Abstract
The cerebellum plays an important role in maintaining balance, posture control, muscle tone, and lower limb coordination in healthy individuals and stroke patients. At the same time, the relationship between cerebellum and motor learning has been widely concerned in recent years. Due to the relatively intact structure preservation and high plasticity after supratentorial stroke, non-invasive neuromodulation targeting the cerebellum is increasingly used to treat abnormal gait in stroke patients. The gamma frequency of transcranial alternating current stimulation (tACS) is commonly used to improve motor learning. It is an essential endogenous EEG oscillation in the gamma range during the swing phase, and rhythmic movement changes in the gait cycle. However, the effect of cerebellar tACS in the gamma frequency band on balance and walking after stroke remains unknown and requires further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Muscle weakness, aberrant muscle tone, joint contractures, and other sequelae caused by a stroke can result in abnormal gait and increase the risk of falling, causing great inconvenience to the daily life activities of the patients. Normal gait is associated with good balance, postural control, adequate muscle strength, and rhythmic motor development. Additionally, patients need to have relevant motor learning skills such as environmental adaptation and information integration [1]. Clinically, most conventional rehabilitation therapies—including physical therapy and occupational therapy—are used to help patients walk more easily by improving muscle strength and muscle tone in the affected limb. However, their abnormal gait pattern is difficult to correct. This abnormal asymmetrical gait may also lead to abnormal joint loading in the lower limb, resulting in joint injury, pain, and deformity [2], forming an irregular gait pattern and raising the risk of falling. For improving abnormal gait, new techniques like neuromodulation targeting the cerebral cortex can be combined to improve the patient’s postural control and motor learning ability from the “top-down” to correct the issue. This is in addition to the traditional “bottom-up” rehabilitation therapy, which reshapes the injured cortex and subcortical network through movement and proprioceptive training of the affected limb. Non-invasive brain stimulation (NIBS) is the primary method for neuromodulation, including transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), etc., producing long-term potentiation by modulating cortical excitability. Transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), and similar techniques alter the excitability of the cerebral cortex to produce long-term potentiation (LTP) or long-term depression (LTP). Long-term potentiation (LTP) or long-term depression (LTD) regulates cortical metabolism and electrophysiological activity by modulating cortical excitability [3], which has been used in treating various neurological diseases [4]. The primary motor cortex has been primarily targeted in recent studies of NIBS interventions for lower extremity dysfunction following stroke, but the effects are uneven [5,6,7,8].
Due to the extensive connection between the cerebellum and the cortex, neocortex, and inferior spinal tracts, the cerebellum can regulate posture control, balance support, and motor learning during walking [9, 10]. Additionally, studies have proved that most patients with supratentorial injuries have structurally sound cerebellum with a high level of plasticity [11,12,13]. Therefore, from the perspective of gait and motor learning theory, researchers have considered the research significance and viability of the cerebellum as a new target to improve gait abnormalities.
In contrast to other non-invasive brain stimulation (NIBS) techniques, transcranial alternating current stimulation (tACS) can selectively influence oscillations at the stimulated frequency through a targeted paradigm of periodic and mild perturbations. This capability allows for a more precise investigation of the mechanistic role of the cerebellum in walking and motor learning. It can aid in selecting methods for reshaping the motor brain network in stroke patients [14]. However, existing literature has only demonstrated the enhancement of lower limb function in healthy individuals with 50 Hz, specifically in the gamma frequency range, tACS, and this approach has yet to be applied to stroke patients.
Therefore, we will summarize the crucial roles of the cerebellum and gamma frequency oscillations in walking and motor learning. This will serve as a foundation to explore the application of gamma frequency cerebellar transcranial alternating current stimulation (tACS) in improving walking function in stroke patients. We conducted a comprehensive search on PubMed, Embase, and Web of Science, using keywords such as “cerebellum,” “gamma oscillations,” “tACS,” “post-stroke cortical reshaping,” “walking,” and “motor learning.” We compiled and categorized the obtained content for presentation in this review.
Cerebellum and Gait
The cerebellum is an anatomically complex region of various structures involved in motor planning, learning, execution, and movement patterning. According to structural and functional neuroimaging studies of the neural basis of balance, the cerebellum is the most critical brain region for controlling balance, and postural control is directly correlated with cerebellar gray matter volume [15,16,17]. Poor balance and coordination and frozen gait can be caused by cerebellar hypoplasia or damage to the cerebellar structures [18]. The cerebellum can maintain balance by integrating somatosensory input from the head and proximal parts of the body and direct connections to the contralateral cortical motor area [5, 19, 20]. Additionally, a study in post-stroke populations has shown a direct correlation between walking ability during recovery and the degree of cerebellar activation [21]. It is clear that the cerebellum plays a crucial regulatory role in balance movements and that each of its various parts is important for postural control and motor regulation.
