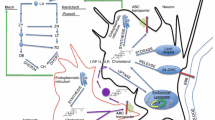
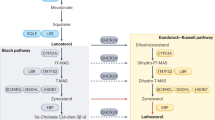
Abstract
Cholesterol is an indispensable component of the cell membrane and plays vital roles in critical physiological processes. Brain cholesterol accounts for a large portion of total cholesterol in the human body, and its content must be tightly regulated to ensure normal brain function. Disorders of cholesterol metabolism in the brain are linked to neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and other atypical cognitive deficits that arise at old age. However, the specific role of cholesterol metabolism disorder in the pathogenesis of neurodegenerative diseases has not been fully elucidated. Statins that are a class of lipid-lowering drugs have been reported to have a positive effect on neurodegenerative diseases. Herein, we reviewed the physiological and pathological conditions of cholesterol metabolism and discussed the possible mechanisms of cholesterol metabolism and statin therapy in neurodegenerative diseases.



Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- PD:
-
Parkinson’s disease
- HD:
-
Huntington’s disease
- BBB:
-
Blood–brain barrier
- ER:
-
Endoplasmic reticulum
- HMG-CoA:
-
3-Hydroxy-3-methylglutaryl CoA
- DE:
-
Desmosterol
- DHCR24:
-
24-Dehydrocholesterol reductase
- DHCR7:
-
7-Dehydrocholesterol reductase
- LT:
-
Lathosterol
- 7D:
-
7-Dehydrocholesterol
- PM:
-
Plasma membrane
- SREBP-2:
-
Sterol regulatory element-binding protein
- SCAP:
-
SREBP cleavage-activating protein
- SRE:
-
Sterol regulatory elements
- HDL:
-
High-density lipoprotein cholesterol
- LDL:
-
Low-density lipoprotein cholesterol
- VLDL:
-
Very-low-density lipoprotein cholesterol
- APOE:
-
Apolipoprotein E
- ABC transporters:
-
ATP-binding cassette (ABC) transporters
- LDLR:
-
Low-density lipoprotein family receptors
- ACAT1/SOAT1:
-
Acyltransferase 1
- CSF:
-
Cerebrospinal fluid
- 24-OHC:
-
24-Hydroxycholesterol
- Aβ:
-
Amyloid-β
- ACID:
-
APP intracellular domain
- APP:
-
Amyloid precursor protein
- 27-OHC:
-
27-Hydroxylcholesterol
- CYP27A1:
-
Sterol 27-hydroxylase
- RAS:
-
Renin–angiotensin system
- PLTP:
-
Phospholipid transporters
- SNpc:
-
Substantia nigra pars compacta
- LBs:
-
Lewy bodies
- ApoA1:
-
Apolipoprotein A-I
- PON1:
-
Oxygenase 1
- VMAT2:
-
Vesicular monoamine transporter 2
- DAT:
-
Dopamine transporter
- mβCD:
-
Methyl-β-cyclodextrin
- LXRs:
-
Liver X receptors
- TH:
-
Tyrosine hydroxylase
- NMDAR1:
-
N-methyl-d-aspartic acid receptor 1
- TNF-α:
-
Tumor necrosis factor-α
- IL-1β:
-
Interleukin-1β
- IL-6:
-
Interleukin-6
- NO:
-
Nitric oxide
References
Dietschy JM, Turley SD (2004) Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res 45:1375–1397. https://doi.org/10.1194/jlr.R400004-JLR200
Vance JE (2012) Dysregulation of cholesterol balance in the brain: contribution to neurodegenerative diseases. Dis Model Mech 5:746–755. https://doi.org/10.1242/dmm.010124
Berg JM, Tymoczko JL, Stryer L (2002) The complex regulation of cholesterol biosynthesis takes place at several levels. In: Biochemistry, 5th edn. W.H. Freeman, New York
Nieweg K, Schaller H, Pfrieger FW (2009) Marked differences in cholesterol synthesis between neurons and glial cells from postnatal rats. J Neurochem 109:125–134. https://doi.org/10.1111/j.1471-4159.2009.05917.x
Saher G, Brügger B, Lappe-Siefke C et al (2005) High cholesterol level is essential for myelin membrane growth. Nat Neurosci 8:468–475. https://doi.org/10.1038/nn1426
Quan G, Xie C, Dietschy JM, Turley SD (2003) Ontogenesis and regulation of cholesterol metabolism in the central nervous system of the mouse. Brain Res Dev Brain Res 146:87–98. https://doi.org/10.1016/j.devbrainres.2003.09.015
DeGrella RF, Simoni RD (1982) Intracellular transport of cholesterol to the plasma membrane. J Biol Chem 257:14256–14262
Kaplan MR, Simoni RD (1985) Transport of cholesterol from the endoplasmic reticulum to the plasma membrane. J Cell Biol 101:446–453. https://doi.org/10.1083/jcb.101.2.446
Heino S, Lusa S, Somerharju P et al (2000) Dissecting the role of the Golgi complex and lipid rafts in biosynthetic transport of cholesterol to the cell surface. Proc Natl Acad Sci 97:8375–8380. https://doi.org/10.1073/pnas.140218797
Leoni V, Caccia C (2015) The impairment of cholesterol metabolism in Huntington disease. Biochim Biophys Acta 1851:1095–1105. https://doi.org/10.1016/j.bbalip.2014.12.018
Anchisi L, Dessì S, Pani A, Mandas A (2013) Cholesterol homeostasis: a key to prevent or slow down neurodegeneration. Front Physiol 3:486. https://doi.org/10.3389/fphys.2012.00486
