Abstract
Background and objective
Several systematic reviews and meta-analyses have been conducted including an analysis to investigate the difference between ethnic groups in the glucose-lowering efficacy of dipeptidyl peptidase-4 (DPP-4) inhibitors. This study assessed the factors related to the glucose-lowering efficacy and explored potential differences among ethnicities; in particular, Japanese subjects were dealt separately from other Asian subjects.
Methods
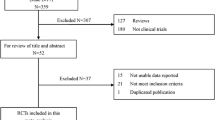
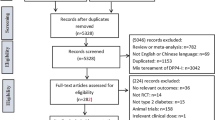
A systematic literature search was conducted using the MEDLINE, EMBASE, Cochrane Central Register, Japan Medical Abstracts Society, and ClinicalTrials.gov databases. Electronically searchable study results from the US/EU/Japanese regulatory approval reviews were also used. The weighted mean difference (WMD) for glycosylated hemoglobin (HbA1c) change from baseline in DPP-4 inhibitors compared with placebo was calculated. Heterogeneity was assessed by using the Q statistic and I 2 statistic. Univariate and multivariate meta-regression analyses were performed.
Results
The literature search identified 79 studies with 91 arms and 25,095 patients. The WMD in the change from baseline of HbA1c between the DPP-4 inhibitor group and placebo group was −0.695% (95% confidence interval −0.734 to −0.656) with considerable heterogeneity (I 2 = 69.7%). In univariate meta-regression, factors including study duration, percentage of males, age, duration of diabetes mellitus, baseline HbA1c values, body mass index, body weight, and percentage of Asian subjects showed associations with the glucose-lowering efficacy. Additionally, studies in Asian subjects, studies in Japanese subjects, and studies conducted in Japan showed relations when three classifications regarding ethnicity and study regions were applied. In multivariate meta-regression, studies in Japanese subjects/studies conducted in Japan as well as the baseline HbA1c values were identified as influencing factors.
Conclusion
These identified factors—Japanese subjects/studies conducted in Japan and the baseline HbA1c values—should be taken into account when planning and conducting future clinical studies.


Similar content being viewed by others
References
World Health Organization. Global report on diabetes. 2016. http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf?ua=1&ua=1. Accessed 14 Jun 2016.
Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–49. doi:10.1016/j.diabres.2013.11.002.
International Diabetes Federation. Diabetes atlas, seventh edition. 2015. http://www.diabetesatlas.org/component/attachments/?task=download&id=116. Accessed 14 Jun 2016.
Survey of the state of diabetes in Japan in 1997 [in Japanese]. 1997. http://www.mhlw.go.jp/toukei/kouhyo/indexkk_4_1.html. Accessed 14 Jun 2016.
National Health and Nutrition Survey Japan, 2012 [in Japanese]. 2012. http://www.mhlw.go.jp/bunya/kenkou/eiyou/h24-houkoku.html. Accessed 14 Jun 2016.
Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response. Diabetes Care. 2013;36(6):1789–96.
DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88(4):787–835. doi:10.1016/j.mcna.2004.04.013.
Kim DJ, Lee MS, Kim KW, Lee MK. Insulin secretory dysfunction and insulin resistance in the pathogenesis of Korean type 2 diabetes mellitus. Metabolism. 2001;50(5):590–3. doi:10.1053/meta.2001.22558.
Qian L, Xu L, Wang X, Fu X, Gu Y, Lin F, et al. Early insulin secretion failure leads to diabetes in Chinese subjects with impaired glucose regulation. Diabetes Metab Res Rev. 2009;25(2):144–9. doi:10.1002/dmrr.922.
Matsumoto K, Miyake S, Yano M, Ueki Y, Yamaguchi Y, Akazawa S, et al. Glucose tolerance, insulin secretion, and insulin sensitivity in nonobese and obese Japanese subjects. Diabetes Care. 1997;20(10):1562–8.
Fukushima M, Usami M, Ikeda M, Nakai Y, Taniguchi A, Matsuura T, et al. Insulin secretion and insulin sensitivity at different stages of glucose tolerance: a cross-sectional study of Japanese type 2 diabetes. Metabolism. 2004;53(7):831–5.