Cerebellar Involvement in Balance and Postural Control in Walking
The cerebellar vermis and nodes are the vestibular cerebellum (cerebellum), and the cerebellar earth and middle portion of the hemispheres are referred to as the spinal cerebellum (old cerebellum). The combined synergy of each part plays a significant role [22] in muscle tone, posture, balance control, and limb coordination. Among them, the cerebellum is one of the supraspinal walking generators for anticipatory postural adjustment, walking initiation, and speed regulation. It is connected to the spinal motor neurons through the brainstem [23]. The sensorimotor cortex, parietal lobe, visual cortex, thalamus, basal ganglia, cerebellum, and subthalamic and midbrain (cuneate and pontine peduncle nuclei), as well as the vestibular, red, and olivary nuclei [24] that descend into the brainstem, are among the neural networks involved in walking. The cerebellum plays a crucial role in walking because of its extensive and intricate connectivity to these structures. According to the classical optimal feedback control theory [25] of postural control, the cerebellum plays a significant role in walking by being able to derive the best flexible multi-joint combinations of movement schemes and output them downward using the basic sensory integration information acquired with brain regions like the sensorimotor cortex, visual cortex, and thalamus as well as the prediction of movement sensations [26] by brain regions like the sensorimotor cortex and parietal cortex. An important location for the integration of feedback data is the vestibular cerebellum. The cerebellar nodes, the earthworm, the lobule, and the pars cerebellar are just a few of the midline and caudal cerebellum structures where vestibular protrusions from the vestibular nuclei can be found. These structures are primarily involved in integrating vestibular information to control the head and trunk position during movement. The spinal motor centers, basal ganglia, and frontal lobes [27] are also affected by the vestibular cerebellum’s projections, allowing for limb coordination and fine-tuning posture during walking through visual [28, 29] and proprioceptive feedback. The cerebellum, cortex, and spinal cord control static postural functions [30] like head and trunk tilt. This circuit can also fine-tune limb movements during walking, allowing the trunk or limbs to return to their original movement patterns and maintain dynamic balance after being perturbed by the outside world. To adapt to internal and external disturbances, this motor pattern depends on a complex interplay between the vestibular, visual, and proprioceptive systems of the spinal cord, supraspinal motor networks, and sensory feedback, in which the cerebellum plays a key role.
Cerebellar Involvement in Motor Learning in Walking
In addition to postural control and balance coordination, the cerebellar structures’ interconnectedness can significantly affect gait in terms of its function in motor learning. First, the cerebellum can be crucial for learning new skills, and studies [31, 32] on animals have revealed that the paramedian lobule of the cerebellum’s cellular dendrites changes as new skills are learned. Lower activity in the cerebellar cortex’s lobules impacts how learned automatic tasks in the upper limbs are carried out [33]. Since “error prediction and correction” has long been thought to be the primary mechanism underlying cerebellar motor learning, olivary nucleus-cerebellar activity has been shown to enhance motor commands, and issue sustained motor commands through synaptic plasticity. The “trigger and store” theory [34] postulates that neural tracts from the olivary nucleus-cerebellar pathway connect to various cerebellum regions (like cerebellar cortical cells and nuclei). Plasticity occurs between these regions at multiple rates to correct or avoid errors, like completing classical associative motor learning, and like the blink reflex. Patients with primary cerebellar hypoplasia have been shown [35] to have impaired blink reflexes, and patients with cerebellar stroke perform significantly worse [36]on continuous finger-tapping tasks and sequential tasks in consolidation when compared to unaffected controls. Learning new cognitive operations is also influenced by the extensive cerebellar-neocortical connectivity 5 that results in cortico-cerebellar plasticity, and this motor learning also takes the form of learning new sequences [37] and predicting and correcting errors [38].
A recent study [39] demonstrates that depending on the pertinent loops and differences in signaling, motor learning patterns in the cerebellar-motor cortex, cerebellar-frontoparietal network, and cerebellar-spinal cord have complex and higher-level influences, including “prediction and measurement of movement” in addition to new skill learning and error prediction correction, and they suggest that NMDA [40] receptor-dominated LTD loops in cerebellar parallel fiber-Purkinje cells [41] (or molecular lamina) are responsible. Thus, the cerebellum can play a significant role in associative motor learning and creating and maintaining sequential movements thanks to connections between the olivary nucleus and cerebellum and the cerebellum and the neocortex. This can be observed throughout the entire gait cycle of a human. Still, it is particularly evident when walking because gait trajectories and speeds must be constantly adjusted to maintain uninterrupted walking movements to accommodate environmental constraints like shifting road and terrain conditions [42].
Progress in the Study of the Effects of Cerebellar NIBS on Walking
Numerous NIBS interventions [43,44,45] targeting the cerebellum have been tested in recent years to enhance motor learning from various perspectives, proving that the cerebellum has multiple effects on motor learning via different pathways. Belkhiria C et al. [46] discovered that when faced with a route change or needing to pass through a scene with narrow terrain, activation of the cerebellar-red nucleus and cerebellar striatum was linked to motor readiness and sustained attention by functional MRI [47]. Koch G et al. [48] used TMS’s intermittent theta burst rhythm stimulation patterns to improve Berg Balance Scale scores and walking function of stroke patients. At the same time, EEG revealed specific activation of the parietal lobe, which is consistent with the combined effect of cerebellar effects on motor and frontoparietal motor learning networks to improve gait. Studies focusing on the modulation of normal and stroke gait at various targets of cerebellar NIBS in combination with traditional rehabilitation techniques like conventional physiotherapy or walking-assisted robots have grown in number [49, 50] This is due to the high plasticity of the cerebellum in movement and motor learning and the non-invasive and user-friendly benefits of NIBS. With bilateral high-accuracy cerebellar tDCS interventions in the dentate nucleus of the cerebellum and VIIb–IX in the lower extremity region of patients with posterior stage stroke, respectively, Solanki D et al. [51] demonstrated improved scores on the 10-m walk test, Time Up and Go, and Berg Balance Scale. The findings of Picelli A’s team [52, 53] lend credence to the notion that augmenting robot-assisted gait training in chronic stroke patients with cathodal tDCS stimulation of the contralateral cerebellar hemisphere and cathodal DC stimulation can be beneficial.