Martin MG, Ahmed T, Korovaichuk A et al (2014) Constitutive hippocampal cholesterol loss underlies poor cognition in old rodents. EMBO Mol Med 6:902–917. https://doi.org/10.15252/emmm.201303711
Kim WS, Weickert CS, Garner B (2008) Role of ATP-binding cassette transporters in brain lipid transport and neurological disease. J Neurochem 104:1145–1166. https://doi.org/10.1111/j.1471-4159.2007.05099.x
Herz J (2009) Apolipoprotein E receptors in the nervous system. Curr Opin Lipidol 20:190–196. https://doi.org/10.1097/MOL.0b013e32832d3a10
Pottier C, Hannequin D, Coutant S et al (2012) High frequency of potentially pathogenic SORL1 mutations in autosomal dominant early-onset Alzheimer disease. Mol Psychiatry 17:875–879. https://doi.org/10.1038/mp.2012.15
Rushworth JV, Griffiths HH, Watt NT, Hooper NM (2013) Prion protein-mediated toxicity of amyloid-β oligomers requires lipid rafts and the transmembrane LRP1. J Biol Chem 288:8935–8951. https://doi.org/10.1074/jbc.M112.400358
Rensen P, Jong MC, Vark LC et al (2000) Internalization of apolipoprotein E by hepatocytes via the LDL receptor is coupled to retroendocytosis. Atherosclerosis 151:239. https://doi.org/10.1016/S0021-9150(00)81081-3
Vance JE, Karten B (2014) Niemann-Pick C disease and mobilization of lysosomal cholesterol by cyclodextrin. J Lipid Res 55:1609–1621. https://doi.org/10.1194/jlr.R047837
Bryleva EY, Rogers MA, Chang CCY et al (2010) ACAT1 gene ablation increases 24(S)-hydroxycholesterol content in the brain and ameliorates amyloid pathology in mice with AD. Proc Natl Acad Sci 107:3081–3086. https://doi.org/10.1073/pnas.0913828107
Liu B, Turley SD, Burns DK et al (2009) Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1-/- mouse. Proc Natl Acad Sci 106:2377–2382. https://doi.org/10.1073/pnas.0810895106
Panzenboeck U et al (2002) ABCA1 and SR-BI are modulators of reverse sterol transport at an in vitro blood-brain barrier constituted of porcine brain capillary endothelial cells. J Biol Chem 277:42781–42789
Lange Y, Ye J, Strebel F (1995) Movement of 25-hydroxycholesterol from the plasma membrane to the rough endoplasmic reticulum in cultured hepatoma cells. J Lipid Res 36:1092–1097
Meaney S, Bodin K, Diczfalusy U, Björkhem I (2002) On the rate of translocation in vitro and kinetics in vivo of the major oxysterols in human circulation: critical importance of the position of the oxygen function. J Lipid Res 43:2130–2135. https://doi.org/10.1194/jlr.M200293-JLR200
Lund EG, Xie C, Kotti T et al (2003) Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J Biol Chem 278:22980–22988. https://doi.org/10.1074/jbc.M303415200
Ramirez DMO, Andersson S, Russell DW (2010) Neuronal expression and subcellular localization of cholesterol 24-hydroxylase in the mouse brain. J Comp Neurol 507(5):1676–1693
Goedert M, Spillantini MG (2006) A century of Alzheimer’s disease. Science 314:777–781. https://doi.org/10.1126/science.1132814
Kivipelto M, Solomon A (2006) Cholesterol as a risk factor for Alzheimer’s disease - epidemiological evidence. Acta Neurol Scand Suppl 185:50–57. https://doi.org/10.1111/j.1600-0404.2006.00685.x
Solomon A, Kåreholt I, Ngandu T et al (2007) Serum cholesterol changes after midlife and late-life cognition: twenty-one-year follow-up study. Neurology 68:751–756. https://doi.org/10.1212/01.wnl.0000256368.57375.b7
Reed B, Villeneuve S, Mack W et al (2014) Associations between serum cholesterol levels and cerebral amyloidosis. JAMA Neurol 71:195. https://doi.org/10.1001/jamaneurol.2013.5390
Popp J, Meichsner S, Kölsch H et al (2013) Cerebral and extracerebral cholesterol metabolism and CSF markers of Alzheimer’s disease. Biochem Pharmacol 86:37–42. https://doi.org/10.1016/j.bcp.2012.12.007
Kivipelto M, Ngandu T, Fratiglioni L et al (2005) Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 62:1556–1560. https://doi.org/10.1001/archneur.62.10.1556
Kuo YM, Emmerling MR, Bisgaier CL et al (1998) Elevated low-density lipoprotein in Alzheimer’s disease correlates with brain abeta 1-42 levels. Biochem Biophys Res Commun 252:711–715. https://doi.org/10.1006/bbrc.1998.9652
Popp J, Lewczuk P, Kölsch H et al (2012) Cholesterol metabolism is associated with soluble amyloid precursor protein production in Alzheimer’s disease. J Neurochem 123:310–316. https://doi.org/10.1111/j.1471-4159.2012.07893.x
Reitz C, Tang M-X, Manly J et al (2008) Plasma lipid levels in the elderly are not associated with the risk of mild cognitive impairment. Dement Geriatr Cogn Disord 25:232–237. https://doi.org/10.1159/000115847