Flatt PR, Bailey CJ, Green BD. Dipeptidyl peptidase IV (DPP IV) and related molecules in type 2 diabetes. Front Biosci. 2008;13:3648–60.
Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014;35(6):992–1019.
Seino Y, Kuwata H, Yabe D. Incretin-based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig. 2016;7(Suppl 1):102–9.
Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013;56(4):696–708. doi:10.1007/s00125-012-2827-3.
Park H, Park C, Kim Y, Rascati KL. Efficacy and safety of dipeptidyl peptidase-4 inhibitors in type 2 diabetes: meta-analysis. Ann Pharmacother. 2012;46(11):1453–69. doi:10.1345/aph.1R041.
Monami M, Cremasco F, Lamanna C, Marchionni N, Mannucci E. Predictors of response to dipeptidyl peptidase-4 inhibitors: evidence from randomized clinical trials. Diabetes Metab Res Rev. 2011;27(4):362–72. doi:10.1002/dmrr.1184.
Esposito K, Cozzolino D, Bellastella G, Maiorino MI, Chiodini P, Ceriello A, et al. Dipeptidyl peptidase-4 inhibitors and HbA1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2011;13(7):594–603. doi:10.1111/j.1463-1326.2011.01380.x.
Esposito K, Chiodini P, Capuano A, Maiorino MI, Bellastella G, Giugliano D. Baseline glycemic parameters predict the hemoglobin A1c response to DPP-4 inhibitors: meta-regression analysis of 78 randomized controlled trials with 20,053 patients. Endocrine. 2014;46(1):43–51. doi:10.1007/s12020-013-0090-0.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi:10.1136/bmj.b2700.
Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, et al. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig. 2012;3(1):39–40.
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2015. http://www.R-project.org/. Accessed 14 Jun 2016.
DeFronzo RA, Fleck PR, Wilson CA, Mekki Q. Alogliptin Study 010 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care. 2008;31(12):2315–7. doi:10.2337/dc08-1035.
Nauck MA, Ellis GC, Fleck PR, Wilson CA, Mekki Q. Alogliptin Study 008 Group. Efficacy and safety of adding the dipeptidyl peptidase-4 inhibitor alogliptin to metformin therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a multicentre, randomised, double-blind, placebo-controlled study. Int J Clin Pract. 2009;63(1):46–55. doi:10.1111/j.1742-1241.2008.01933.x.
Pratley RE, Reusch JE, Fleck PR, Wilson CA, Mekki Q. Alogliptin Study 009 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin added to pioglitazone in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Curr Med Res Opin. 2009;25(10):2361–71. doi:10.1185/03007990903156111.
Pratley RE, Kipnes MS, Fleck PR, Wilson C, Mekki Q. Alogliptin Study 007 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes inadequately controlled by glyburide monotherapy. Diabetes Obes Metab. 2009;11(2):167–76. doi:10.1111/j.1463-1326.2008.01016.x.
Seino Y, Fujita T, Hiroi S, Hirayama M, Kaku K. Efficacy and safety of alogliptin in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, dose-ranging comparison with placebo, followed by a long-term extension study. Curr Med Res Opin. 2011;27(9):1781–92. doi:10.1185/03007995.2011.599371.
Kaku K, Itayasu T, Hiroi S, Hirayama M, Seino Y. Efficacy and safety of alogliptin added to pioglitazone in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label long-term extension study. Diabetes Obes Metab. 2011;13(11):1028–35. doi:10.1111/j.1463-1326.2011.01460.x.
Seino Y, Fujita T, Hiroi S, Hirayama M, Kaku K. Alogliptin plus voglibose in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label, long-term extension. Curr Med Res Opin. 2011;27(Suppl 3):21–9. doi:10.1185/03007995.2011.614936.
Seino Y, Hiroi S, Hirayama M, Kaku K. Efficacy and safety of alogliptin added to sulfonylurea in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label, long-term extension study. J Diabetes Investig. 2012;3(6):517–25. doi:10.1111/j.2040-1124.2012.00226.x.
Seino Y, Miyata Y, Hiroi S, Hirayama M, Kaku K. Efficacy and safety of alogliptin added to metformin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label, long-term extension study. Diabetes Obes Metab. 2012;14(10):927–36. doi:10.1111/j.1463-1326.2012.01620.x.