Gamma Band Has a Significant Impact on Movement and Walking
EEG oscillations produced by neurons during brain activity are typically divided into five bands: beta (13–30 Hz), alpha (8–13 Hz), theta (4–8 Hz), delta (1–4 Hz), and gamma (> 30 Hz) [54]. Among them, inhibitory interneurons and pyramidal cells [55] interact to produce EEG oscillations in the gamma band. Gamma oscillations affect perception, movement, memory, and emotion differently through excitation-inhibition and inhibition-inhibition control of the specific synaptic connections between inhibitory interneurons and their peripheral excitatory neurons. Gamma rhythms are categorized as low gamma (25–40 Hz), medium gamma (40–65 Hz), and high gamma (65–85 Hz) [56] by the various options for spatial information processing in the primary visual cortex. The basal ganglia–thalamic–cortex is the primary circuit of human movement. And localized single-point transient gamma oscillations can be activated and form a gamma oscillation network [51] in multiple brain regions by coupling phase and amplitude, thus creating coherence with theta waves [57] in key brain regions such as hippocampal pus and prefrontal to influence motor learning. After a stroke, the cortical network oscillations’ excitation and inhibition are out of balance and out of sync, affecting the injured lesion locally [58] and the functional connectivity between distal cortical loops. Exogenous gamma neuromodulation has been shown to improve blood flow, motor function [59] and brain reorganization in the acute phase through cross-regional interference with spontaneous EEG oscillatory networks in cortical regions. Gamma frequency oscillations are believed to play a significant role in typical walking and the motor initiation and learning processes associated with walking.
It has been demonstrated that the gamma band oscillations in the brain itself influence gait throughout the cycle and activate various brain areas to create stable walking networks. Brain EEG detected ERD-ERS (event-related desynchronization (ERD); event-related synchronization (ERS)) lateralization [60]in the cerebellar beta-gamma frequency band during gait initiation, i.e., during leg swing and the double-support phase [61]. ERD-ERS was also found in the primary motor cortex, premotor cortex, and thalamus throughout the gait cycle. Additionally, restricted rhythmic oscillation of the upper limb during walking reduces beta-gamma ERD-ERS in the supplementary motor area (SMA) [62]. Additionally, the gait cycle and the coupling of the high and low gamma frequency bands in the sensorimotor cortex are closely related, and modifications to their coupling form affect the gait rhythm [63]. According to another study [64], the pontocerebellar pedunculopontine nucleus exhibits intrinsic oscillations in the gamma frequency band, which is crucial for subcortical rhythmic walking. Therefore, we postulate that the initiation of walking, the transition and coordination of the swing and support phases, and the maintenance of rhythm in walking are all significantly influenced by the brain’s gamma-band oscillations.
The process of regaining motor function after a stroke is broadly analogous [65] to that of motor learning, and in normal subjects, activated cortical regions, particularly the cerebellum, play a significant and influential role in the later functional recovery [66]. Rhythmic oscillations between regions of the sensorimotor cortex [67], parietal lobe, and primary motor cortex [68] can be modulated and synergized by the cerebellum, thus regulating walking-related factors such as sensory input and muscle tone during movement. It has been demonstrated that [69] the thalamus, motor-sensory cortical areas, and motor cortical areas can all be excited by gamma-band oscillations in the cerebellum via specific neuronal synaptic structures.
Gamma-Band Cerebellar tACS for Improving Motor Function
Overview of tACS
TACS, a noninvasive neuromodulation technique, uses weak sinusoidal electric fields to modify cortical activity. Through a more focused stimulation paradigm, tACS periodic, weak perturbations can selectively affect oscillations at the applied stimulus frequency [70]. Exogenous sinusoidal alternating current (tACS) of various frequencies directly interacts with neuronal activity sustained during rest or in the task state, impacting oscillations in particular regional brain networks by entraining, synchronizing, or interfering with them [71]. However, the underlying mechanisms by which it modifies the spatiotemporal dynamics of large-scale cortical network dynamics are still up for discussion. The effects of tACS are highly variable, with different pulse frequencies, phases, and duration of action modulated to bring the cortex into excitation-inhibition balance [72], in contrast to the NIBS approach, which produces sustained, long-duration effects by altering the excitability of the cerebral cortex, including TMS and tDCS, thereby regulating cortical metabolism and electrophysiological activity [73]and targeting the threshold of neuronal membrane potential [74].
The contraindications of tACS as a type of transcutaneous electrical stimulation (tES) can be found in the list of clinical contraindications for tES, which includes patients with epilepsy, metal in the head, pacemakers, and other implanted materials [75]. Even though tDCS and tACS fall under the same category of tES, differences in their mechanisms of action and protocol design (including current intensity and target setting) result in differences in their tolerance, discomfort, and safety. For example, the anodal stimulation of tDCS tends to cause varying degrees of skin discomfort, such as tingling, itching, and burning when the current intensity reaches 2 mA, mainly due to mild transient skin effects caused by the vascular expansion effect induced by tES [76]. At the same time, tACS had lower skin discomfort and no significant difference compared to sham stimulation [77]. Due to the activation of the retinal neural firing caused by the placement of the tACS electrode in the frontal and occipital regions, which occurs only during the intervention [78], “optical hallucinations” or “phosphonic responses” are caused. These are perceptions of flickering light without actual visual input. The ipsilateral mandible was suggested as a secondary electrode placement for cerebellar tACS based on findings from a recent study [79] on electrode placement for cerebellar tACS that the cerebellar tACS intervention caused little skin sensation and high-intensity currents in the eye only when the electrodes were positioned close to the frontal and orbital regions, resulting in “optical hallucinations” as a side effect during the tACS intervention. As a result, cerebellar tACS is a painless and secure treatment option. To ensure the safety of the patient’s treatment, the treatment process also necessitates observation, measurement, and questioning of the patient’s blood pressure, heart rate, or any discomfort that may be present. This is followed by a prompt explanation or adjustment of the treatment plan to the patient's tolerance.
