC) using ANCOVA similar to the primary analysis, and with the same terms as the primary analysis model plus the term “baseline iPPG”.
Exploratory analyses were carried out to determine the influence of the following factors or covariates on the adjusted mean change from baseline in HbA1c, “use of insulin”; “Asia subregion”; “baseline BMI”. Regression and correlation analyses were used to investigate the relationship between baseline BMI and change from baseline in HbA1c at week 24.
Safety analyses were conducted on the treated set, which comprised all patients who were treated with at least one dose of study medication. Adverse events were summarized using descriptive statistics without any additional formal inferential statistical analysis.
Results
Demographics and Clinical Characteristics at Baseline
The FAS (efficacy) population comprised 2065 Asian patients (linagliptin, n = 1404; placebo, n = 661); the treated (safety) set comprised 2681 Asian patients (linagliptin, n = 1842; placebo, n = 839) (Table 2). In the FAS, mean standard deviation (SD) age, HbA1c, and BMI were 54.7 (10.0) years, 8.2 (0.9)%, and 26.0 (3.9) kg/m2, respectively. Most patients (59.0%) were from East Asia and half had the disease for more than 5 years. Approximately 50% had estimated glomerular filtration rate (eGFR) <90 mL/min/1.73 m2, most of whom (43% of all patients) had mild renal impairment (eGFR, 60 to <90 mL/min/1.73 m2). Approximately 50% of patients were using two or more OADs at enrollment. The linagliptin and placebo groups had similar profiles, except for higher insulin use in the placebo group (14.1%) than in the linagliptin group (6.6%). Mean (SD) exposure to linagliptin was 185 (75) days and to placebo was 204 (105) days (median, 170 days in each group).
Efficacy
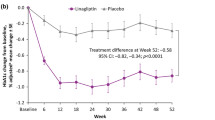
At 24 weeks, the adjusted mean ± standard error (SE) change from baseline in HbA1c was −0.77 ± 0.02% with linagliptin and −0.04 ± 0.03% with placebo, with a placebo-corrected difference of −0.73 ± 0.04% [95% confidence interval (CI) −0.81, −0.65; P < 0.0001] (Supplementary Fig. S1). Linagliptin was superior to placebo in reducing HbA1c stratified by Asian regional subpopulation [East Asians, −0.68 ± 0.05% (95% CI −0.78, −0.58; P < 0.0001); Southeast Asians, −0.90 ± 0.11% (95% CI −1.12, −0.68; P < 0.0001); South Asians, −0.75 ± 0.11% (95% CI −0.96, −0.54; P < 0.0001); Fig. 1a], BMI categories [<25 kg/m2, −0.82 ± 0.06% (95% CI −0.94, −0.70; P < 0.0001]; ≥25 kg/m2, −0.65 ± 0.06% (95% CI −0.76, −0.54; P < 0.0001); Fig. 1b], age categories [<65 years, −0.71 ± 0.05% (95% CI −0.80, −0.62; P < 0.0001); ≥65 years, −0.81 ± 0.10% (95% CI −1.01, −0.60; P < 0.0001); Fig. 1c], and eGFR categories [<90 mL/min/1.73 m2, −0.71 ± 0.06% 95% CI (−0.82, −0.60; P < 0.0001); ≥90 mL/min/1.73 m2, −0.75 ± 0.06% (95% CI −0.87, −0.64; P < 0.0001); Fig. 1d]. In our exploratory analysis, linagliptin tended to have greater efficacy in the Southeast Asian subpopulation, patients with BMI < 25 kg/m2, and those ≥65 years compared with their comparative counterparts. Exploratory analyses showed that the adjusted mean HbA1c change from baseline in the overall population remained the same when each of the covariates “use of insulin”, “Asia subregion”, or “baseline BMI” was added separately to the ANCOVA model. No significant interaction was found when the interaction term of each of these covariates with treatment was added to the analysis model (Supplementary Table S1).