Pan C, Han P, Ji Q, Li C, Lu J, Yang J, et al. Efficacy and safety of alogliptin in patients with type 2 diabetes mellitus: a multicentre randomized double-blind placebo-controlled phase 3 study in mainland China, Taiwan, and Hong Kong. J Diabetes. 2016;. doi:10.1111/1753-0407.12425 [Epub ahead of print].
Forst T, Uhlig-Laske B, Ring A, Graefe-Mody U, Friedrich C, Herbach K, et al. Linagliptin (BI 1356), a potent and selective DPP-4 inhibitor, is safe and efficacious in combination with metformin in patients with inadequately controlled type 2 diabetes. Diabet Med. 2010;27(12):1409–19. doi:10.1111/j.1464-5491.2010.03131.x.
Del Prato S, Barnett AH, Huisman H, Neubacher D, Woerle HJ, Dugi KA. Effect of linagliptin monotherapy on glycaemic control and markers of beta-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2011;13(3):258–67. doi:10.1111/j.1463-1326.2010.01350.x.
Taskinen MR, Rosenstock J, Tamminen I, Kubiak R, Patel S, Dugi KA, et al. Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2011;13(1):65–74. doi:10.1111/j.1463-1326.2010.01326.x.
Owens DR, Swallow R, Dugi KA, Woerle HJ. Efficacy and safety of linagliptin in persons with type 2 diabetes inadequately controlled by a combination of metformin and sulphonylurea: a 24-week randomized study. Diabet Med. 2011;28(11):1352–61. doi:10.1111/j.1464-5491.2011.03387.x.
Lewin AJ, Arvay L, Liu D, Patel S, von Eynatten M, Woerle HJ. Efficacy and tolerability of linagliptin added to a sulfonylurea regimen in patients with inadequately controlled type 2 diabetes mellitus: an 18-week, multicenter, randomized, double-blind, placebo-controlled trial. Clin Ther. 2012;34(9):1909–19 e15. doi: 10.1016/j.clinthera.2012.07.008.
Kawamori R, Inagaki N, Araki E, Watada H, Hayashi N, Horie Y, et al. Linagliptin monotherapy provides superior glycaemic control versus placebo or voglibose with comparable safety in Japanese patients with type 2 diabetes: a randomized, placebo and active comparator-controlled, double-blind study. Diabetes Obes Metab. 2012;14(4):348–57. doi:10.1111/j.1463-1326.2011.01545.x.
Ross SA, Rafeiro E, Meinicke T, Toorawa R, Weber-Born S, Woerle HJ. Efficacy and safety of linagliptin 2.5 mg twice daily versus 5 mg once daily in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, placebo-controlled trial. Curr Med Res Opin. 2012;28(9):1465–74. doi:10.1185/03007995.2012.714360.
Haak T, Meinicke T, Jones R, Weber S, von Eynatten M, Woerle HJ. Initial combination of linagliptin and metformin improves glycaemic control in type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2012;14(6):565–74. doi:10.1111/j.1463-1326.2012.01590.x.
Barnett AH, Patel S, Harper R, Toorawa R, Thiemann S, von Eynatten M, et al. Linagliptin monotherapy in type 2 diabetes patients for whom metformin is inappropriate: an 18-week randomized, double-blind, placebo-controlled phase III trial with a 34-week active-controlled extension. Diabetes Obes Metab. 2012;14(12):1145–54. doi:10.1111/dom.12011.
Bajaj M, Gilman R, Patel S, Kempthorne-Rawson J. Lewis-D’Agostino D, Woerle HJ. Linagliptin improved glycaemic control without weight gain or hypoglycaemia in patients with type 2 diabetes inadequately controlled by a combination of metformin and pioglitazone: a 24-week randomized, double-blind study. Diabet Med. 2014;31(12):1505–14. doi:10.1111/dme.12495.
Chen Y, Ning G, Wang C, Gong Y, Patel S, Zhang C, et al. Efficacy and safety of linagliptin monotherapy in Asian patients with inadequately controlled type 2 diabetes mellitus: a multinational, 24-week, randomized, clinical trial. J Diabetes Investig. 2015;6(6):692–8. doi:10.1111/jdi.12346.