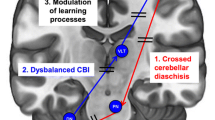
Research Progress on Cerebellar tACS in the Gamma Band
Early in 1995, it was found that a conditional stimulation of 90% AMT given to the contralateral cerebellum 5 ~ 7 ms before M1 stimulation could reduce MEP, and the reduction of MEP was related to the intensity of the conditioned stimulation, by Ugawa Y et al [80, 81]. They discovered that this reduced MEP and that the degree of the MEP reduction correlated with the intensity of the conditioned stimulus. One of the main ways the cerebellum is involved in motor function is through a phenomenon known as cerebellum-brain inhibition (CBI). This phenomenon is thought to be formed by the formation of a dentate-thalamic pathway dominated by Purkinje cells (PC) in the motor areas of the cerebellum for the inhibition of the contralateral motor cortex, which can be used to determine the strength of cerebellar-cortical connections, primarily involving parallel fibers, PC, and Golgi [82]. CBI increased, and MEP decreased after 300-Hz tACS, while CBI decreased, MEP increased, and finger-tapping task time decreased in healthy subjects after 50-Hz true-stimulation tACS, according to research by Naro A et al. [83, 84] using parallel fiber, PC, and Golgi firing frequencies of 10 Hz, 50 Hz, and 300 Hz to control 50-Hz sham-stimulation tACS on the cerebellum, respectively. Only 50 Hz caused an increase in MEP in the ipsilateral lower extremities when the same team applied the same tACS frequency setting to the lower extremities of healthy subjects. Based on this finding, the researchers hypothesized that 50-Hz ctACS might prevent PC-forming cerebellar LTD from increasing MEP 14. Based on the results of the studies mentioned above [35, 36], we can conclude that cerebellar tACS intervention in the 50-Hz gamma band has effects on cerebellar PC that go beyond simple LTD interference. There is insufficient evidence that PC is involved in CBI [85], so CBI is primarily used to explain cerebellar-cortical connections and is not sufficient, although the gamma band of tACS is thought to induce LTD-like plasticity changes [86] in the primary motor circuits of the brain. Additionally, different Purkinje cell synapses have other functions that include facilitation-inhibition and inhibition-inhibition. These functions can selectively inhibit peripheral muscles unrelated to motor production from improving cortical signaling to active muscles during locomotion, making cerebellum activation a convergent enhancement. Therefore, we prefer 50-Hz gamma band cerebellar tACS on walking, which may be connected to the cerebellum’s influence on walking balance, enhanced postural regulation before and during exercise, and the gamma band itself.
It has been shown that [87, 88] tACS in the gamma band of the M1-cerebellum improves visuomotor tracking ability in healthy individuals compared to M1 alone, as well as an increase in the number and speed of muscle fiber recruitment, representing motor ability.
Exogenous gamma oscillations produced by tACS in the primary motor cortex can significantly decrease the abduction reaction time [89] of the nonlethal thumb, whereas the coherence of cerebellar gamma-band oscillations with theta oscillations at 4–8 Hz in the hippocampus itself dominates skill learning and memory retrieval. This indicates that gamma-band changes in the cerebellum not only support the gamma band’s ability to synchronize motor-related brain regions like sensory and motor cortices [90] but also further improve motor performance by enhancing the activation of neural networks related to motor learning, such as vision and memory. This aligns with the earlier assertion that tACS intervention in the cerebellum’s gamma band can activate the cross-regional brain area. Improved upper limb function has been achieved using 50-Hz gamma cerebellar tACS, which uses gamma frequency band entrainment of the cerebellar inhibitory pathway Purkinje cell-parallel fibers. This technique enhances the learning of fixed-sequence movements and subsequently improves the mobility of patterned activities in daily life [91]. The SMA cerebellum was a better target than the supplementary motor area in another trial [92] of gamma-band tACS intervention on the upper extremity in healthy subjects.
Studies on the upper extremity have demonstrated that tACS in the gamma band acting on the cerebellum can support motor relearning in post-stroke patients either through its oscillations or through coherence with oscillations in other frequency bands in adjacent brain regions of the circuit [93], although tACS has received less attention about the use of motor learning theory to improve walking. Therefore, we hypothesize that tACS intervention in the gamma band can have a corresponding effect on gait and posture by activating the motor learning network, and its theory and mechanism are worth further exploring and have some research significance and clinical value. We summarize the current gamma band in motion literature in Table 1.
Summary and Prospect
The goal of clinical treatment has been to improve postural control, and correct and prevent the occurrence of gait abnormalities so that patients can better reintegrate into their families and society. Rehabilitation of walking and balance after stroke has been the focus of clinical treatment. The cerebellum, which plays a crucial role in balance, postural control, muscle tone, and lower limb coordination in both healthy individuals and stroke patients, has recently gained attention as a second NIBS target after the primary motor cortex due to its relatively intact structural preservation, high plasticity, and these characteristics in patients after episodic stroke. It has been demonstrated that the gamma frequency of tACS is an exogenous frequency band that enhances motor learning. The gamma band is also an important EEG-generating segment of the cerebellum itself at the beginning of the swing phase of the gait cycle and during rhythmic movement changes. The gamma-band cerebellar tACS has been demonstrated to enhance lower limb MEP and upper limb function in healthy people. However, no study has been able to explain how repeated cerebellar tACS at gamma frequency interventions in stroke patients affect the neural circuits linked to abnormal gait, postural control, balance, and motor learning, or what the long-lasting effects are. Is there a particular frequency or band with the best efficacy for movement or motor learning with multiple targets, numerous NIBS approaches, combined interventions with conventional rehabilitation approaches targeting the affected neuromuscular, and various wavelengths in the gamma band? Additionally, the cerebellum’s anatomical makeup and functional mechanisms are intricate, and high-precision cerebellar neuromodulation is an excellent way to explain available mechanisms that merit more research.
Data Availability
This review paper does not involve any clinical data.