Mielke MM, Zandi PP, Sjögren M et al (2005) High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology 64:1689–1695. https://doi.org/10.1212/01.WNL.0000161870.78572.A5
Anstey KJ, Ashby-Mitchell K, Peters R (2017) Updating the evidence on the association between serum cholesterol and risk of late-life dementia: review and meta-analysis. J Alzheimers Dis 56:215–228. https://doi.org/10.3233/JAD-160826
Heverin M, Bogdanovic N, Lütjohann D et al (2004) Changes in the levels of cerebral and extracerebral sterols in the brain of patients with Alzheimer’s disease. J Lipid Res 45:186–193. https://doi.org/10.1194/jlr.M300320-JLR200
Mason RP, Shoemaker WJ, Shajenko L et al (1992) Evidence for changes in the Alzheimer’s disease brain cortical membrane structure mediated by cholesterol. Neurobiol Aging 13:413–419. https://doi.org/10.1016/0197-4580(92)90116-f
Sparks DL (1997) Coronary artery disease, hypertension, ApoE, and cholesterol: a link to Alzheimer’s disease? Ann N Y Acad Sci 826:128–146. https://doi.org/10.1111/j.1749-6632.1997.tb48466.x
Eckert GP, Cairns NJ, Maras A et al (2000) Cholesterol modulates the membrane-disordering effects of beta-amyloid peptides in the hippocampus: specific changes in Alzheimer’s disease. Dement Geriatr Cogn Disord 11:181–186. https://doi.org/10.1159/000017234
Xiong H, Callaghan D, Jones A et al (2008) Cholesterol retention in Alzheimer’s brain is responsible for high β- and γ-secretase activities and Aβ production. Neurobiol Dis 29:422–437. https://doi.org/10.1016/j.nbd.2007.10.005
Papassotiropoulos A, Lütjohann D, Bagli M et al (2002) 24S-hydroxycholesterol in cerebrospinal fluid is elevated in early stages of dementia. J Psychiatr Res 36:27–32. https://doi.org/10.1016/s0022-3956(01)00050-4
Testa G, Staurenghi E, Zerbinati C et al (2016) Changes in brain oxysterols at different stages of Alzheimer’s disease: their involvement in neuroinflammation. Redox Biol 10:24–33. https://doi.org/10.1016/j.redox.2016.09.001
Yu A, Zhang X, Wang Y et al (2019) Longitudinal and nonlinear relations of dietary and serum cholesterol in midlife with cognitive decline: results from EMCOA study. Mol Neurodegener 14:51. https://doi.org/10.1186/s13024-019-0353-1
Whitmer RA, Sidney S, Selby J et al (2005) Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64:277–281. https://doi.org/10.1212/01.WNL.0000149519.47454.F2
Kirsch C, Eckert GP, Koudinov AR, Müller WE (2003) Brain cholesterol, statins and Alzheimer’s disease. Pharmacopsychiatry 36(Suppl 2):S113–S119. https://doi.org/10.1055/s-2003-43058
Thériault P, ElAli A, Rivest S (2016) High fat diet exacerbates Alzheimer’s disease-related pathology in APPswe/PS1 mice. Oncotarget 7:67808–67827. https://doi.org/10.18632/oncotarget.12179
Ledreux A, Wang X, Schultzberg M et al (2016) Detrimental effects of a high fat/high cholesterol diet on memory and hippocampal markers in aged rats. Behav Brain Res 312:294–304. https://doi.org/10.1016/j.bbr.2016.06.012
Walker JM, Dixit S, Saulsberry AC et al (2017) Reversal of high fat diet-induced obesity improves glucose tolerance, inflammatory response, β-amyloid accumulation and cognitive decline in the APP/PSEN1 mouse model of Alzheimer’s disease. Neurobiol Dis 100:87–98. https://doi.org/10.1016/j.nbd.2017.01.004
Lehtisalo J, Levälahti E, Lindström J et al (2019) Dietary changes and cognition over 2 years within a multidomain intervention trial—the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER). Alzheimers Dement 15:410–417. https://doi.org/10.1016/j.jalz.2018.10.001
Ngandu T, Lehtisalo J, Solomon A et al (2015) A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385:2255–2263. https://doi.org/10.1016/S0140-6736(15)60461-5
Rosenberg A, Ngandu T, Rusanen M et al (2018) Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: the FINGER trial. Alzheimers Dement J Alzheimers Assoc 14:263–270. https://doi.org/10.1016/j.jalz.2017.09.006
Pfrieger FW (2003) Cholesterol homeostasis and function in neurons of the central nervous system. Cell Mol Life Sci CMLS 60:1158–1171. https://doi.org/10.1007/s00018-003-3018-7
Wood WG, Igbavboa U, Eckert GP et al (2005) Is hypercholesterolemia a risk factor for Alzheimer’s disease? Mol Neurobiol 31:185–192. https://doi.org/10.1385/MN:31:1-3:185
Sonnino S, Prinetti A (2013) Membrane domains and the “lipid raft” concept. Curr Med Chem 20:4–21
Hayashi H, Igbavboa U, Hamanaka H et al (2002) Cholesterol is increased in the exofacial leaflet of synaptic plasma membranes of human apolipoprotein E4 knock-in mice. Neuroreport 13:383–386. https://doi.org/10.1097/00001756-200203250-00004