Wang W, Yang J, Yang G, Gong Y, Patel S, Zhang C, et al. Efficacy and safety of linagliptin in Asian patients with type 2 diabetes mellitus inadequately controlled by metformin: a multinational 24-week, randomized clinical trial. J Diabetes. 2016;8(2):229–37. doi:10.1111/1753-0407.12284.
Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes. Diabetes Obes Metab. 2008;10(5):376–86. doi:10.1111/j.1463-1326.2008.00876.x.
Rosenstock J, Aguilar-Salinas C, Klein E, Nepal S, List J, Chen R, et al. Effect of saxagliptin monotherapy in treatment-naive patients with type 2 diabetes. Curr Med Res Opin. 2009;25(10):2401–11. doi:10.1185/03007990903178735.
DeFronzo RA, Hissa MN, Garber AJ, Luiz Gross J, Yuyan Duan R, Ravichandran S, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care. 2009;32(9):1649–55. doi:10.2337/dc08-1984.
Hollander P, Li J, Allen E, Chen R, CV181-013 Investigators. Saxagliptin added to a thiazolidinedione improves glycemic control in patients with type 2 diabetes and inadequate control on thiazolidinedione alone. J Clin Endocrinol Metab. 2009;94(12):4810–9. doi:10.1210/jc.2009-0550.
Chacra AR, Tan GH, Apanovitch A, Ravichandran S, List J, Chen R, et al. Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: a randomised controlled trial. Int J Clin Pract. 2009;63(9):1395–406. doi:10.1111/j.1742-1241.2009.02143.x.
Yang W, Pan CY, Tou C, Zhao J, Gause-Nilsson I. Efficacy and safety of saxagliptin added to metformin in Asian people with type 2 diabetes mellitus: a randomized controlled trial. Diabetes Res Clin Pract. 2011;94(2):217–24. doi:10.1016/j.diabres.2011.07.035.
Pan CY, Yang W, Tou C, Gause-Nilsson I, Zhao J. Efficacy and safety of saxagliptin in drug-naive Asian patients with type 2 diabetes mellitus: a randomized controlled trial. Diabetes Metab Res Rev. 2012;28(3):268–75. doi:10.1002/dmrr.1306.
Frederich R, McNeill R, Berglind N, Fleming D, Chen R. The efficacy and safety of the dipeptidyl peptidase-4 inhibitor saxagliptin in treatment-naive patients with type 2 diabetes mellitus: a randomized controlled trial. Diabetol Metab Syndr. 2012;4(1):36. doi:10.1186/1758-5996-4-36.
White JL, Buchanan P, Li J, Frederich R. A randomized controlled trial of the efficacy and safety of twice-daily saxagliptin plus metformin combination therapy in patients with type 2 diabetes and inadequate glycemic control on metformin monotherapy. BMC Endocr Disord. 2014;14:17. doi:10.1186/1472-6823-14-17.
Moses RG, Kalra S, Brook D, Sockler J, Monyak J, Visvanathan J, et al. A randomized controlled trial of the efficacy and safety of saxagliptin as add-on therapy in patients with type 2 diabetes and inadequate glycaemic control on metformin plus a sulphonylurea. Diabetes Obes Metab. 2014;16(5):443–50. doi:10.1111/dom.12234.
Seino Y. Efficacy and safety of saxagliptin in Japanese patients with type 2 diabetes—two multi-centre, randomized, double-blind, placebo-controlled studies [in Japanese]. Jpn Pharmacol Ther. 2014;42(7):503–18.
Matthaei S, Catrinoiu D, Celiński A, Ekholm E, Cook W, Hirshberg B, et al. Randomized, double-blind trial of triple therapy with saxagliptin add-on to dapagliflozin plus metformin in patients with type 2 diabetes. Diabetes Care. 2015;38(11):2018–24. doi:10.2337/dc15-0811.
AstraZeneca. Evaluate saxagliptin in adult indian patients with type 2 diabetes inadequate glycemic control [ClinicalTrials.gov identifier NCT00918879]. US National Institutes of Health, ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT00918879?term=NCT00918879&rank=1. Accessed 7 Jul 2016.