a Placebo-corrected adjusted mean change in HbA1c with linagliptin in regional subpopulations*. Adjusted mean change in HbA1c b by BMI subgroup, c age, d by eGFR (all data are FAS-LOCF). BMI body mass index, CI confidence interval, eGFR estimated glomerular filtration rate, FAS full analysis set, HbA1c glycated hemoglobin, LOCF last observation carried forward, SE standard error, SD standard deviation. *East Asians: China, Korea, Taiwan, Hong Kong, and Japan; Southeast Asians: Indonesia, Malaysia, Philippines, Singapore, and Thailand; South Asians: India
At week 24, the adjusted mean ± SE change from baseline in FPG in the pooled Asian population was −10.42 ± 0.93 mg/dL and 4.86 ± 1.36 with linagliptin and placebo, respectively, with a placebo-corrected difference of −15.28 mg/dL (95% CI −18.54, −12.03; P < 0.0001). The placebo-corrected differences in FPG levels for East Asia was −13.91 mg/dL (95% CI −18.12, −9.71; P < 0.0001); Southeast Asia, −13.48 mg/dL (95% CI −21.11, −5.84; P = 0.0006); South Asia, −17.69 mg/dL (95% CI −25.11, −10.26; P < 0.0001).
During the 24-week treatment period, the largest reductions occurred during the first 6–12 weeks and were maintained until week 24 (Supplementary Figs. S2a, b). In a subset of patients who underwent meal tolerance tests (n = 40), treatment with linagliptin improved iPPG (placebo-corrected mean change from baseline with linagliptin, −45.0 mg/dL; 95% CI −71.6, −18.4; P = 0.0014; Supplementary Fig. S3). Exploratory analyses showed that the adjusted mean change from baseline levels of iPPG in the overall population was not significantly affected by any of the evaluated covariates: “use of insulin”, “Asia subregion”, or “baseline BMI” (Supplementary Table S2). No clinically relevant increase in body weight was observed with linagliptin (data not shown).
Safety
Overall adverse events and drug-related adverse events occurred at similar frequencies with both treatments (Supplementary Table S3). Approximately 3% of patients in each group discontinued treatment because of adverse events. The frequency of serious adverse events was low and similar between the placebo and linagliptin groups (4.5% vs. 3.0%). One of these events was reported as life-threatening and possibly drug-related (acute myocardial infarction in the linagliptin group). No cases of pancreatitis or pancreatic cancer were reported in either treatment group. There was one death in the placebo group (acute myocardial infarction and cardiovascular death). After adjusting for time exposed to study drugs, the incidence rates were similar between the study groups (Supplementary Table S3).
The frequency of investigator-reported hypoglycemia with linagliptin was comparable to placebo (8.3% vs. 9.5%). Less than 1.0% of patients in either group experienced severe hypoglycemia (0.3% vs. 0.1%). More hypoglycemic events occurred among patients treated with sulfonylurea and/or insulin (Supplementary Fig. S4). Severe hypoglycemia was experienced by 0.6% of linagliptin and 0.3% of placebo patients treated with sulfonylurea and/or insulin and was not reported in patients receiving other background therapies.
In a subgroup analysis of elderly patients (≥65 years) treated with linagliptin (n = 358) or placebo (n = 160), 53.6% of the linagliptin group and 61.9% of placebo group reported an adverse event. Serious adverse events and drug-related adverse events were reported by 4.5% and 12.6% of the linagliptin group. The respective frequencies were 6.9% and 17.5% in the placebo group. The incidence of hypoglycemia in the linagliptin group was 9.5% versus 18.1% in the placebo group, mainly among patients treated with sulfonylurea and/or insulin.
