Abstract
Current evidence supports the beneficial role of phytoestrogens in metabolic diseases, but their influences on spontaneous motor and anxiety behaviors plus neuroprotective effects have still not been completely elucidated. With the present study, neuro-behavioral activities were correlated to daidzein (DZ)-dependent expression changes of a high affinity catalytic receptor for several neurotrophins, and namely tropomyosin-related kinase B receptor (TrkB) in the cerebellar cortex of high-fat diet (HFD) hamsters (Mesocricetus auratus). Indeed, these changes appear to be tightly linked to altered plasma lipid profiles as shown by reduced low-density lipoproteins plus total cholesterol levels in DZ-treated obesity hamsters accounting for increased spontaneous locomotor together with diminished anxiety activities in novel cage (NCT) and light/dark box (LDT) tests. For this latter case, the anxiolytic-like hamsters spent more time in the light compartment, which was retained the aversive area of the LDT box. As for the evaluation of the neurotrophin receptor site, significantly elevated TrkB levels were also detected, for the first time, in the cerebellum of obese hamsters treated with DZ. In this condition, such a treatment widely led to an overall improvement of HFD-induced neurodegeneration damages, above all in the Purkinje and granular layers of the cerebellum. In this context, the notably active TrkB signaling events occurring in a DZ-dependent manner may turn out to be a key neuroprotective element capable of restoring normal emotional and spontaneously linked locomotor behaviors regulated by cerebellar cortical areas especially in obesity-related conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The growing increase regarding the incidence of obesity has attracted scientific interests aimed towards the identification of novel natural compounds capable of modulating food intake that may constitute a valid pharmacological alternative for the treatment of obesity and metabolic syndromes. From recent studies, it seems that dietary supplements are becoming more and more appropriate for the management of obesity and other metabolic disorders as suggested by some polyphenolic extracts reducing the accumulation of intracellular lipids, oxidative stress, and inflammation through the regulation of different metabolic pathways [1, 2]. Of the different bioactive isoflavones, daidzein (DZ), which is abundant in soy, has shown to exert beneficial effects on adiposity with a subsequent reduction of adipose tissue deposition stimulating lipolysis via the activation of the hormone-sensitive lipase [3]. This isoflavone accounts for improved mood, behavior, and cognitive functions especially in anxious plus depressed conditions [4, 5]. Interestingly, DZ has shown to attenuate cerebral inflammation and oxidative stress [6], along with influencing the intestinal bacterial composition [7], as well as being involved with the induction of apoptotic activity of cancer cell [8]. These polyphenols are also known to actively exert a protective role in high-fat diet (HFD) conditions, which represents one of the major health concern responsible for neuronal complications in telencephalon plus hypothalamic areas [9] as suggested by smaller cortical thickness and reduction of gray matter volume under such conditions [10]. As a consequence, polyphenols tend to promote an important functional role especially when they are involved with neuroprotective measures following neurodegenerative alterations or with neuronal dysfunctions that are typical of metabolic obesity states [11].
It has been suggested that neuronal factors, such as neurotrophins, are involved not only with the balance of lipid and glucose levels but are also for the regulation of energy expenditure and cardiovascular homeostasis [12, 13]. Among these, the brain-derived neurotrophic factor (BDNF) and its catalytic receptor tropomyosin-related kinase B receptor (TrkB), which are widely expressed throughout the brain, seem to play a crucial role on synaptic transmission and plasticity events during both the developmental and adulthood stages [14, 15]. Altered expression levels of neurotrophins and Trk receptors resulted to be modified by the ingestion of flavonoids. It appears that the activation of MAP kinase plus PI3 kinase intracellular signaling pathways [16] and the incremented synthesis of NGF together with BDNF improve not only cognitive events [17, 18] but also motor disorders [19].
Based on the above indications, behavioral effects of HFD enriched with simple carbohydrates and lipids ± the phytoestrogen DZ were assessed to evaluate exploratory, emotional plus locomotor activities of the Syrian golden hamster (Mesocricetus auratus) when exposed to light/dark test (LDT) and novel cage test (NCT). Amino cupric silver stain (ACS) techniques were applied to determine neuronal damages in the different layers of the hamster cerebellum cortex. Additionally, TrkB expression activities were estimated in the cerebellum cortex of HFD hamsters with respect to animals that received a standard diet aimed to establish the potential role of cerebellum TrkB on emotional and motor performances in HFD hamsters treated with DZ. The selection of this brain area was based on recent evidences corroborating that obesity and body mass are tightly related to a lower volume of brain gray matter, especially in areas like the cerebellum that play a key role in executing motor activities [20]. Indeed, this region, which is involved with the coordination of regulatory processes, sequencing, plus interhemispheric exchanges [21], the control of cognition [22] and sensory emotional activities [23, 24] may turn out to be a pivotal neuronal station for the recovery of behavioral performances in obesity-related disorders.