Igbavboa U, Avdulov NA, Schroeder F, Wood WG (1996) Increasing age alters transbilayer fluidity and cholesterol asymmetry in synaptic plasma membranes of mice. J Neurochem 66:1717–1725. https://doi.org/10.1046/j.1471-4159.1996.66041717.x
Burns MP, Igbavboa U, Wang L et al (2006) Cholesterol distribution, not total levels, correlate with altered amyloid precursor protein processing in statin-treated mice. NeuroMolecular Med 8:319–328. https://doi.org/10.1385/nmm:8:3:319
Cutler RG, Kelly J, Storie K et al (2004) Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci U S A 101:2070–2075. https://doi.org/10.1073/pnas.0305799101
Kang J, Lemaire HG, Unterbeck A et al (1987) The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 325:733–736. https://doi.org/10.1038/325733a0
Kojro E, Gimpl G, Lammich S et al (2001) Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the α-secretase ADAM 10. Proc Natl Acad Sci U S A 98:5815–5820. https://doi.org/10.1073/pnas.081612998
Seubert P, Oltersdorf T, Lee MG et al (1993) Secretion of beta-amyloid precursor protein cleaved at the amino terminus of the beta-amyloid peptide. Nature 361:260–263. https://doi.org/10.1038/361260a0
Vetrivel KS, Thinakaran G (2010) Membrane rafts in Alzheimer’s disease beta-amyloid production. Biochim Biophys Acta 1801:860–867. https://doi.org/10.1016/j.bbalip.2010.03.007
Marquer C, Devauges V, Cossec J-C et al (2011) Local cholesterol increase triggers amyloid precursor protein-Bace1 clustering in lipid rafts and rapid endocytosis. FASEB J Off Publ Fed Am Soc Exp Biol 25:1295–1305. https://doi.org/10.1096/fj.10-168633
Barrett PJ, Song Y, Van Horn WD et al (2012) The amyloid precursor protein has a flexible transmembrane domain and binds cholesterol. Science 336:1168–1171. https://doi.org/10.1126/science.1219988
Makarov M, Grossard M (2008) Corrections and clarifications: efficient inhibition of the Alzheimer’s disease β-secretase by membrane targeting. Science 321:912–912
van der Kant R, Langness VF, Herrera CM et al (2019) Cholesterol metabolism is a druggable axis that independently regulates tau and amyloid-β in iPSC-derived Alzheimer’s disease neurons. Cell Stem Cell 24:363-375.e9. https://doi.org/10.1016/j.stem.2018.12.013
Kunkle BW, Grenier-Boley B, Sims R et al (2019) Author correction: genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet 51:1423–1424. https://doi.org/10.1038/s41588-019-0495-7
Liu C-C, Kanekiyo T, Xu H, Bu G (2013) Apolipoprotein E and Alzheimer disease: risk, mechanisms, and therapy. Nat Rev Neurol 9:106–118. https://doi.org/10.1038/nrneurol.2012.263
Harold D, Abraham R, Hollingworth P et al (2009) Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet 41(10):1088–1093
Lambert J-C, Heath S, Even G et al (2009) Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet 41:1094–1099. https://doi.org/10.1038/ng.439
Corder EH, Saunders AM, Risch NJ et al (1994) Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet 7:180–184. https://doi.org/10.1038/ng0694-180
Poirier J (1994) Apolipoprotein E in animal models of CNS injury and in Alzheimer’s disease. Trends Neurosci 17:525–530. https://doi.org/10.1016/0166-2236(94)90156-2
Rapp A, Gmeiner B, Hüttinger M (2006) Implication of apoE isoforms in cholesterol metabolism by primary rat hippocampal neurons and astrocytes. Biochimie 88:473–483. https://doi.org/10.1016/j.biochi.2005.10.007
Gong J-S, Kobayashi M, Hayashi H et al (2002) Apolipoprotein E (ApoE) isoform-dependent lipid release from astrocytes prepared from human ApoE3 and ApoE4 knock-in mice. J Biol Chem 277:29919–29926. https://doi.org/10.1074/jbc.M203934200
Ellis RJ, Olichney JM, Thal LJ et al (1996) Cerebral amyloid angiopathy in the brains of patients with Alzheimer’s disease: the CERAD experience, part XV. Neurology 46:1592–1596. https://doi.org/10.1212/wnl.46.6.1592
Fleisher AS, Chen K, Liu X et al (2013) Apolipoprotein E ε4 and age effects on florbetapir positron emission tomography in healthy aging and Alzheimer disease. Neurobiol Aging 34:1–12. https://doi.org/10.1016/j.neurobiolaging.2012.04.017
Brecht WJ, Harris FM, Chang S et al (2004) Neuron-specific apolipoprotein E4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J Neurosci 24:2527–2534. https://doi.org/10.1523/JNEUROSCI.4315-03.2004
Thambisetty M, Simmons A, Velayudhan L et al (2010) Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch Gen Psychiatry 67:739–748. https://doi.org/10.1001/archgenpsychiatry.2010.78