Discussion
In this pooled analysis, we have confirmed the efficacy and safety of linagliptin in an Asian multiethnic population, irrespective of BMI, age, and renal function. Early age of diagnosis and therefore long disease duration as well as high risk for renal dysfunction in Asian T2D patients may have implications for the selection of antidiabetes drugs [3, 5, 6, 19, 20]. DPP-4 inhibitors augment prandial insulin secretion and suppress glucagon with low risk of hypoglycemia [21]. Meta-analysis suggested that Asians or subjects with low BMI had favorable response to DPP-4 inhibition [22, 23]. Given that beta-cell insufficiency is a prominent feature in Asian populations with T2D [24, 25], DPP-4 inhibitors can have therapeutic advantages over other drug classes in these patients.
In this pooled analysis of 11 trials, 24-weeks’ treatment with linagliptin reduced HbA1c by 0.7% after correction for placebo effect with reduction in both FPG and iPPG in the entire and subregional populations. Data from 15 trials also confirmed the low occurrence of adverse events and the risk of hypoglycemia was mainly limited to patients treated with sulfonylurea or insulin therapy. In elderly subjects aged ≥65 years, linagliptin reduced HbA1c by 0.8% with low risk of adverse events.
These results confirmed an earlier analysis of pooled Asian data from four studies [18]. Exploratory analyses such as pooled analyses reinforce the efficacy and safety findings of individual trials, improve the detection of adverse events that occur at low frequencies, and provide a larger database for performing subgroup and treatment interactions. In two phase 3 trials that exclusively recruited patients in China, Malaysia, and the Philippines [16, 17], which evaluated linagliptin as monotherapy [16] or as add-on to metformin [17] also confirmed the efficacy and safety of linagliptin. Consistent with the overall linagliptin phase 3 clinical development program, all these Asian trials included a significant proportion of patients with renal impairment in whom linagliptin was efficacious, safe, and well tolerated [26].
In a multiethnic population, Asian individuals had a lower insulinogenic index than White populations, which might contribute to the high prevalence of T2D in Asian populations [27]. Other researchers have reported a linear relationship between BMI and beta-cell volume in Asian populations [28]. Although the World Health Organization [29] used ≥25 kg/m2 to define overweight, in South Asian males, a cutoff of 22.6 kg/m2 has been proposed to define obesity since these subjects had a fat percentage equivalent to that of a White person with 30 kg/m2 [30]. The American Diabetes Association now recommends a cut point of 23 kg/m2 (rather than 25 kg/m2) for screening Asian patients for T2D [31]. In the present analysis, although Asian subjects with a BMI < 25 kg/m2 had a numerically greater reduction in HbA1c than those with a high BMI, further exploratory analyses suggested that the HbA1c-lowering efficacy of linagliptin was largely unaffected by baseline BMI. Indeed, the relationship between BMI and response to antidiabetes drugs such as DPP-4 inhibitors remains inconclusive with some reports showing an association [22, 23, 32] while others have not [33].
In this Asian multiethnic pooled analysis, Southeast Asian patients appeared to have greater reductions in HbA1c than other ethnic subgroups, but statistical analyses did not reveal interaction between ethnicity and treatment effect. On the other hand, other researchers have reported interethnic differences among Asians in the prevalence of T2D, distributions of risk factors (e.g., BMI, waist circumference), and beta-cell function [34, 35], as well as differences in HbA1c responses to treatment [36,37,38]. While more studies are needed to explore these subethnicity differences, our results and others showed comparable efficacy and safety of linagliptin in Japanese, Asian (non-Japanese), and White patients with T2D [39].
In our analysis, the efficacy of linagliptin in lowering iPPG may be particularly relevant to Asians with high carbohydrate intake and therefore high and fluctuating postprandial blood glucose excursion [16, 40, 41]. Hyperglycemia and glycemic variability might impair beta-cell function. Thus, it is plausible that lowering iPPG levels with linagliptin may slow the decline in beta-cell function [3, 42]. Indeed, previous reports have indicated improvements in beta-cell function with linagliptin treatment [18].