Materials and Methods
Animals
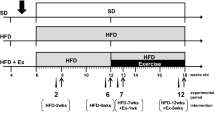
Adult male Syrian golden hamsters (Mesocricetus auratus; Envigo Laboratories, Udine-Italy, n = 50) weighing 120–180 g were housed individually under a temperature-controlled environment (22–24 °C), at a 14-h light/10-h dark cycle (lights on 07:00 a.m.) and humidity (60%) conditions with food and water available ad libitum. After 1 week of acclimatization, they were randomly assigned to the following groups: (1) group I (control group, CTRL; n = 18): hamsters were fed with the standard laboratory rodent chow diet some for 14 (n = 6), others for 16 consecutive weeks (n = 12); (2) group II (HFD group, n = 16), of which some were fed with HFD (60% of calories from fat; analytical components: crude protein 23.00%, crude oils and fats 34.00%, crude fiber 5.00%, crude ash 5.50%, carbohydrates 27.3%; Envigo Laboratories, Udine-Italy) for 14 consecutive weeks (n = 6), while others for 16 consecutive weeks (n = 10) to induce obesity; (3) group III (HFD + DZ group, n = 16): some hamsters were fed with HFD for 14 weeks while others for 16 consecutive weeks, in which DZ (200 mg/kg diet; 98% DZ; Santa Cruz Biotechnology) was added during the last 2 weeks (n = 6, HFD + DZ; 2 w) or 4 weeks (n = 10, HFD + DZ; 4 w) of treatment [25]. The effects of the hyperlipidic diet on body weight of each hamster were monitored weekly to assure the state of obesity. The effects of only DZ group were studied in a previous work [5] and so was not handled in the present paper.
After the last behavioral session, hamsters were sacrificed by decapitation and blood was collected (hamsters fasted overnight prior to the blood collection), for the evaluation of some serological markers (i.e., total cholesterol, TC; low-density lipoprotein cholesterol, LDL, and high-density lipoprotein cholesterol, HDL). The concentration was determined using enzymatic colorimetric methods (CHOD-PAP; GPO-PAP; GOD POD) according to manufacturer’s protocol (Biogramma srl; Biotecnica Instruments, Rome-Italy) plus modifications.
Animal maintenance and experimental procedures were carried out in compliance with ethical provisions for Care and Use of Laboratory Animals reported in the legislative law n° 26 (04–03-2014) and authorized by the National Committee of the Italian Ministry of Health. Efforts were made to minimize animal suffering and reduce the number of experiments.
Behavioral Tests
After the diet, all animals individually were subjected to light/dark (LDT) and novel cage (NCT) tests. The behavioral procedures were conducted during the light phase (between 2:00 and 6:00 p.m.) in a sound-isolated room and video-taped via high resolution Waterproof Action Camera (DBPOWER- SJ4000 SPORTS HD DV) mounted vertically above the chamber. The data were analyzed with the specific software EthoLog (version 2.2.5; Visual Basic, São Paulo, Brazil). At the end of each test, the apparatus was cleaned with 0.1% acetic acid to remove all traces of previously tested animals.
Light–Dark Test (LDT)
LDT apparatus consists of a box with two compartments: a first arena composed of a small and dark opaque familiar compartment (16 × 16 × 16 cm) containing a black glass while the second arena containing a large translucent and white illuminated compartment (25 × 25 × 30 cm), considered the unfamiliar environment. The compartments were connected by a communicating door (7 × 7 cm), which allowed hamsters to freely move from one compartment to the other.
Prior to the behavioral observations, animals were subjected to a brief period of habituation in the LDT apparatus; then, the hamster was placed in the center of the light compartment with their head opposite to the opening and was allowed to explore the two compartments for 300 seconds (sec). During this period, the following behavioral measures were recorded: light permanence (time spent in the light compartment), dark permanence (time spent in the dark compartment), latency to dark (the first latency to enter the dark compartment), rearing (time spent standing on hind legs), and risk assessment (time in which head and/or body extends from the dark to the light compartment); number of transitions between compartments and risk assessment-number. The advantage of using this apparatus is linked to the presence of an unfamiliar environment so that the hamster is placed in front of a natural conflict deriving from the necessity to explore a novel environment (spontaneous exploratory behavior) and the innate aversion to an open and brightly illuminated arena. In the latter case, it was the presence of light and not darkness that were responsible for the induction of stress-like conditions for hamsters, as shown by the percentage of time spent in the dark compartment. Moreover, the exploratory behavior in the light compartment was also used as an indicator of anxiety. Similarly, the number of crossings between compartments provide an estimation of the anxiety level if measured without changes of spontaneous locomotor activity [26].
Novel Cage Test (NCT)
To investigate spontaneous locomotor activity [27], hamsters were placed individually at the beginning of the test in the center of a novel, clean, transparent plexiglass cage (50 × 50 × 30 cm), under light intensity of approximately 200 lx without water and food. The behavior was recorded with a video for 5 min (300 sec) and categorized as mobility (movement in the cage), rearing (standing on hind limbs), wall-rearing (standing on hind limbs and touching the walls of the cage with forelimbs), sniffing (sniffing sawdust), digging (digging sawdust with forelimbs, often kicking it away with hind limbs), grooming (licking own fur, sometimes using forepaws, passing them over the nose with a series of brief, horizontal movements), and climbing (escape behavior). Following the final behavioral session, hamsters were anesthetized, and blood plus brains were taken and processed for biochemical analysis.