De Roeck A, Van Broeckhoven C, Sleegers K (2019) The role of ABCA7 in Alzheimer’s disease: evidence from genomics, transcriptomics and methylomics. Acta Neuropathol (Berl) 138:201–220. https://doi.org/10.1007/s00401-019-01994-1
Kunkle BW, Grenier-Boley B, Sims R et al (2019) Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet 51:414–430. https://doi.org/10.1038/s41588-019-0358-2
Nugent AA, Lin K, van Lengerich B et al (2020) TREM2 regulates microglial cholesterol metabolism upon chronic phagocytic challenge. Neuron 105:837-854.e9. https://doi.org/10.1016/j.neuron.2019.12.007
Björkhem I (2006) Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J Intern Med 260:493–508. https://doi.org/10.1111/j.1365-2796.2006.01725.x
Gamba P, Testa G, Gargiulo S et al (2015) Oxidized cholesterol as the driving force behind the development of Alzheimer’s disease. Front Aging Neurosci 7:119. https://doi.org/10.3389/fnagi.2015.00119
Ali Z, Heverin M, Olin M et al (2013) On the regulatory role of side-chain hydroxylated oxysterols in the brain. Lessons from CYP27A1 transgenic and Cyp27a1−/− mice. J Lipid Res 54:1033–1043. https://doi.org/10.1194/jlr.M034124
Saeed AA, Genové G, Li T et al (2014) Effects of a disrupted blood-brain barrier on cholesterol homeostasis in the brain. J Biol Chem 289:23712–23722. https://doi.org/10.1074/jbc.M114.556159
Lütjohann D, von Bergmann K (2003) 24S-hydroxycholesterol: a marker of brain cholesterol metabolism. Pharmacopsychiatry 36(Suppl 2):S102–S106. https://doi.org/10.1055/s-2003-43053
Maioli S, Båvner A, Ali Z et al (2013) Is it possible to improve memory function by upregulation of the cholesterol 24S-hydroxylase (CYP46A1) in the brain? PLoS One 8:e68534. https://doi.org/10.1371/journal.pone.0068534
Brown J, Theisler C, Silberman S et al (2004) Differential expression of cholesterol hydroxylases in Alzheimer’s disease. J Biol Chem 279:34674–34681. https://doi.org/10.1074/jbc.M402324200
Famer D, Meaney S, Mousavi M et al (2007) Regulation of alpha- and beta-secretase activity by oxysterols: cerebrosterol stimulates processing of APP via the alpha-secretase pathway. Biochem Biophys Res Commun 359:46–50. https://doi.org/10.1016/j.bbrc.2007.05.033
Simons K, Ehehalt R (2002) Cholesterol, lipid rafts, and disease. J Clin Invest 110:597–603. https://doi.org/10.1172/JCI16390
Meaney S, Heverin M, Panzenboeck U et al (2007) Novel route for elimination of brain oxysterols across the blood-brain barrier: conversion into 7alpha-hydroxy-3-oxo-4-cholestenoic acid. J Lipid Res 48:944–951. https://doi.org/10.1194/jlr.M600529-JLR200
Shafaati M, Marutle A, Pettersson H et al (2011) Marked accumulation of 27-hydroxycholesterol in the brains of Alzheimer’s patients with the Swedish APP 670/671 mutation. J Lipid Res 52:1004–1010. https://doi.org/10.1194/jlr.M014548
Yau JLW, Rasmuson S, Andrew R et al (2003) Dehydroepiandrosterone 7-hydroxylase CYP7B: predominant expression in primate hippocampus and reduced expression in Alzheimer’s disease. Neuroscience 121:307–314. https://doi.org/10.1016/s0306-4522(03)00438-x
Mateos L, Akterin S, Gil-Bea F-J et al (2009) Activity-regulated cytoskeleton-associated protein in rodent brain is down-regulated by high fat diet in vivo and by 27-hydroxycholesterol in vitro. Brain Pathol Zurich Switz 19:69–80. https://doi.org/10.1111/j.1750-3639.2008.00174.x
Björkhem I, Cedazo-Minguez A, Leoni V, Meaney S (2009) Oxysterols and neurodegenerative diseases. Mol Asp Med 30:171–179. https://doi.org/10.1016/j.mam.2009.02.001
Ismail M-A-M, Mateos L, Maioli S et al (2017) 27-Hydroxycholesterol impairs neuronal glucose uptake through an IRAP/GLUT4 system dysregulation. J Exp Med 214:699–717. https://doi.org/10.1084/jem.20160534
Heverin M, Maioli S, Pham T et al (2015) 27-Hydroxycholesterol mediates negative effects of dietary cholesterol on cognition in mice. Behav Brain Res 278:356–359. https://doi.org/10.1016/j.bbr.2014.10.018
Mateos L, Ismail M-A-M, Gil-Bea F-J et al (2011) Upregulation of brain renin angiotensin system by 27-hydroxycholesterol in Alzheimer’s disease. J Alzheimers Dis JAD 24:669–679. https://doi.org/10.3233/JAD-2011-101512
Cedazo-Mínguez A, Ismail M-A-M, Mateos L (2011) Plasma cholesterol and risk for late-onset Alzheimer’s disease. Expert Rev Neurother 11:495–498. https://doi.org/10.1586/ern.11.36
Mateos L, Ismail M-A-M, Gil-Bea F-J et al (2011) Side chain-oxidized oxysterols regulate the brain renin-angiotensin system through a liver X receptor-dependent mechanism. J Biol Chem 286:25574–25585. https://doi.org/10.1074/jbc.M111.236877