References
Hamacher D, Herold F, Wiegel P, Hamacher D, Schega L. Brain activity during walking: a systematic review. Neurosci Biobehav Rev. 2015;57:310–27.
Li S, Francisco GE, Zhou P. Post-stroke hemiplegic gait: new perspective and insights. Front Physiol. 2018;9:1021.
Lisanby SH, Luber B, Perera T, Sackeim HA. Transcranial magnetic stimulation: applications in basic neuroscience and neuropsychopharmacology. Int J Neuropsychopharmacol. 2000;3(3):259–73.
Gaojing X, Yi W. Research progress on the effect of new rehabilitation treatment techniques on brain plasticity after stroke. Chin J Phys Med Rehabil. 2019;41(2):150–3.
Veldema J, Gharabaghi A. Non-invasive brain stimulation for improving gait, balance, and lower limbs motor function in stroke. J Neuroeng Rehabil. 2022;19(1):84.
Cheng HL, Lin CH, Tseng SH, Peng CW, Lai CH. Effectiveness of repetitive transcranial magnetic stimulation combined with visual feedback training in improving neuroplasticity and lower limb function after chronic stroke: a pilot study. Biology (Basel). 2023;12(4):515.
Kuwahara W, Sasaki S, Yamamoto R, Kawakami M, Kaneko F. The effects of robot-assisted gait training combined with non-invasive brain stimulation on lower limb function in patients with stroke and spinal cord injury: a systematic review and meta-analysis. Front Hum Neurosci. 2022;16: 969036.
Krogh S, Jønsson AB, Aagaard P, Kasch H. Efficacy of repetitive transcranial magnetic stimulation for improving lower limb function in individuals with neurological disorders: a systematic review and meta-analysis of randomized sham-controlled trials. J Rehabil Med. 2022;54:jrm00256.
Bostan AC, Dum RP, Strick PL. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci. 2013;17(5):241–54.
0 Rodríguez-Takeuchi SY, Baena-Caldas GP, Orejuela-Zapata JF, Granados Sánchez AM. Analysis of the pattern of functional activation of the cerebellum and its topographical correlation. Análisis del patrón de activación funcional del cerebelo y su correlación topográfica. Radiologia (Engl Ed). 2020;62(4):298–305.
Rehme AK, Eickhoff SB, Rottschy C, Fink GR, Grefkes C. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. Neuroimage. 2012;59(3):2771–82.
Ntakou EA, Nasios G, Nousia A, Siokas V, Messinis L, Dardiotis E. Targeting cerebellum with non-invasive transcranial magnetic or current stimulation after cerebral hemispheric stroke-insights for corticocerebellar network reorganization: a comprehensive review. Healthcare (Basel). 2022;10(12):2401.
Marinho V, Pinto GR, Bandeira J, Oliveira T, Carvalho V, Rocha K, Magalhães F, de Sousa VG, Bastos VH, Gupta D, Orsini M, Teixeira S. Impaired decision-making and time perception in individuals with stroke: behavioral and neural correlates. Rev Neurol (Paris). 2019;175(6):367–76.
Naro A, Milardi D, Cacciola A, Russo M, Sciarrone F, La Rosa G, Bramanti A, Bramanti P, Calabrò RS. What do we know about the influence of the cerebellum on walking ability? Promising findings from transcranial alternating current stimulation. Cerebellum. 2017;16(4):859–67.
Drijkoningen D, Leunissen I, Caeyenberghs K, Hoogkamer W, Sunaert S, Duysens J, Swinnen SP. Regional volumes in brain stem and cerebellum are associated with postural impairments in young brain-injured patients. Hum Brain Mapp. 2015;36(12):4897–909.
Zabihhosseinian M, Yielder P, Berkers V, Ambalavanar U, Holmes M, Murphy B. Neck muscle fatigue impacts plasticity and sensorimotor integration in cerebellum and motor cortex in response to novel motor skill acquisition. J Neurophysiol. 2020;124(3):844–55.
Richard A, Van Hamme A, Drevelle X, Golmard JL, Meunier S, Welter ML. Contribution of the supplementary motor area and the cerebellum to the anticipatory postural adjustments and execution phases of human gait initiation. Neuroscience. 2017;358:181–9.
Jung JH, Kim BH, Chung SJ, Yoo HS, Lee YH, Baik K, Ye BS, Sohn YH, Lee JM, Lee PH. Motor cerebellar connectivity and future development of freezing of gait in de novo Parkinson’s disease. Mov Disord. 2020;35(12):2240–9.
Rodríguez-Takeuchi SY, Baena-Caldas GP, Orejuela-Zapata JF, Granados Sánchez AM. Analysis of the pattern of functional activation of the cerebellum and its topographical correlation. Radiologia (Engl Ed). 2020;62(4):298–305.
Bukhari Q, Ruf SF, Guell X, Whitfield-Gabrieli S, Anteraper S. Interaction between cerebellum and cerebral cortex, evidence from dynamic causal modeling. Cerebellum. 2022;21(2):225–33.
Lee HA, Kim DH. Brain connectivity affecting gait function after unilateral supratentorial stroke. Brain Sci. 2021;11(7):870.
Cabaraux P, Agrawal SK, Cai H, Calabro RS, Casali C, Damm L, Doss S, Habas C, Horn AKE, Ilg W, Louis ED, Mitoma H, Monaco V, Petracca M, Ranavolo A, Rao AK, Ruggieri S, Schirinzi T, Serrao M, Summa S, Strupp M, Surgent O, Synofzik M, Tao S, Terasi H, Torres-Russotto D, Travers B, Roper JA, Manto M. Consensus paper: ataxic gait. Cerebellum. 2023;22(3):394–430.
Matsugi A. Do changes in spinal reflex excitability elicited by transcranial magnetic stimulation differ based on the site of cerebellar stimulation? Somatosens Mot Res. 2018;35(2):80–5.