Protein Extraction and Western Blotting
The cerebellum was removed and stored at − 80 °C until western blotting (n = 36). Frozen cerebellar tissues (n = 6 from each experimental group) were homogenized on ice in a lysis buffer (20 mM Tris–HCl, pH 7.6, 15 mM Triton X-100, 10% glycerol, 2 mM EDTA) containing a cocktail of protease (Roche) and phosphatase (Sigma) inhibitors. Samples were centrifuged at 13,000 rpm at 4 °C for 20 min and the supernatant was collected, stored at − 80 °C for future immunoblotting analyses. Protein concentrations were determined using the Bradford protein assay (Bio-Rad, Hercules, CA). Equal amounts of protein per sample (20 µg) were separated by electrophoresis on 8% and 10% Tris–glycine gels with 4% stacking gels; then, they were transferred to nitrocellulose membranes and the membranes were blocked with 5% serum albumin for 1 h. The nitrocellulose membranes were incubated overnight at 4 °C with the following antibodies: rabbit monoclonal anti TrkB (80E3; EuroClone, Milano, Italy) and goat polyclonal anti β-actin antibody (C-20; sc-7396, 1:2000, Santa Cruz Biotechnology). Blots were then incubated with the appropriate horseradish peroxidase (HRP) conjugated goat anti-rabbit IgG (HRP-linked antibody; EuroClone, Milano, Italy) secondary antibody and developed using enhanced chemiluminescence detection system (Bio-Rad Laboratories). Optical density (O.D.) was determined using the NIH Image J software. Protein expression was normalized to the β -actin protein.
Cerebellar Histological Analyses
Amino Cupric Silver (ACS) Stain
In order to evaluate the argyrophilic reaction, an ACS staining technique was used to determine cerebellar fields that featured advanced damaged cell bodies, dendrites, axons, and terminals of both HFD and DZ treated groups subjected to a diet for 16 consecutive weeks. For this part, cerebral sections (30 µm) were obtained from each of the above experimental groups: CTRL (n = 4), HFD (n = 4) and HFD ± DZ (n = 4), at an interval of 240 µm for ACS procedures as previously described [28]. The estimation of damaged limbic neuronal fields required the volume (defined as Vref) of three layers of cerebellar cortex, and namely the molecular layer (ML), Purkinje layer (PL), and granular layer (GL) by using the following formula:
In this calculation, Ns represents the number of stained damaged neurons; N is the number of damaged neurons in a single section; Vsection is the volume of a single section; n is the number of sections; Vref is the total volume of above brain regions.
For general histological observation, some cerebellar sections (16 µm) of CTRL animals (n = 2) were stained with Nissl dye.
Statistical Analysis
The behavioral data and the number of degenerating cells were expressed in % ± S.E.M. while molecular biochemical data were expressed as OD ± S.E.M. Data of HFD hamsters were evaluated and compared with CTRLs (*) and to HFD (letters) using a two-way ANOVA followed by post hoc Newman-Keuls multiple range test when p < 0.05; *, a p < 0.05; **, b p < 0.01; ***, c p < 0.001.
Results
Effects of DZ on Anxiety-like Response in LDT
HFD hamsters treated with DZ displayed improved body weight with respect to CTRLs, as indicated in our previous work [5]. At the same time, they exhibited anxiety-like behaviors in LDT exploration paradigm, which were attenuated in the presence of DZ. In particular, the different behavioral evaluations (F(2,15) = 3.69; p < 0.05) resulted to be reduced in HFD hamsters as indicated by moderate diminished levels of permanence in light compartment (− 45%) and of rearing (− 33%) with respect to CTRLs (Fig. 1A). Conversely, HFD hamsters treated with DZ for 2 w spent more time (+ 51%) in the light compartment with respect to CTRLs and this turned out to be extremely higher when compared to HFD hamsters (+ 147%; Fig. 1A). In addition, HFD + DZ 2w hamsters explicated more transitions between the two compartments (+ 200%) while contemporarily reducing both the time (− 66%; Fig. 1B) and the number of times (− 43%) of risk assessment.
Evaluation of the anxiolytic activity of DZ using the LDT on A) time (sec ± S.E.M., compared to the total duration of the test of 300 sec) spent in the light compartment and in the dark compartment, latency to enter the dark compartment, rearing (time spent standing on the hind legs), and risk assessment (time in which incomplete head and/or body extends that dips from the dark to the light compartment); B) number (average of the number of times ± S.E.M.) of transitions between compartments and risk assessment in CTRL, HFD, and HFD + DZ 2w hamster groups. Statistical differences were evaluated by using a two-way ANOVA followed by post hoc Newman-Keul’s multiple range test when p-value < 0.05. *p < 0.05, **p < 0.01, ***p < 0.001. *Significant difference compared with CTRL group; a, b, c Significant difference of HFD + DZ 2w compared with HFD group
It was worthy to note that HFD hamsters after 4 w of DZ treatment (Fig. 2A and B) continued to exhibit similar but somewhat greater behavioral trends (F(2,15) = 3.75; p < 0.05). Even in this case, HFD hamsters spent less time (− 54%) in light compartment along with showing a diminished rearing activity (− 48%), when compared to CTRLs while despite HFD + DZ hamsters having spent extremely more time in the light compartment (+ 215%), displayed a reduction of both permanence (− 60%) and number of risk assessment with respect to HFD hamsters. Moreover, HFD + DZ4w animals exhibited extremely more transitions between the two compartments with respect to HFD hamsters (+ 100%) whereas it was not of the same entity when compared to CTRLs (+ 33%). Additionally, a moderate decrease (− 49%) in the first latency to enter the dark compartment was also detected.