Marwarha G, Dasari B, Prasanthi JRP et al (2010) Leptin reduces the accumulation of Aβ and phosphorylated tau induced by 27-hydroxycholesterol in rabbit organotypic slices. J Alzheimers Dis JAD 19:1007–1019. https://doi.org/10.3233/JAD-2010-1298
Fassbender K, Simons M, Bergmann C et al (2001) Simvastatin strongly reduces levels of Alzheimer’s disease β-amyloid peptides Aβ42 and Aβ40 in vitro and in vivo. Proc Natl Acad Sci U S A 98:5856–5861. https://doi.org/10.1073/pnas.081620098
Lin F-C, Chuang Y-S, Hsieh H-M et al (2015) Early statin use and the progression of Alzheimer disease. Medicine (Baltimore) 94:47. https://doi.org/10.1097/MD.0000000000002143
Kurinami H, Sato N, Shinohara M et al (2008) Prevention of amyloid beta-induced memory impairment by fluvastatin, associated with the decrease in amyloid beta accumulation and oxidative stress in amyloid beta injection mouse model. Int J Mol Med 21:531–537
Li L, Cao D, Kim H et al (2006) Simvastatin enhances learning and memory independent of amyloid load in mice. Ann Neurol 60:729–739. https://doi.org/10.1002/ana.21053
Petanceska SS, DeRosa S, Olm V et al (2002) Statin therapy for Alzheimer’s disease: will it work? J Mol Neurosci MN 19:155–161. https://doi.org/10.1007/s12031-002-0026-2
Chauhan NB, Siegel GJ, Feinstein DL (2004) Effects of lovastatin and pravastatin on amyloid processing and inflammatory response in TgCRND8 brain. Neurochem Res 29:1897–1911. https://doi.org/10.1023/b:nere.0000042217.90204.8d
Paris D, Townsend KP, Humphrey J et al (2002) Statins inhibit A beta-neurotoxicity in vitro and A beta-induced vasoconstriction and inflammation in rat aortae. Atherosclerosis 161:293–299. https://doi.org/10.1016/s0021-9150(01)00660-8
Abad-Rodriguez J, Ledesma MD, Craessaerts K et al (2004) Neuronal membrane cholesterol loss enhances amyloid peptide generation. J Cell Biol 167:953–960. https://doi.org/10.1083/jcb.200404149
Puglielli L, Konopka G, Pack-Chung E et al (2001) Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid beta-peptide. Nat Cell Biol 3:905–912. https://doi.org/10.1038/ncb1001-905
Hutter-Paier B, Huttunen HJ, Puglielli L et al (2004) The ACAT inhibitor CP-113,818 markedly reduces amyloid pathology in a mouse model of Alzheimer’s disease. Neuron 44:227–238. https://doi.org/10.1016/j.neuron.2004.08.043
Wolozin B, Kellman W, Ruosseau P et al (2000) Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol 57:1439–1443. https://doi.org/10.1001/archneur.57.10.1439
Wolozin B, Wang SW, Li N-C et al (2007) Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease. BMC Med 5:20. https://doi.org/10.1186/1741-7015-5-20
Haag MDM, Hofman A, Koudstaal PJ et al (2009) Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J Neurol Neurosurg Psychiatry 80:13–17. https://doi.org/10.1136/jnnp.2008.150433
Feldman HH, Doody RS, Kivipelto M et al (2010) Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology 74:956–964. https://doi.org/10.1212/WNL.0b013e3181d6476a
Sparks DL, Sabbagh MN, Connor DJ et al (2005) Atorvastatin for the treatment of mild to moderate Alzheimer disease: preliminary results. Arch Neurol 62:753–757. https://doi.org/10.1001/archneur.62.5.753
Friedhoff LT, Cullen EI, Geoghagen NS, Buxbaum JD (2001) Treatment with controlled-release lovastatin decreases serum concentrations of human beta-amyloid (A beta) peptide. Int J Neuropsychopharmacol 4:127–130. https://doi.org/10.1017/S1461145701002310
Rockwood K, Kirkland S, Hogan DB et al (2002) Use of lipid-lowering agents, indication bias, and the risk of dementia in community-dwelling elderly people. Arch Neurol 59:223–227. https://doi.org/10.1001/archneur.59.2.223
Rea TD, Breitner JC, Psaty BM et al (2005) Statin use and the risk of incident dementia: the Cardiovascular Health Study. Arch Neurol 62:1047–1051. https://doi.org/10.1001/archneur.62.7.1047
Wahner AD, Bronstein JM, Bordelon YM, Ritz B (2008) Statin use and the risk of Parkinson’s disease. Neurology 70:1418–1422. https://doi.org/10.1212/01.wnl.0000286942.14552.51
Tysnes O-B, Storstein A (2017) Epidemiology of Parkinson’s disease. J Neural Transm Vienna Austria 1996 124:901–905. https://doi.org/10.1007/s00702-017-1686-y
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet Lond Engl 386:896–912. https://doi.org/10.1016/S0140-6736(14)61393-3
Sweeney P, Park H et al (2017) Protein misfolding in neurodegenerative diseases: implications and strategies. Transl Neurodegener 6:6. https://doi.org/10.1186/s40035-017-0077-5