Kiehn O. Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci. 2016;17(4):224–38.
Todorov E, Jordan MI. Optimal feedback control as a theory of motor coordination. Nat Neurosci. 2002;5(11):1226–35.
Morelli N, Hoch M. A proposed postural control theory synthesizing optimal feedback control theory, postural motor learning, and cerebellar supervision learning. Percept Mot Skills. 2020;127(6):1118–33.
Voogd J, Glickstein M. The anatomy of the cerebellum. Trends Neurosci. 1998;21(9):370–5.
van Es DM, van der Zwaag W, Knapen T. Topographic maps of visual space in the human cerebellum. Curr Biol. 2019;29(10):1689–94.
Mizelle JC, Oparah A, Wheaton LA. Reliability of visual and somatosensory feedback in skilled movement: the role of the cerebellum. Brain Topogr. 2016;29(1):27–41.
Schniepp R, Möhwald K, Wuehr M. Gait ataxia in humans: vestibular and cerebellar control of dynamic stability. J Neurol. 2017;264:87–92.
Li DB, Yao J, Sun L, Wu B, Li X, Liu SL, Hou JM, Liu HL, Sui JF, Wu GY. Reevaluating the ability of cerebellum in associative motor learning. Sci Rep. 2019;9(1):6029.
González-Tapia D, González-Ramírez MM, Vázquez-Hernández N, González-Burgos I. Motor learning induces plastic changes in Purkinje cell dendritic spines in the rat cerebellum. Neurologia (Engl Ed). 2020;35(7):451–7.
Balsters JH, Ramnani N. Cerebellar plasticity and the automation of first-order rules. J Neurosci. 2011;31(6):2305–12.
Lang EJ, Apps R, Bengtsson F, Cerminara NL, De Zeeuw CI, Ebner TJ, Heck DH, Jaeger D, Jörntell H, Kawato M, Otis TS, Ozyildirim O, Popa LS, Reeves AM, Schweighofer N, Sugihara I, Xiao J. The roles of the olivocerebellar pathway in motor learning and motor control. A consensus paper Cerebellum. 2017;16(1):230–52.
Wu B, Yao J, Wu GY, Li X, Gao WJ, Zhang RW, Sui JF. Absence of associative motor learning and impaired time perception in a rare case of complete cerebellar agenesis. Neuropsychologia. 2018;117:551–7.
Hermsdorf F, Fricke C, Stockert A, Classen J, Rumpf JJ. Motor performance but neither motor learning nor motor consolidation are impaired in chronic cerebellar stroke patients. Cerebellum. 2020;19(2):275–85.
Magon S, Pfister A, Laura G, Lüthi M, Papadopoulou A, Kappos L, Sprenger T. Short timescale modulation of cortical and cerebellar activity in the early phase of motor sequence learning: an fMRI study. Brain Imaging Behav. 2020;14(6):2159–75.
Carey MR, Myoga MH, McDaniels KR, Marsicano G, Lutz B, Mackie K, Regehr WG. Presynaptic CB1 receptors regulate synaptic plasticity at cerebellar parallel fiber synapses. J Neurophysiol. 2011;105(2):958–63.
Hull C. Prediction signals in the cerebellum: beyond supervised motor learning. Elife. 2020;9: e54073.
Kono M, Kakegawa W, Yoshida K, Yuzaki M. Interneuronal NMDA receptors regulate long-term depression and motor learning in the cerebellum. J Physiol. 2019;597(3):903–20.
Nagao S. Ocular reflex adaptation as an experimental model of cerebellar learning – in memory of Masao Ito -. Neuroscience. 2021;462:191–204.
Yiou E, Artico R, Teyssedre CA, et al. Anticipatory postural control of stability during gait initiation over obstacles of different height and distance made under reaction-time and self-initiated instructions. Front Hum Neurosci. 2016;10:449.
Liebrand M, Karabanov A, Antonenko D, Flöel A, Siebner HR, Classen J, Krämer UM, Tzvi E. Beneficial effects of cerebellar tDCS on motor learning are associated with altered putamen-cerebellar connectivity: a simultaneous tDCS-fMRI study. Neuroimage. 2020;223: 117363.
Ortelli P, Ferrazzoli D, Maestri R, Saltuari L, Kofler M, Alibardi A, Koch G, Spampinato D, Castagna A, Sebastianelli L, Versace V. Experimental protocol to test explicit motor learning-cerebellar theta burst stimulation. Front Rehabil Sci. 2021;2: 720184.
Matsugi A, Nishishita S, Yoshida N, Tanaka H, Douchi S, Bando K, Tsujimoto K, Honda T, Kikuchi Y, Shimizu Y, Odagaki M, Nakano H, Okada Y, Mori N, Hosomi K, Saitoh Y. Impact of repetitive transcranial magnetic stimulation to the cerebellum on performance of a ballistic targeting movement. Cerebellum. 2023;22(4):680–97.
Belkhiria C, Mssedi E, Habas C, Driss T, de Marco G. Collaboration of cerebello-rubral and cerebello-striatal loops in a motor preparation task. Cerebellum. 2019;18(2):203–11.
Marchal V, Sellers J, Pélégrini-Issac M, Galléa C, Bertasi E, Valabrègue R, Lau B, Leboucher P, Bardinet E, Welter ML, Karachi C. Deep brain activation patterns involved in virtual gait without and with a doorway: An fMRI study. PLoS ONE. 2019;14(10): e0223494.
Koch G, Bonnì S, Casula EP, Iosa M, Paolucci S, Pellicciari MC, Cinnera AM, Ponzo V, Maiella M, Picazio S, Sallustio F, Caltagirone C. Effect of cerebellar stimulation on gait and balance recovery in patients with hemiparetic stroke: a randomized clinical trial. JAMA Neurol. 2019;76(2):170–8.