Evaluation of the anxiolytic activity of DZ using the LDT on A) time (sec ± S.E.M., compared to the total duration of the test of 300 sec) spent in the light compartment and in the dark compartment, latency to enter the dark compartment, rearing, and risk assessment; B) number (average of the number of times ± S.E.M.) of transitions between compartments and risk assessment-number in CTRL, HFD, and HFD + DZ 4w hamster groups. Statistical calculations were handled as reported in Fig. 1
Evaluation of Locomotor Activity in NCT
For analyzing further spontaneous exploration activity in a non-aversive environment (cage), NCT was also evaluated. In this case a low reactivity (F(2,15) = 3.87; p < 0.05) appeared to be induced by HFD as suggested by HFD group spending less time (~ − 33%) performing rearing, wall rearing (Fig. 3A and B) and sniffing (Fig. 3B) behaviors with respect to CTRLs. Conversely, HFD + DZ2w hamsters spent more time in rearing (+ 200%) when compared to CTRLs, while on the other hand performed more wall rearing plus climbing (~ + 60%) together with a moderate sniffing activity (+ 42%), this time with respect to HFD. Such an effect widely improved (F(2,15) = 6. 90; p < 0.01) after a 4-week DZ diet (Fig. 3B) as indicated by an extremely evident rearing (+ 270%) and climbing (+ 225%) activities when compared to the HFD group hamsters. These animals also displayed high dipping (+ 62%) and wall rearing (− 41%) along with a moderate grooming activity (− 33%), which proved to be more frequent than in HFD hamsters.
Evaluation of the spontaneous locomotor activity in A) CTRL, HFD, and HFD + DZ 2w and B) CTRL, HFD, and HFD + DZ 4w hamster groups using the NCT on time (sec ± S.E.M. compared to the total duration of the test of 300 sec) spent in: mobility (movement in the cage), rearing (standing on hind limbs), wall-rearing (standing on hind limbs and touching the walls of the cage with forelimbs), sniffing (sniffing sawdust), digging (digging sawdust with forelimbs, often kicking it away with hind limbs), grooming (licking own fur, sometimes using forepaws, passing them over the nose with a series of brief, horizontal movements), and climbing (escape behavior). Statistical calculations were handled as reported in Fig. 1
Effects of DZ on Cerebellar TrkB Expression
The expression of TrkB protein in the cerebellum of HFD hamsters resulted to be moderately downregulated (− 50%; p < 0.05) with respect to CTRLs (Fig. 4A and B), while a notable up-regulation was instead reported for HFD + DZ2w (+ 75%; p < 0.01). The increment of TrkB levels turned out to be extremely higher (+ 220%) in HFD + DZ2w when compared to HFD hamsters. Furthermore, and in a very evident fashion, hamsters treated with DZ continued to improve the largely reduced expression levels of TrkB in HFD especially after 4 weeks of diet (Fig. 4B) as indicated by its extremely elevated up-regulation with respect to CTRL (+ 200%) and this up-regulation resulted to be even greater (+ 700%) when the expression levels of HFD + DZ were compared, this time to those of HFD hamsters.
Western blot analysis of TrkB expression (O.D. ± S.E.M.) in cerebellar cortex of A) CTRL, HFD, and HFD + DZ 2w and B) CTRL, HFD, and HFD + DZ 4w hamsters. C) Representative image of coronal cerebellar cortex sections stained with (I) Nissl and (II; III; IV) ACS. Photograms of ACS showing dark damaged neuronal perikarya that indicate the different levels of neurodegeneration (arrows; Scale bar = 100 µm). D) Estimation of neurodegeneration (% ± S.E.M.) of degenerated neurons in ML, PL, and GL of HFD, and HFD + DZ 4w groups with respect to CTRL. Statistical calculations were handled as reported in Fig. 1. Abbreviations: ML, molecular layer; PL, Purkinje layer; GL, granular layer
Effects of DZ on Plasma Lipid Profiles
From the plasma lipidic differences in HFD group (Table 1), it appeared that TC levels were significantly increased (+ 130%), with a very evident (+ 190%) and moderate (+ 33%) increase of LDL and HDL levels, respectively, than CTRLs. In contrast, HFD + DZ 4w hamsters appeared to supply a somewhat consistent reduction (− 74%) of the harmful cholesterol component (LDL) together with a moderate reduction (− 35%) of TC.