Karimi-Moghadam A, Charsouei S, Bell B, Jabalameli MR (2018) Parkinson disease from Mendelian forms to genetic susceptibility: new molecular insights into the neurodegeneration process. Cell Mol Neurobiol 38:1153–1178. https://doi.org/10.1007/s10571-018-0587-4
Wei Q, Wang H, Tian Y et al (2013) Reduced serum levels of triglyceride, very low density lipoprotein cholesterol and apolipoprotein B in Parkinson’s disease patients. PLoS ONE 8. https://doi.org/10.1371/journal.pone.0075743
Huang X, Chen H, Miller WC et al (2007) Lower low density lipid cholesterol levels are associated with Parkinson’s disease. Mov Disord Off J Mov Disord Soc 22:377–381. https://doi.org/10.1002/mds.21290
Swanson CR, Berlyand Y, Xie SX et al (2015) Plasma ApoA1 associates with age at onset and motor severity in early Parkinson disease patients. Mov Disord Off J Mov Disord Soc 30:1648–1656. https://doi.org/10.1002/mds.26290
Qiang JK, Wong YC, Siderowf A et al (2013) Plasma apolipoprotein A1 as a biomarker for Parkinson’s disease. Ann Neurol 74:119–127. https://doi.org/10.1002/ana.23872
Teunissen CE, Lütjohann D, Bergmann KV et al (2003) Combination of serum markers related to several mechanisms in Alzheimer’s disease. Neurobiol Aging 24:893–902. https://doi.org/10.1016/S0197-4580(03)00005-8
Sohmiya M, Tanaka M, Tak NW et al (2004) Redox status of plasma coenzyme Q10 indicates elevated systemic oxidative stress in Parkinson’s disease. J Neurol Sci 223:161–166. https://doi.org/10.1016/j.jns.2004.05.007
de Lau LML, Koudstaal PJ, Albert H, Breteler MMB (2006) Serum cholesterol levels and the risk of Parkinson’s disease. Am J Epidemiol 10. https://doi.org/10.1093/aje/kwj283
Rozani V, Gurevich T, Giladi N et al (2018) Higher serum cholesterol and decreased Parkinson’s disease risk: a statin-free cohort study. Mov Disord. https://doi.org/10.1002/mds.27413
Hu G, Antikainen R, Jousilahti P et al (2008) Total cholesterol and the risk of Parkinson disease. Neurology 70:1972–1979. https://doi.org/10.1212/01.wnl.0000312511.62699.a8
Hu G (2009) Total cholesterol and the risk of Parkinson’s disease: a review for some new findings. Park Dis 2010:836962. https://doi.org/10.4061/2010/836962
Peter S et al (2002) Cerebrospinal fluid 24S-hydroxycholesterol is increased in patients with Alzheimer’s disease compared to healthy controls. Neurosci Lett. https://doi.org/10.1016/S0304-3940(02)00164-7
Lee C-YJ, Seet RCS, Huang SH et al (2009) Different patterns of oxidized lipid products in plasma and urine of dengue fever, stroke, and Parkinson’s disease patients: cautions in the use of biomarkers of oxidative stress. Antioxid Redox Signal 11:407–420. https://doi.org/10.1089/ars.2008.2179
Björkhem I, Lövgren-Sandblom A, Leoni V et al (2013) Oxysterols and Parkinson’s disease: evidence that levels of 24S-hydroxycholesterol in cerebrospinal fluid correlates with the duration of the disease. Neurosci Lett 555:102–105. https://doi.org/10.1016/j.neulet.2013.09.003
Huang X, Chen PC, Poole C (2004) APOE-[epsilon]2 allele associated with higher prevalence of sporadic Parkinson disease. Neurology 62:2198–2202. https://doi.org/10.1212/01.wnl.0000130159.28215.6a
Wakabayashi K, Kakita A, Hayashi S et al (1998) Apolipoprotein E epsilon4 allele and progression of cortical Lewy body pathology in Parkinson’s disease. Acta Neuropathol (Berl) 95:450–454. https://doi.org/10.1007/s004010050824
Huang X, Chen P, Kaufer DI et al (2006) Apolipoprotein E and dementia in Parkinson disease: a meta-analysis. Arch Neurol 63:189–193. https://doi.org/10.1001/archneur.63.2.189
Williams-Gray CH, Goris A, Saiki M et al (2009) Apolipoprotein E genotype as a risk factor for susceptibility to and dementia in Parkinson’s disease. J Neurol 256:493–498. https://doi.org/10.1007/s00415-009-0119-8
Gao J, Huang X, Park Y et al (2011) Apolipoprotein E genotypes and the risk of Parkinson disease. Neurobiol Aging 32:2106.e1-2106.e6. https://doi.org/10.1016/j.neurobiolaging.2011.05.016
Federoff M, Jimenez-Rolando B, Nalls MA, Singleton AB (2012) A large study reveals no association between APOE and Parkinson’s disease. Neurobiol Dis 46:389–392. https://doi.org/10.1016/j.nbd.2012.02.002
Marwarha G, Ghribi O (2015) Does the oxysterol 27-hydroxycholesterol underlie Alzheimer’s disease-Parkinson’s disease overlap? Exp Gerontol 68:13–18. https://doi.org/10.1016/j.exger.2014.09.013
Jeong S-M, Jang W, Shin DW (2019) Association of statin use with Parkinson’s disease: dose-response relationship. Mov Disord Off J Mov Disord Soc 34:1014–1021. https://doi.org/10.1002/mds.27681