Wessel MJ, Hummel FC. Non-invasive cerebellar stimulation: a promising approach for stroke recovery? Cerebellum. 2018;17(3):359–71.
Parikh V, Medley A, Chung YC, Goh HT. Optimal timing and neural loci: a scoping review on the effect of non-invasive brain stimulation on post-stroke gait and balance recovery. Top Stroke Rehabil. 2021;3:1–17.
Solanki D, Rezaee Z, Dutta A, Lahiri U. Investigating the feasibility of cerebellar transcranial direct current stimulation to facilitate post-stroke overground gait performance in chronic stroke: a partial least-squares regression approach. J Neuroeng Rehabil. 2021;18(1):18.
Picelli A, Chemello E, Castellazzi P, Filippetti M, Brugnera A, Gandolfi M, Waldner A, Saltuari L, Smania N. Combined effects of cerebellar transcranial direct current stimulation and transcutaneous spinal direct current stimulation on robot-assisted gait training in patients with chronic brain stroke: a pilot, single blind, randomized controlled trial. Restor Neurol Neurosci. 2018;36(2):161–71.
Picelli A, Brugnera A, Filippetti M, Mattiuz N, Chemello E, Modenese A, Gandolfi M, Waldner A, Saltuari L, Smania N. Effects of two different protocols of cerebellar transcranial direct current stimulation combined with transcutaneous spinal direct current stimulation on robot-assisted gait training in patients with chronic supratentorial stroke: a single blind, randomized controlled trial. Restor Neurol Neurosci. 2019;37(2):97–107.
Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A. 2007;104(32):13170–5.
Buzsáki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–25.
Guan A, Wang S, Huang A, Qiu C, Li Y, Li X, Wang J, Wang Q, Deng B. The role of gamma oscillations in central nervous system diseases: mechanism and treatment. Front Cell Neurosci. 2022;16: 962957.
Lara GA, Alekseichuk I, Turi Z, Lehr A, Antal A, Paulus W. Perturbation of theta-gamma coupling at the temporal lobe hinders verbal declarative memory. Brain Stimul. 2018;11(3):509–17.
Disrupted functional network integrity and flexibility after stroke. relation to motor impairments. Neuroimage Clin. 2018;9(19):883–91.
Balbi M, Xiao D, Jativa Vega M, Hu H, Vanni MP, Bernier LP, LeDue J, MacVicar B, Murphy TH. Gamma frequency activation of inhibitory neurons in the acute phase after stroke attenuates vascular and behavioral dysfunction. Cell Rep. 2021;34(5): 108696.
Zhao M, Bonassi G, Samogin J, Taberna GA, Pelosin E, Nieuwboer A, Avanzino L, Mantini D. Frequency-dependent modulation of neural oscillations across the gait cycle. Hum Brain Mapp. 2022;43(11):3404–15.
Kooiman VGM, van Keeken HG, Maurits NM, Weerdesteyn V, Solis-Escalante T. Rhythmic neural activity is comodulated with short-term gait modifications during first-time use of a dummy prosthesis: a pilot study. J Neuroeng Rehabil. 2020;17(1):134.
Weersink JB, Maurits NM, de Jong BM. EEG time-frequency analysis provides arguments for arm swing support in human gait control. Gait Posture. 2019;70:71–8.
Seeber M, Scherer R, Wagner J, Solis-Escalante T, Müller-Putz GR. High and low gamma EEG oscillations in central sensorimotor areas are conversely modulated during the human gait cycle. Neuroimage. 2015;112:318–26.
Garcia-Rill E. Neuroepigenetics of arousal: gamma oscillations in the pedunculopontine nucleus. J Neurosci Res. 2019;97(12):1515–20.
Panyakaew P, Cho HJ, Srivanitchapoom P, Popa T, Wu T, Hallett M. Cerebellar brain inhibition in the target and surround muscles during voluntary tonic activation. Eur J Neurosci. 2016;43(8):1075–81.
Ossmy O, Mukamel R. Perception as a route for motor skill learning: perspectives from neuroscience. Neuroscience. 2018;382:144–53.
Georgescu EL, Georgescu IA, Zahiu CDM, Şteopoaie AR, Morozan VP, Pană AŞ, Zăgrean AM, Popa D. Oscillatory cortical activity in an animal model of dystonia caused by cerebellar dysfunction. Front Cell Neurosci. 2018;12:390.
Lindeman S, Hong S, Kros L, Mejias JF, Romano V, Oostenveld R, Negrello M, Bosman LWJ, De Zeeuw CI. Cerebellar Purkinje cells can differentially modulate coherence between sensory and motor cortex depending on region and behavior. Proc Natl Acad Sci U S A. 2021;118(2): e2015292118.
Amo C, De Santiago L, Zarza Luciáñez D, et al. Induced gamma band activity from EEG as a possible index of training-related brain plasticity in motor tasks. PLoS ONE. 2017;12(10): e0186008.
Ali MM, Sellers KK, Fröhlich F. Transcranial alternating current stimulation modulates large-scale cortical network activity by network resonance. J Neurosci. 2013;33(27):11262–75.
Antonenko D, Faxel M, Grittner U, Lavidor M, Flöel A. Effects of transcranial alternating current stimulation on cognitive functions in healthy young and older adults. Neural Plast. 2016;2016:4274127.
Khatoun A, Asamoah B, Mc LM. Simultaneously excitatory and inhibitory effects of transcranial alternating current stimulation revealed using selective pulse-train stimulation in the rat motor cortex. J Neurosci. 2017;37(39):9389–402.
Numssen O, van der Burght CL, Hartwigsen G. Revisiting the focality of non-invasive brain stimulation - implications for studies of human cognition. Neurosci Biobehav Rev. 2023;149: 105154.