Cerebellar Histological Changes Following HFD and DZ Treatments
From the histological analyses of the cerebellar cortical layers in CTRLs stained with Nissl (Fig. 4C, I) as well as for ACS labeled sections (Fig. 4C, II), it was possible to observe specific neuronal damages in the different cerebellar regions. In this case, the cerebellar cortex of HFD hamsters exhibited altered neuronal histological conditions as indicated by the evident argyrophilic reaction (Fig. 4C, III) that supplied dense dark granules in the molecular (ML), Purkinje cell (PL), and granular (GL) layers. In particular, evident neuronal damages were detected (Fig. 4D) in the Purkinje cell layer (PL; + 103%) of HFD hamsters with respect to CTRLs. As for the other regions, only a significantly high density of granules was observed in the molecular (ML; + 62%) and granular layers (GL; + 78%). Conversely, the argyrophilic reaction in HFD hamsters fed with DZ for 4 weeks was moderately reduced (~ 30%) in the same above cortical layers with respect to CTRLs. An even stronger reduction of the dark granules seemed to characterize PL (− 30%) and GL (− 42%) layers of HFD 4w-treated hamsters with respect to HFD group, if we consider the heavily dense levels in obese hamsters with respect to CTRLs.
Discussion
The aim of the present study was to examine the role of HFD, separately, and in combination with DZ towards behavioral performances along with indications, obtained for the first time on the expression levels of the high-affinity catalytic receptor for several neurotrophins in the hamster cerebellar cortex. The results tend to corroborate an evident effect of HFD not only towards exploratory and anxiety-related behaviors but also for expression levels of TrkB in the cerebellar cortex. It was noteworthy that the altered expression levels of this neurotrophic factor appeared to be tightly related with neuronal death in the cortical layers thus supporting the hypothesis that the cerebellum may turn out to be a pivotal station controlling emotional reactions [29] as well as being involved with the explication of neurodegenerative mechanisms occurring in states of obesity [30].
From a behavioral point of view, HFD hamsters rapidly displayed a reduction of spontaneous locomotor behavior as indicated by a decrease in behavioral reactivity, e.g., decreased time of rearing and wall rearing, when hamsters were placed in a novel clean cage during NCT, which was similar to that reported for HFD 57BL/6 J mice [31]. It was not unusual to observe altered explorative performances concomitantly with other anxiety-related motor activities in HFD treated animals as suggested by a decrease of time spent in the light chamber of the LDT box considered the aversive site [32]. However, when HFD hamsters were treated with DZ, an inverse situation of HFD-induced behavioral performances occurred in both NCT plus LDT apparatus, which resulted to be in line with other polyphenols reducing anxiety states and restoring spontaneous locomotor activity and exploration-like behaviors in obese rats [33]. In this context, it appears that the potential role of dietary phytoestrogens towards the restoration of explorative behaviors in obese animals [29] may be accomplished via its anxiolytic-like effects [34] due to reduced depressive-like behaviors plus enhanced synaptic plasticity processes [35] occurring above all in motor-controlling brain regions.
The recovery of behavioral activities by phytoestrogens may also be due to their ability of re-establishing lipidic levels as suggested by the accumulating data on dietary polyphenols playing a vital role on lipoprotein metabolism [36, 37]. Such feature seems to derive from the antioxidant as well as anxiolytic properties of phytoestrogens [34], despite the mechanisms by which these compounds regulate behavioral changes in the presence of reduced lipidic levels in obesity conditions have not yet been fully elucidated. In our work, the various behavioral alterations in HFD hamsters like those provided by other studies [5, 38, 39] have shown to be related to elevated hyperlipidemia levels [40, 41]. This feature appears to go in the same direction of elevated total cholesterol and LDL levels exhibited also in our obese animal model, conditions which are tightly linked with neurodegenerative syndromes like Alzheimer’s disease or other atypical cognitive deficits [42, 43].
Interestingly, the variations of our behavioral data seemed to be tightly correlated to decreased expression of TrkB in the cerebellum, brain site that is known to be, via reciprocal connections with the prefrontal cortex, not only a key motor controlling site but recently has been also shown to influence appetite signals in obesity conditions [44]. The importance of the neurotrophic factor in the cerebellum is further supported by this major neurotrophic factor, acting as a mitogenic and chemotactic factor, after birth, stimulating the cerebellar cell precursors of GL to proliferate, migrate, and maturate along with the dendritic development of PL plus being highly expressed in such a brain region of adults [45]. Indeed, visceral adiposity in obese animals has shown to modify cerebellar morphological-functional changes that consequently interfere with motor, cognitive, and emotional processes [46]. It has also been shown that obesity and psychosocial stress tend to account for a heavy toll on the bioavailability of BDNF that is required for its adequate interaction with TrkB receptor and thus assuring an appropriate regulatory role on neuroplasticity events necessary for neuronal cells to cope with stressful conditions [47]. The highlighting, for the first time, of anxiety-like and altered spontaneous locomotor behaviors in LDT and NCT being related to low cerebellar expression levels of TrkB in HFD hamsters may bring us closer to the understanding of altered motor activities in obesity states. Indeed, the ameliorated expression of BDNF has been shown to occur in several neurodegenerative diseases, including cerebellar pathologies such as spinocerebellar ataxia type 6 [48], schizophrenia, and bipolar disorder state [49, 50]. Thus, TrkB changes in cerebellum may result to be either a cause or a consequential effect of obesity thereby enhancing a dysregulation capacity in exploratory and anxiety behaviors. Under such a condition, DZ might be inducing anxiolytic-like effects because this phytoestrogen by interacting with β estrogen receptor site tends to exert a restoration effect not only for anxiolytic states deriving from altered levels of this estrogenic site but more importantly for environmental exploration performances [51]. In this context, the increased cerebellar levels of TrkB in HFD-fed hamsters after dietary addition of DZ may constitute another element underlying the importance of this brain station operating under anxiety-like conditions. Furthermore, the reduction of HFD-induced neuronal death in cerebellar cortex, which is evident 16 weeks after diet, may represent a crucial step concerning the involvement of DZ on the restoration of the cerebellar cortical areas through neurotrophic-dependent neuronal plasticity events in HFD-induced brain impairments [52, 53]. At the same time, DZ-dependent TrkB protective effects in the cerebellum propose this brain region as a key site for the recovery of motor-related behavioral functions especially in obesity conditions.