Ristic S, Byiers S, Foley J, Holmes D. Improved glycaemic control with dipeptidyl peptidase-4 inhibition in patients with type 2 diabetes: vildagliptin (LAF237) dose response. Diabetes Obes Metab. 2005;7(6):692–8. doi:10.1111/j.1463-1326.2005.00539.x.
Dejager S, Razac S, Foley JE, Schweizer A. Vildagliptin in drug-naive patients with type 2 diabetes: a 24-week, double-blind, randomized, placebo-controlled, multiple-dose study. Horm Metab Res. 2007;39(3):218–23. doi:10.1055/s-2007-970422.
Pi-Sunyer FX, Schweizer A, Mills D, Dejager S. Efficacy and tolerability of vildagliptin monotherapy in drug-naive patients with type 2 diabetes. Diabetes Res Clin Pract. 2007;76(1):132–8. doi:10.1016/j.diabres.2006.12.009.
Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30(4):890–5. doi:10.2337/dc06-1732.
Garber AJ, Schweizer A, Baron MA, Rochotte E, Dejager S. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab. 2007;9(2):166–74. doi:10.1111/j.1463-1326.2006.00684.x.
Garber AJ, Foley JE, Banerji MA, Ebeling P, Gudbjornsdottir S, Camisasca RP, et al. Effects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulphonylurea. Diabetes Obes Metab. 2008;10(11):1047–56. doi:10.1111/j.1463-1326.2008.00859.x.
Kikuchi M, Abe N, Kato M, Terao S, Mimori N, Tachibana H. Vildagliptin dose-dependently improves glycemic control in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2009;83(2):233–40. doi:10.1016/j.diabres.2008.10.006.
Kikuchi M, Haneda M, Koya D, Tobe K, Onishi Y, Couturier A, et al. Efficacy and tolerability of vildagliptin as an add-on to glimepiride in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2010;89(3):216–23. doi:10.1016/j.diabres.2010.04.017.
Kikuchi M, Iwamoto Y, Inagaki N, Yoshioka T, Mimori N, Ebina H. Clinical evaluation of vildagliptin in patients with type 2 diabetes [in Japanese]. J New Rem Clin. 2010;59(2):121–36.
Pan C, Xing X, Han P, Zheng S, Ma J, Liu J, et al. Efficacy and tolerability of vildagliptin as add-on therapy to metformin in Chinese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14(8):737–44. doi:10.1111/j.1463-1326.2012.01593.x.
Odawara M, Hamada I, Suzuki M. Efficacy and safety of vildagliptin as add-on to metformin in Japanese patients with type 2 diabetes mellitus. Diabetes Ther. 2014;5(1):169–81. doi:10.1007/s13300-014-0059-x.
Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia. 2006;49(11):2564–71. doi:10.1007/s00125-006-0416-z.
Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE, et al. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29(12):2632–7. doi:10.2337/dc06-0703.
Charbonnel B, Karasik A, Liu J, Wu M. Meininger G; Sitagliptin Study 020 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29(12):2638–43. doi:10.2337/dc06-0706.
Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28(10):1556–68. doi:10.1016/j.clinthera.2006.10.007.
Hanefeld M, Herman GA, Wu M, Mickel C, Sanchez M, Stein PP, et al. Once-daily sitagliptin, a dipeptidyl peptidase-4 inhibitor, for the treatment of patients with type 2 diabetes. Curr Med Res Opin. 2007;23(6):1329–39. doi:10.1185/030079907X188152.
Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE, Sitagliptin 036 Study Group. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007;30(8):1979–87. doi:10.2337/dc07-0627.
Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9(5):733–45. doi:10.1111/j.1463-1326.2007.00744.x.
Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract. 2007;61(1):171–80. doi:10.1111/j.1742-1241.2006.01246.x.
Raz I, Chen Y, Wu M, Hussain S, Kaufman KD, Amatruda JM, et al. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin. 2008;24(2):537–50. doi:10.1185/030079908X260925.
Scott R, Loeys T, Davies MJ, Engel SS. Sitagliptin Study 801 Group. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2008;10(10):959–69. doi:10.1111/j.1463-1326.2007.00839.x.
Nonaka K, Kakikawa T, Sato A, Okuyama K, Fujimoto G, Kato N, et al. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2008;79(2):291–8. doi:10.1016/j.diabres.2007.08.021.