In common with all pooled analyses, the present study is limited by the inclusion of data from different clinical studies. However, this analysis was based on individual patient data from a large clinical development program in which the methodological approach was similar across all trials. Despite the relatively large sample size, volunteer effects and ethnic diversity mean that the results may not be fully generalizable to other Asian populations. Without inclusion of White patients, the relative efficacy between non-Asian and Asian populations has not been investigated, although in most of the primary studies, ethnicity was often adjusted in the final analysis. This latest pooled analysis provides additional data on the safety and efficacy of linagliptin in Asian populations.
Conclusion
In this global epidemic of T2D, Asian populations are disproportionately affected. Given the importance of beta-cell insufficiency and renal dysfunction in these Asian patients, as well as their high carbohydrate intake with associated high glucose excursions, linagliptin has a particular place in therapy for these high-risk subjects, irrespective of age, BMI, renal function, or subethnicity.
References
Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–49.
Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet. 2010;375(9712):408–18.
Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301(20):2129–40.
Baskar V, Kamalakannan D, Holland MR, Singh BM. Does ethnic origin have an independent impact on hypertension and diabetic complications? Diabetes Obes Metab. 2006;8(2):214–9.
Li J, Dong Y, Wu T, et al. Differences between Western and Asian type 2 diabetes patients in the incidence of vascular complications and mortality: a systematic review of randomized controlled trials on lowering blood glucose. J Diabetes. 2016; 8(6):824–33.
Lanting LC, Joung IM, Mackenbach JP, Lamberts SW, Bootsma AH. Ethnic differences in mortality, end-stage complications, and quality of care among diabetic patients: a review. Diabetes Care. 2005;28(9):2280–8.
Thiers FA, Sinskey AJ, Berndt ER. Trends in the globalization of clinical trials. Nat Rev Drug Discov. 2008;7(1):13–4.
Deacon CF. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes Metab. 2011;13(1):7–18.
Boehringer Ingelheim Pharmaceuticals Inc. Tradjenta (package insert). Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc. 2015.
Gomis R, Espadero RM, Jones R, Woerle HJ, Dugi KA. Efficacy and safety of initial combination therapy with linagliptin and pioglitazone in patients with inadequately controlled type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2011;13(7):653–61.
Del Prato S, Barnett AH, Huisman H, Neubacher D, Woerle HJ, Dugi KA. Effect of linagliptin monotherapy on glycaemic control and markers of beta-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2011;13(3):258–67.
Taskinen MR, Rosenstock J, Tamminen I, et al. Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2011;13(1):65–74.
Owens DR, Swallow R, Dugi KA, Woerle HJ. Efficacy and safety of linagliptin in persons with type 2 diabetes inadequately controlled by a combination of metformin and sulphonylurea: a 24-week randomized study. Diabet Med. 2011;28(11):1352–61.
Kawamori R, Inagaki N, Araki E, et al. Linagliptin monotherapy provides superior glycaemic control versus placebo or voglibose with comparable safety in Japanese patients with type 2 diabetes: a randomized, placebo and active comparator-controlled, double-blind study. Diabetes Obes Metab. 2012;14(4):348–57.
Zeng Z, Yang JK, Tong N, et al. Efficacy and safety of linagliptin added to metformin and sulphonylurea in Chinese patients with type 2 diabetes: a sub-analysis of data from a randomised clinical trial. Curr Med Res Opin. 2013;29(8):921–9.
Chen Y, Ning G, Wang C, et al. Efficacy and safety of linagliptin monotherapy in Asian patients with inadequately controlled type 2 diabetes mellitus: a multinational, 24-week, randomized, clinical trial. J Diabetes Investig. 2015;6(6):692–8.
Wang W, Yang J, Yang G, et al. Efficacy and safety of linagliptin in Asian patients with type 2 diabetes mellitus inadequately controlled by metformin: a multinational 24-week, randomized clinical trial. J Diabetes. 2016;8(2):229–37.
Zeng Z, Choi DS, Mohan V, et al. Efficacy and safety of linagliptin as monotherapy or add-on treatment in Asian patients with suboptimal glycemic control: a pooled analysis. Curr Med Res Opin. 2015;31(1):99–106.