Conclusion
In summary, our data support the important role played by all three cortical layers of the cerebellum in obese hamsters as suggested by the altered locomotor plus anxiety-like responses as well as being involved with neurodegenerative events during such a syndrome. Following the administration of DZ, hamsters rapidly displayed an evident preference for the light compartment, plus transiting more frequently between the two compartments as well as reduced spontaneous exploration in a non-aversive environment. Treatment with this phytoestrogen also pointed to an extremely elevated increase of TrkB expression in cerebellum cortex thus inverting the effects caused by obesity conditions and namely inflammatory-induced depression-like behaviors [54]. Overall, these first neuroprotective effects of DZ in the cerebellar layers strongly corroborate its major role in altered locomotor and emotional behavioral performances and this may open new interests on how the manipulation of BDNF and its main intracellular signaling mechanisms may contribute to the introduction of new and innovative drug strategies for treating such neurodegenerative syndromes.
References
Chiva-Blanch G, Badimon L. Effects of polyphenol intake on metabolic syndrome: current evidence from human trials. Ox Med Cell Long. 2017; 5812401.
Kang GG, Francis N, Hill R, Waters D, Blanchard C, Santhakumar AB. Dietary polyphenols and gene expression in molecular pathways associated with type 2 diabetes mellitus: a review. Int J Mol Sci. 2019;21:140.
Guo Y, Wu G, Su X, Yang H, Zhang J. Antiobesity action of a daidzein derivative on male obese mice induced by a high-fat diet. Nutr Res. 2009;29:656–63.
Sandini TM, Reis-Silva TM, Moreira N, Bernardi MM, Lebrun I, Spinosa HS. Effects of isoflavones on behavior, estradiol, glutamate, and GABA levels in intact middle-aged female rats. Nutr Neurosci. 2019;22:805–16.
Alò R, Fazzari G, Zizza M, Avolio E, Di Vito A, Bruno R, et al. Daidzein pro-cognitive effects coincided with changes of brain neurotensin1 receptor and interleukin-10 expression levels in obese hamsters. Neurotox Res. 2021;39:645–57.
Pang D, Yang C, Luo Q, Li C, Liu W, Li L, Zou Y, Feng B, Chen Z, Huang C. Soy isoflavones improve the oxidative stress induced hypothalamic inflammation and apoptosis in high fat diet-induced obese male mice through PGC1-alpha pathway. Aging (Albany NY). 2020;12:8710–27.
Shabbir U, Tyagi A, Elahi F, Aloo SO, Oh DH. The potential role of polyphenols in oxidative stress and inflammation induced by gut microbiota in Alzheimer’s disease. Antioxidants (Basel). 2021;10:1370.
Li M, Cai Q, Gao Y-T, Franke AA, Zhang X, Zhao Y, Wen W, Lan Q, Rothman N, Shyr Y, Shu X-O, Zheng W, Yang G. Phytoestrogens and lung cancer risk: a nested case-control study in never-smoking Chinese women. Am J Clin Nutr. 2022;115:643–51.
Wang H, Wang B, Yin H, Zhang G, Yu L, Kong X, Yuan H, Fang X, Liu Q, Liu C, Shi L. Reduced neurotrophic factor level is the early event before the functional neuronal deficiency in high-fat diet induced obese mice. Metab Brain Dis. 2017;32(1):247–57.
Gómez-Apo E, Mondragón-Maya A, Ferrari-Díaz M, Silva-Pereyra J. Structural brain changes associated with overweight and obesity. J Obes; 2021; 2021:6613385.
Shay J, Elbaz HA, Lee I, Zielske SP, Malek MH, Hüttemann M. Molecular mechanisms and therapeutic effects of (−)-epicatechin and other polyphenols in cancer, inflammation, diabetes, and neurodegeneration. Oxid Med Cell Longev. 2015:181260.
László A, Lénárt L, Illésy L, Fekete A, Nemcsik J. The role of neurotrophins in psychopathology and cardiovascular diseases: psychosomatic connections. J Neural Transm. 2019;126:265–78.