Mohan V, Yang W, Son HY, Xu L, Noble L, Langdon RB, et al. Efficacy and safety of sitagliptin in the treatment of patients with type 2 diabetes in China, India, and Korea. Diabetes Res Clin Pract. 2009;83(1):106–16. doi:10.1016/j.diabres.2008.10.009.
Iwamoto Y, Taniguchi T, Nonaka K, Okamoto T, Okuyama K, Arjona Ferreira JC, et al. Dose-ranging efficacy of sitagliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Endocr J. 2010;57(5):383–94.
Kashiwagi A, Kadowaki T, Tajima N, Nonaka K, Taniguchi T, Nishii M, et al. Sitagliptin added to treatment with ongoing pioglitazone for up to 52 weeks improves glycemic control in Japanese patients with type 2 diabetes. J Diabetes Investig. 2011;2(5):381–90. doi:10.1111/j.2040-1124.2011.00120.x.
Barzilai N, Guo H, Mahoney EM, Caporossi S, Golm GT, Langdon RB, et al. Efficacy and tolerability of sitagliptin monotherapy in elderly patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Curr Med Res Opin. 2011;27(5):1049–58. doi:10.1185/03007995.2011.568059.
Tajima N, Kadowaki T, Odawara M, Nishii M, Taniguchi T, Arjona Ferreira JC. Addition of sitagliptin to ongoing glimepiride therapy in Japanese patients with type 2 diabetes over 52 weeks leads to improved glycemic control. Diabetol Int. 2011;2(1):32–44. doi:10.1007/s13340-011-0022-2.
Yang W, Guan Y, Shentu Y, Li Z, Johnson-Levonas AO, Engel SS, et al. The addition of sitagliptin to ongoing metformin therapy significantly improves glycemic control in Chinese patients with type 2 diabetes. J Diabetes. 2012;4(3):227–37. doi:10.1111/j.1753-0407.2012.00213.x.
Nicolle LE, Capuano G, Ways K, Usiskin K. Effect of canagliflozin, a sodium glucose co-transporter 2 (SGLT2) inhibitor, on bacteriuria and urinary tract infection in subjects with type 2 diabetes enrolled in a 12-week, phase 2 study. Curr Med Res Opin. 2012;28(7):1167–71. doi:10.1185/03007995.2012.689956.
Fonseca V, Staels B, Morgan JD 2nd, Shentu Y, Golm GT, Johnson-Levonas AO, et al. Efficacy and safety of sitagliptin added to ongoing metformin and pioglitazone combination therapy in a randomized, placebo-controlled, 26-week trial in patients with type 2 diabetes. J Diabetes Complications. 2013;27(2):177–83. doi:10.1016/j.jdiacomp.2012.09.007.
Dobs AS, Goldstein BJ, Aschner P, Horton ES, Umpierrez GE, Duran L, et al. Efficacy and safety of sitagliptin added to ongoing metformin and rosiglitazone combination therapy in a randomized placebo-controlled 54-week trial in patients with type 2 diabetes. J Diabetes. 2013;5(1):68–79. doi:10.1111/j.1753-0407.2012.00223.x.
Roden M, Weng J, Eilbracht J, Delafont B, Kim G, Woerle HJ, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1(3):208–19. doi:10.1016/s2213-8587(13)70084-6.
Lavalle-Gonzalez FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56(12):2582–92. doi:10.1007/s00125-013-3039-1.
Tajima N, Kadowaki T, Okamoto T, Sato A, Okuyama K, Minamide T, et al. Sitagliptin added to voglibose monotherapy improves glycemic control in patients with type 2 diabetes. J Diabetes Investig. 2013;4(6):595–604. doi:10.1111/jdi.12116.
Kadowaki T, Tajima N, Odawara M, Nishii M, Taniguchi T, Ferreira JC. Addition of sitagliptin to ongoing metformin monotherapy improves glycemic control in Japanese patients with type 2 diabetes over 52 weeks. J Diabetes Investig. 2013;4(2):174–81. doi:10.1111/jdi.12001.