Li LM, Violante IR, Leech R, Ross E, Hampshire A, Opitz A, Rothwell JC, Carmichael DW, Sharp DJ. Brain state and polarity dependent modulation of brain networks by transcranial direct current stimulation. Hum Brain Mapp. 2019;40(3):904–15.
Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, Cohen LG, Fregni F, Herrmann CS, Kappenman ES, Knotkova H, Liebetanz D, Miniussi C, Miranda PC, Paulus W, Priori A, Reato D, Stagg C, Wenderoth N, Nitsche MA. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. 2016;127(2):1031–48.
Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, Edwards DJ, Valero-Cabre A, Rotenberg A, Pascual-Leone A, Ferrucci R, Priori A, Boggio PS, Fregni F. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 2012;5(3):175–95.
Fertonani A, Ferrari C, Miniussi C. What do you feel if I apply transcranial electric stimulation? Safety, sensations and secondary induced effects. Clin Neurophysiol. 2015;126(11):2181–8.
Evans ID, Palmisano S, Loughran SP, Legros A, Croft RJ. Frequency-dependent and montage-based differences in phosphene perception thresholds via transcranial alternating current stimulation. Bioelectromagnetics. 2019;40(6):365–74.
Sadeghihassanabadi F, Misselhorn J, Gerloff C, Zittel S. Optimizing the montage for cerebellar transcranial alternating current stimulation (tACS): a combined computational and experimental study. J Neural Eng. 2022;19(2).
Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann Neurol. 1995;37(6):703–13.
Spampinato DA, Celnik PA, Rothwell JC. Cerebellar-motor cortex connectivity: one or two different networks? J Neurosci. 2020;40(21):4230–9.
Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78(3–5):272–303.
Naro A, Leo A, Russo M, Cannavò A, Milardi D, Bramanti P, Calabrò RS. Does transcranial alternating current stimulation induce cerebellum plasticity? Feasibility, safety and efficacy of a novel electrophysiological approach. Brain Stimul. 2016;9(3):388–95.
Naro A, Bramanti A, Leo A, Manuli A, Sciarrone F, Russo M, Bramanti P, Calabrò RS. Effects of cerebellar transcranial alternating current stimulation on motor cortex excitability and motor function. Brain Struct Funct. 2017;222(6):2891–906.
Manto M, Argyropoulos GPD, Bocci T, Celnik PA, Corben LA, Guidetti M, Koch G, Priori A, Rothwell JC, Sadnicka A, Spampinato D, Ugawa Y, Wessel MJ, Ferrucci R. Consensus paper: novel directions and next steps of non-invasive brain stimulation of the cerebellum in health and disease. Cerebellum. 2022;21(6):1092–122.
Guerra A, Suppa A, Asci F, De Marco G, D’Onofrio V, Bologna M, Di Lazzaro V, Berardelli A. LTD-like plasticity of the human primary motor cortex can be reversed by γ-tACS. Brain Stimul. 2019;12(6):1490–9.
Miyaguchi S, Inukai Y, Matsumoto Y, Miyashita M, Takahashi R, Otsuru N, Onishi H. Effects on motor learning of transcranial alternating current stimulation applied over the primary motor cortex and cerebellar hemisphere. J Clin Neurosci. 2020;78:296–300.
Miyaguchi S, Otsuru N, Kojima S, Saito K, Inukai Y, Masaki M, Onishi H. Transcranial Alternating current stimulation with gamma oscillations over the primary motor cortex and cerebellar hemisphere improved visuomotor performance. Front Behav Neurosci. 2018;12:132.
Akkad H, Dupont-Hadwen J, Kane E, Evans C, Barrett L, Frese A, Tetkovic I, Bestmann S, Stagg CJ. Increasing human motor skill acquisition by driving theta-gamma coupling. Elife. 2021;10: e67355.
Minc D, Machado S, Bastos VH, Machado D, Cunha M, Cagy M, Budde H, Basile L, Piedade R, Ribeiro P. Gamma band oscillations under influence of bromazepam during a sensorimotor integration task: an EEG coherence study. Neurosci Lett. 2010;469(1):145–9.
Giustiniani A, Tarantino V, Bonaventura RE, Smirni D, Turriziani P, Oliveri M. Effects of low-gamma tACS on primary motor cortex in implicit motor learning. Behav Brain Res. 2019;376: 112170.
Miyaguchi S, Inukai Y, Mitsumoto S, Otsuru N, Onishi H. Gamma-transcranial alternating current stimulation on the cerebellum and supplementary motor area improves bimanual motor skill. Behav Brain Res. 2022;424: 113805.
Giustiniani A, Tarantino V, Bracco M, Bonaventura RE, Oliveri M. Functional role of cerebellar gamma frequency in motor sequences learning: a tACS study. Cerebellum. 2021;20(6):913–21.
Funding
This work was supported by the National Key Research and Development Program of China (2018YFC2001600); National Natural Science Fund (82272612); Shanghai Clinical Research Center for Rehabilitation Medicine (21MC1930200).
Author information
Authors and Affiliations
Contributions
TF, LZ, and YW are co-first authors of this manuscript.
TF, LZ, and YW conceptualized and designed the study, and drafted the initial manuscript. LT, XC, and YL reviewed and revised the manuscript. CS supervised the whole procedure. All the authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethics Approval
This article does not involve any clinical data, so there is no ethics involved.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Feng, T., Zhang, L., Wu, Y. et al. Exploring the Therapeutic Effects and Mechanisms of Transcranial Alternating Current Stimulation on Improving Walking Ability in Stroke Patients via Modulating Cerebellar Gamma Frequency Band—a Narrative Review. Cerebellum 23, 1593–1603 (2024). https://doi.org/10.1007/s12311-023-01632-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-023-01632-3