Fonseca-Portilla R, Krell-Roesch J, Shaibi GQ, Caselli RJ, Mandarino LJ, Zhang N, et al. Brain-derived neurotrophic factor and its associations with metabolism and physical activity in a Latino sample. Metab Syndr Rel Dis. 2019;17:75–80.
Bartkowska K, Paquin A, Gauthier AS, Kaplan DR, Miller FD. Trk signaling regulates neural precursor cell proliferation and differentiation during cortical development. Development. 2007;134:4369–80.
Vilar M, Mira H. Regulation of neurogenesis by neurotrophins during adulthood: expected and unexpected roles. Front Neurosc. 2016;10:26.
Neshatdoust S, Saunders C, Castle SM, Vauzour D, Williams C, Butler L, et al. High-flavonoid intake induces cognitive improvements linked to changes in serum brain-derived neurotrophic factor: two randomised, controlled trials. Nutr Healthy Aging. 2016;4:81–93.
Bensalem J, Servant L, Alfos S, Gaudout D, Layé S, Pallet V, et al. Dietary polyphenol supplementation prevents alterations of spatial navigation in middle-aged mice. Front Behav Neurosci. 2016;10:9.
Moosavi F, Hosseini R, Saso L, Firuzi O. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des Dev Ther. 2016;10:23–42.
Wurzelmann M, Romeika J, Sun D. Therapeutic potential of brain-derived neurotrophic factor (BDNF) and a small molecular mimic of BDNF for traumatic brain injury. Neural Regen Res. 2017;12:7–12.
García M, Lázaro E, López-Paz JF, Martínez O, Pérez M, Berrocoso S, et al. Cognitive functioning in chiari malformation type I without posterior fossa surgery. The Cerebellum. 2018;1:11.
Ben-Soussan TD, Avirame K, Glicksohn J, Goldstein A, Harpaz Y, Ben-Schachar M. Changes in cerebellar activity and inter-hemispheric coherence accompany improved reading capacity following Quadrato Motor Training. Front Syst Neurosci. 2014;8:81.
Schmahmann JD, Guell X, Stoodley CJ. Halko mathe theory and neuroscience of cerebellar cognition. Ann Rew Neurosci. 2019;42:337–64.
Xu X-M, Jiao Y, Tang T-Y, Zhang J, Lu C-Q, Luan Y, et al. Dissociation between cerebellar and cerebral neural activities in humans with long-term bilateral sensorineural hearing loss. Neural Plast. 2019;2019:8354849.
Pierce JE, Péron J. The basal ganglia and the cerebellum in human emotion. Soc Cogn Affect Neurosci. 2020;15:599–613.
Zeng S, Tai F, Zhai P, Yuan A, Jia R, Zhang X. Effect of daidzein on anxiety, social behavior and spatial learning in male Balb/cJ mice. Pharmacol Biochem Behav. 2010;96:16–23.
Bourin M, Hascoët M. The mouse lingh/dark box test. Eur Pharmacol. 2003;463:55–65.
Marques JM, Olsson IA, Ogren SO, Dahlborn K. Evaluation of exploration and risk assessment in pre-weaning mice using the novel cage test. Physiol Behav. 2008;93:139–47.
Alò R, Zizza M, Fazzari G, Facciolo RM, Canonaco M. Genistein modifies hamster behavior and expression of inflammatory factors following subchronic unpredictable mild stress. Neuroendocrinol. 2019;108:98–108.
Beuriat PA, Cohen-Zimerman S, Smith GNL, Krueger F, Gordon B, Grafman J. A new insight on the role of the cerebellum for executive functions and emotion processing in adults. Front Neurol. 2020;11: 593490.
Samson M, Claassen DO. Neurodegeneration and the cerebellum. Neurodegener Dis. 2017;17:155–65.
Han J, Nepal P, Odelade A, Freely FD, Belton DM, Graves JL Jr, et al. High-fat diet-induced weight gain, behavioral deficits, and dopamine changes in young C57BL/6J mice. Front Nutr. 2021;7:591161.
Van Leuven S, Carey A, Squiccimara L, Pintea G. The impact of obesity and consumption of a high fat diet on anxiety-like behavior in mice. Curr Develop Nutr. 2020;4:1239.
Arika WM, Kibiti CM, Njagi JM, Ngugi MP. Effects of DCM leaf extract of Gnidia glauca (Fresen) on locomotor activity, anxiety, and exploration-like behaviors in high-fat diet-induced obese rats. Behav Neurol. 2019;2019:7359235.
Es-Safi I, Mechchate H, Amaghnouje A, Kamaly OMA, Jawhari FZ, Imtara H, et al. The potential of parsley polyphenols and their antioxidant capacity to help in the treatment of depression and anxiety: an in vivo subacute study. Molecules. 2021;26:2009.
Lu C, Gao R, Zhang Y, Jiang N, Chen Y, Sun J, et al. S-equol, a Metabolite of dietary soy isoflavones, alleviates lipopolysaccharide-induced depressive-like behavior in mice by inhibiting neuroinflammation and enhancing synaptic plasticity. Food Funct. 2021;12:5770–8.