Woodward M, Zhang X, Barzi F, et al. The effects of diabetes on the risks of major cardiovascular diseases and death in the Asia-Pacific region. Diabetes Care. 2003;26(2):360–6.
Wu AY, Kong NC, de Leon FA, et al. An alarmingly high prevalence of diabetic nephropathy in Asian type 2 diabetic patients: the MicroAlbuminuria Prevalence (MAP) Study. Diabetologia. 2005;48(1):17–26.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–705.
Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013;56(4):696–708.
Yagi S, Aihara K, Akaike M, et al. Predictive factors for efficacy of dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes mellitus. Diabetes Metab J. 2015;39(4):342–7.
Fukushima M, Usami M, Ikeda M, et al. Insulin secretion and insulin sensitivity at different stages of glucose tolerance: a cross-sectional study of Japanese type 2 diabetes. Metabolism. 2004;53(7):831–5.
Chan WB, Tong PC, Chow CC, et al. The associations of body mass index, C-peptide and metabolic status in Chinese type 2 diabetic patients. Diabet Med. 2004;21(4):349–53.
Shah A, Kanaya AM. Diabetes and associated complications in the South Asian population. Curr Cardiol Rep. 2014;16(5):476.
Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013;36(6):1789–96.
Yoon KH, Ko SH, Cho JH, et al. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003;88(5):2300–8.
WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63.
Gray LJ, Yates T, Davies MJ, et al. Defining obesity cut-off points for migrant South Asians. PLoS One. 2011;6(10):e26464.
American Diabetes Association. Standards of medical care in diabetes—2015: summary of revisions. Diabetes Care. 2015;38(Suppl):S4.
Nomiyama T, Akehi Y, Takenoshita H, et al. Contributing factors related to efficacy of the dipeptidyl peptidase-4 inhibitor sitagliptin in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2012;95(2):e27–8.
Cai X, Han X, Luo Y, Ji L. Efficacy of dipeptidyl-peptidase-4 inhibitors and impact on beta-cell function in Asian and Caucasian type 2 diabetes mellitus patients: a meta-analysis. J Diabetes. 2015;7(3):347–59.
Stewart SL, Dang J, Chen MS Jr. Diabetes prevalence and risk factors in four Asian American communities. J Community Health. 2016;41(6):1264–73.
Tan VM, Lee YS, Venkataraman K, et al. Ethnic differences in insulin sensitivity and beta-cell function among Asian men. Nutr Diabetes. 2015;5:e173.
Ji L, Min KW, Oliveira J, Lew T, Duan R. Comparison of efficacy and safety of two starting insulin regimens in non-Asian, Asian Indian, and East Asian patients with type 2 diabetes: a post hoc analysis of the PARADIGM study. Diabetes Metab Syndr Obes. 2016;9:243–9.
Tan NC, Barbier S, Lim WY, Chia KS. 5-Year longitudinal study of determinants of glycemic control for multi-ethnic Asian patients with type 2 diabetes mellitus managed in primary care. Diabetes Res Clin Pract. 2015;110(2):218–23.
Fujita K, Kaneko M, Narukawa M. Factors related to the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors: a systematic review and meta-analysis focusing on ethnicity and study regions. Clin Drug Investig. 2016;37:219–32.
Sarashina A, Friedrich C, Crowe S, et al. Comparable pharmacodynamics, efficacy, and safety of linagliptin 5 mg among Japanese, Asian and white patients with type 2 diabetes. J Diabetes Investig. 2016;7(5):744–50.
Wang JS, Tu ST, Lee IT, et al. Contribution of postprandial glucose to excess hyperglycaemia in Asian type 2 diabetic patients using continuous glucose monitoring. Diabetes Metab Res Rev. 2011;27(1):79–84.
Wu W, Li Y, Chen X, et al. Effect of linagliptin on glycemic control in Chinese patients with newly-diagnosed, drug-naive type 2 diabetes mellitus: a randomized controlled trial. Med Sci Monit. 2015;21:2678–84.