Amin NB, Aggarwal N, Pall D, Paragh G, Denney WS, Le V, et al. Two dose-ranging studies with PF-04937319, a systemic partial activator of glucokinase, as add-on therapy to metformin in adults with type 2 diabetes. Diabetes Obes Metab. 2015;17(8):751–9. doi:10.1111/dom.12474.
Ji L, Han P, Wang X, Liu J, Zheng S, Jou YM, et al. Randomized clinical trial of the safety and efficacy of sitagliptin and metformin co-administered to Chinese patients with type 2 diabetes mellitus. J Diabetes Investig. 2016;7(5):727–36. doi:10.1111/jdi.12511.
Moses RG, Round E, Shentu Y, Golm GT, O’Neill EA, Gantz I, et al. A randomized clinical trial evaluating the safety and efficacy of sitagliptin added to the combination of sulfonylurea and metformin in patients with type 2 diabetes mellitus and inadequate glycemic control. J Diabetes. 2016;8(5):701–11. doi:10.1111/1753-0407.12351.
Merck Sharp & Dohme Corp. Clinical trial to evaluate the safety and efficacy of the addition of sitagliptin in participants with type 2 diabetes mellitus receiving acarbose monotherapy (MK-0431-130) [ClinicalTrials.gov identifier NCT01177384]. US National Institutes of Health, ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01177384?term=NCT01177384&rank=1. Accessed 7 Jul 2016.
Boehringer Ingelheim/Eli Lilly and Company. Safety and efficacy of empagliflozin (BI 10773) and sitagliptin versus placebo over 76 weeks in patients with type 2 diabetes [ClinicalTrials.gov identifier NCT01289990]. US National Institutes of Health, ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01289990?term=NCT01289990&rank=1. Accessed 7 Jul 2016.
Pfizer. Study of safety and efficacy of PF-04991532 in subjects with type 2 diabetes [ClinicalTrials.gov identifier NCT01338870]. US National Institutes of Health, ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01338870?term=NCT01338870&rank=1. Accessed 7 Jul 2016.
Pfizer. Study of safety and efficacy of PF-04991532 in subjects with type 2 diabetes mellitus [ClinicalTrials.gov identifier NCT01336738]. US National Institutes of Health, ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01336738?term=NCT01336738&rank=1. Accessed 7 Jul 2016.
Merck Sharp & Dohme Corp. A study in China evaluating the safety and efficacy of adding sitagliptin to stable therapy with sulfonylurea with or without metformin in participants with type 2 diabetes mellitus (T2DM) (MK-0431-253) [ClinicalTrials.gov identifier NCT01590771]. US National Institutes of Health, ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01590771?term=NCT01590771&rank=1. Accessed 7 Jul 2016.
Merck Sharp & Dohme Corp. Omarigliptin (MK-3102) clinical trial – placebo- and sitagliptin-controlled monotherapy study in japanese patients with type 2 diabetes mellitus (MK-3102-020) [ClinicalTrials.gov identifier NCT01703221]. US National Institutes of Health, ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01703221?term=NCT01703221&rank=1. Accessed 7 Jul 2016.
Bloomgarden ZT, Dodis R, Viscoli CM, Holmboe ES, Inzucchi SE. Lower baseline glycemia reduces apparent oral agent glucose-lowering efficacy: a meta-regression analysis. Diabetes Care. 2006;29(9):2137–9. doi:10.2337/dc06-1120.
Deacon CF. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes Metab. 2011;13(1):7–18. doi:10.1111/j.1463-1326.2010.01306.x.
Singh AK. Incretin response in Asian type 2 diabetes: are Indians different? Indian J Endocrinol Metab. 2015;19(1):30–8. doi:10.4103/2230-8210.146861.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this manuscript was not supported by any external funding.
Conflict of interest
The authors have no conflicts of interest that are directly relevant to this research. Kayo Fujita is an employee of Pfizer Japan Inc.
Rights and permissions
About this article
Cite this article
Fujita, K., Kaneko, M. & Narukawa, M. Factors Related to the Glucose-Lowering Efficacy of Dipeptidyl Peptidase-4 Inhibitors: A Systematic Review and Meta-Analysis Focusing on Ethnicity and Study Regions. Clin Drug Investig 37, 219–232 (2017). https://doi.org/10.1007/s40261-016-0478-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-016-0478-8