Das J, Ramani R, Suraju MO. Polyphenol compounds and PKC signaling. Biochim Biophys Acta. 2016;1860:2107–21.
Joseph SV, Edirisinghe I, Burton-Freeman BM. Fruit polyphenols: a review of anti-inflammatory effects in humans. Crit Rev Food Sci Nutr. 2016;56:419–44.
Addison O, Marcus RL, Lastayo PC, Ryan AS. Int J Endocrinol Review. 2014;2014:309570.
Ajayia AM, John KA, Emmanuel IB, Chidebe EO, Adedapo ADA. High-fat diet-induced memory impairment and anxiety-like behavior in rats attenuated by peel extract of Ananas comosus fruit via atheroprotective, antioxidant and anti-inflammatory actions. Metabolism Open. 2021;9:100077.
Carlsson CM, Nondahl DM, Klein BE, McBride PE, Sager MA, Schubert CR, et al. Increased atherogenic lipoproteins are associated with cognitive impairment: effects of statins and subclinical atherosclerosis. Alzheimer Dis Assoc Disord. 2009;23:11–7.
Han Q, Yeung SC, Ip MSM, Mak JCW. Dysregulation of cardiac lipid parameters in high-fat high-cholesterol diet-induced rat model. Lipids Health Dis. 2018;17:255.
Wang HL, Wang YY, Liu XG, Kuo SH, Liu N, Song QY, et al. Cholesterol, 24-hydroxycholesterol, and hydroxycholesterol as surrogate biomarkers in cerebrospinal fluid in mild cognitive impairment and Alzheimer’s disease: a meta-analysis. J Alzheimers Dis. 2016;51:45–55.
Dai L, Zou L, Meng L, Qiang G, Yan M, Zhang Z. Cholesterol metabolism in neurodegenerative diseases: molecular mechanisms and therapeutic targets. Mol Neurobiol. 2021;58:2183–201.
Marron EM, Viejo-Sobera R, Cuatrecasas G, Redolar-Ripoll D, Lorda PG, Datta A, et al. Prefronto-cerebellar neuromodulation affects appetite in obesity. Int J Obes. 2019;43:2119–24.
Camuso S, La Rosa PG, Fiore MT, Canterini S. Pleiotropic effects of BDNF on the cerebellum and hippocampus: implications for neurodevelopmental disorders. Neurobiol Dis. 2022;163:105606.
Raschpichler M, Straatman K, Schroeter ML, Arelin K, Schlögl H, Fritzsch D, et al. Abdominal fat distribution and its relationship to brain changes: the differential effects of age on cerebellar structure and function: a cross-sectional, exploratory study. BMJ Open. 2013;3:e001915.
Agrimi J, Spalletti C, Baroni C, Keceli G, Zhu G, Caragnano A, et al. Obese mice exposed to psychosocial stress display cardiac and hippocampal dysfunction associated with local brain-derived neurotrophic factor depletion. EBioMed. 2019;47:384–401.
Sheeler C, Rosa JG, Borgenheimer E, Mellesmoen A, Rainwater O, Cvetanovic M. Post-symptomatic delivery of brain-derived neurotrophic factor (BDNF) ameliorates spinocerebellar ataxia type 1 (SCA1) pathogenesis. Cerebellum. 2021;20:420–9.
Soontornniyomkij B, Everall IP, Chana G, Tsuang MT, Achim CL, Soontornniyomkij V. Tyrosine kinase B protein expression is reduced in the cerebellum of patients with bipolar disorder. J Affect Disord. 2011;133:646–54.
Li Y, Peng C, Guo X, You JJ, Yadav HP. Expression of brain-derived neurotrophic factor and tyrosine kinase B in cerebellum of poststroke depression rat model. Chin Med J. 2015;128:2926–31.
Le Moëne O, Stavarache M, Ogawa S, Musatov S, Ågmo A. Estrogen receptors α and β in the central amygdala and the ventromedial nucleus of the hypothalamus: sociosexual behaviors, fear and arousal in female rats during emotionally challenging events. Behav Brain Res. 2019;367:128–42.
Moosavi F, Hosseini R, Saso L, Firuzi O. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des Devel Ther. 2016;10:23–42.
Grynkiewicz G. Isoflavone research towards healthcare applications. Cancer Metastasis Treat. 2020;6:48.
Sugiyama A, Kato H, Takakura H, Osawa S, Maeda Y, Izawa T. Effects of physical activity and melatonin on brain-derived neurotrophic factor and cytokine expression in the cerebellum of high-fat-diet-fed rats. Neuropsychopharmacol Rep. 2020;40:291–6.
Funding
Open access funding provided by Università della Calabria within the CRUI-CARE Agreement. This work was supported by funds provided by the Italian University Research Ministry (MIUR) that granted financial assistance for the Doctorate Research Program in “Life Science.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alò, R., Fazzari, G., Zizza, M. et al. Emotional and Spontaneous Locomotor Behaviors Related to cerebellar Daidzein-dependent TrkB Expression Changes in Obese Hamsters. Cerebellum 22, 698–707 (2023). https://doi.org/10.1007/s12311-022-01432-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-022-01432-1