Ramachandran A, Snehalatha C, Vijay V. Low risk threshold for acquired diabetogenic factors in Asian Indians. Diabetes Res Clin Pract. 2004;65(3):189–95.
Lewin AJ, Arvay L, Liu D, Patel S, von Eynatten M, Woerle HJ. Efficacy and tolerability of linagliptin added to a sulfonylurea regimen in patients with inadequately controlled type 2 diabetes mellitus: an 18-week, multicenter, randomized, double-blind, placebo-controlled trial. Clin Ther. 2012;34(9):1909–19.e15.
Yki-Jarvinen H, Rosenstock J, Duran-Garcia S, et al. Effects of adding linagliptin to basal insulin regimen for inadequately controlled type 2 diabetes: a ≥52-week randomized, double-blind study. Diabetes Care. 2013;36(12):3875–81.
McGill JB, Sloan L, Newman J, et al. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment: a 1-year, randomized, double-blind, placebo-controlled study. Diabetes Care. 2013;36(2):237–44.
Haak T, Meinicke T, Jones R, Weber S, von Eynatten M, Woerle HJ. Initial combination of linagliptin and metformin improves glycaemic control in type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2012;14(6):565–74.
Bajaj M, Gilman R, Patel S, Kempthorne-Rawson J, Woerle HJ. Linagliptin improved glycemic control without weight gain or hypoglycemia in patients with type 2 diabetes inadequately controlled by a combination of metformin and pioglitazone. Diabetes. 2013;62(Suppl 1):A283.
Ross SA, Rafeiro E, Meinicke T, Toorawa R, Weber-Born S, Woerle HJ. Efficacy and safety of linagliptin 2.5 mg twice daily versus 5 mg once daily in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, placebo-controlled trial. Curr Med Res Opin. 2012;28(9):1465–74.
Barnett AH, Huisman H, Jones R, von Eynatten M, Patel S, Woerle HJ. Linagliptin for patients aged 70 years or older with type 2 diabetes inadequately controlled with common antidiabetes treatments: a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382(9902):1413–23.
Laakso M, Rosenstock J, Groop PH, et al. Treatment with the dipeptidyl peptidase-4 inhibitor linagliptin or placebo followed by glimepiride in patients with type 2 diabetes with moderate to severe renal impairment: a 52-week, randomized, double-blind clinical trial. Diabetes Care. 2015;38(2):e15–7.
Acknowledgements
This study was supported by Boehringer Ingelheim, Eli Lilly and Company, and the Diabetes Alliance. Article processing charges and open access fees were funded by Boehringer Ingelheim. Medical writing assistance was supported financially by Boehringer Ingelheim, and was provided by Paul MacCallum, PhD and Jennifer Garrett, MB BS of Envision Scientific Solutions during the preparation of this manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Disclosures
Guang Ning is a member of the speaker bureau and advisory board and has received meeting support and honoraria from Boehringer Ingelheim. Guang Ning’s institution, Ruijin Hospital, Shanghai Jiao Tong University Hospital School of Medicine, has also received research grants from Boehringer Ingelheim. Juliana CN Chan is a member of the advisory board and speaker bureau and has received meeting support and honoraria from Boehringer Ingelheim. Juliana CN Chan’s insititution, the Chinese University of Hong Kong, has also received research grants from Boehringer Ingelheim. Uwe Hehnke is an employee of Boehringer Ingelheim Pharma GmbH & Co. KG. Jisoo Lee is an employee of Boehringer Ingelheim Pharma GmbH & Co. KG. Tushar Bandgar has nothing to disclose.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all trial participants.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/EDD8F0600A676521.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ning, G., Bandgar, T., Hehnke, U. et al. Efficacy and Safety of Linagliptin in 2681 Asian Patients Stratified by Age, Obesity, and Renal Function: A Pooled Analysis of Randomized Clinical Trials. Adv Ther 34, 2150–2162 (2017). https://doi.org/10.1007/s12325-017-0595-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-017-0595-7