Kimura T, Hasegawa M, Takano O (2002) The effect of dopamine on serum lipid concentration after propofol administration. Masui 51:286–288
de Oliveira J, Moreira ELG, Mancini G et al (2013) Diphenyl diselenide prevents cortico-cerebral mitochondrial dysfunction and oxidative stress induced by hypercholesterolemia in LDL receptor knockout mice. Neurochem Res 38:2028–2036. https://doi.org/10.1007/s11064-013-1110-4
Thirumangalakudi L, Prakasam A, Zhang R et al (2008) High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J Neurochem 106:475–485. https://doi.org/10.1111/j.1471-4159.2008.05415.x
Morris JK, Bomhoff GL, Stanford JA, Geiger PC (2010) Neurodegeneration in an animal model of Parkinson’s disease is exacerbated by a high-fat diet. Am J Physiol - Regul Integr Comp Physiol 299:R1082–R1090. https://doi.org/10.1152/ajpregu.00449.2010
Visanji NP, Lang AE, Kovacs GG (2019) Beyond the synucleinopathies: alpha synuclein as a driving force in neurodegenerative comorbidities. Transl Neurodegener 8:28. https://doi.org/10.1186/s40035-019-0172-x
Fabelo N, Martín V, Santpere G et al (2011) Severe alterations in lipid composition of frontal cortex lipid rafts from Parkinson’s disease and incidental Parkinson’s disease. Mol Med 17:1107–1118. https://doi.org/10.2119/molmed.2011.00119
Ida E, Nath S et al (2017) Impact of high cholesterol in a Parkinson’s disease model: prevention of lysosomal leakage versus stimulation of α-synuclein aggregation. Eur J Cell Biol. https://doi.org/10.1016/j.ejcb.2017.01.002
Abbott CA, Mackness MI, Kumar S et al (1995) Serum paraoxonase activity, concentration, and phenotype distribution in diabetes mellitus and its relationship to serum lipids and lipoproteins. Arterioscler Thromb Vasc Biol 15:1812–1818. https://doi.org/10.1161/01.atv.15.11.1812
Prasanthi JR, Huls A, Thomasson S et al (2009) Differential effects of 24-hydroxycholesterol and 27-hydroxycholesterol on β-amyloid precursor protein levels and processing in human neuroblastoma SH-SY5Y cells. Mol Neurodegener 4:1. https://doi.org/10.1186/1750-1326-4-1
Balazs Z, Panzenboeck U, Hammer A et al (2004) Uptake and transport of high-density lipoprotein (HDL) and HDL-associated alpha-tocopherol by an in vitro blood-brain barrier model. J Neurochem 89:939–950. https://doi.org/10.1111/j.1471-4159.2004.02373.x
Emamzadeh FN, Allsop D (2017) α-Synuclein interacts with lipoproteins in plasma. J Mol Neurosci 63:165. https://doi.org/10.1007/s12031-017-0967-0
Shahmoradian SH, Genoud C, Graffmeyer A et al (2017) Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat Neurosci 22:1099–1109. https://doi.org/10.1038/s41593-019-0423-2
Halliday GM, Ophof A, Broe M et al (2005) Alpha-synuclein redistributes to neuromelanin lipid in the substantia nigra early in Parkinson’s disease. Brain J Neurol 128:2654–2664. https://doi.org/10.1093/brain/awh584
Boassa D, Berlanga ML, Yang MA et al (2013) Mapping the subcellular distribution of α-synuclein in neurons using genetically encoded probes for correlated light and electron microscopy: implications for Parkinson’s disease pathogenesis. J Neurosci 33:2605–2615. https://doi.org/10.1523/JNEUROSCI.2898-12.2013
Scott D, Roy S (2012) α-Synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis. J Neurosci 32(30):10129–10135. https://doi.org/10.1523/JNEUROSCI.0535-12.2012
Nakamura K, Mori F, Tanji K et al (2015) Isopentenyl diphosphate isomerase, a cholesterol synthesizing enzyme, is localized in Lewy bodies. Neuropathol Off J Jpn Soc Neuropathol 35:432–440. https://doi.org/10.1111/neup.12204
Baron P, Crews L, Koob AO et al (2008) Statins reduce neuronal alpha-synuclein aggregation in in vitro models of Parkinson’s disease. J Neurochem 105:1656–1667. https://doi.org/10.1111/j.1471-4159.2008.05254.x
Krüger R, Vieira-Saecker AM, Kuhn W et al (1999) Increased susceptibility to sporadic Parkinson’s disease by a certain combined alpha-synuclein/apolipoprotein E genotype. Ann Neurol 45:611–617. https://doi.org/10.1002/1531-8249(199905)45:5<611::aid-ana9>3.0.co;2-x
Fantini J, Carlus D, Yahi N (2011) The fusogenic tilted peptide (67-78) of α-synuclein is a cholesterol binding domain. Biochim Biophys Acta 1808:2343–2351. https://doi.org/10.1016/j.bbamem.2011.06.017
Hsiao J-HT, Halliday GM et al (2017) α-Synuclein regulates neuronal cholesterol efflux. Molecules 22(10):1769
Sui Y-T, Bullock KM, Erickson MA et al (2014) Alpha synuclein is transported into and out of the brain by the blood-brain barrier. Peptides 62:197–202. https://doi.org/10.1016/j.peptides.2014.09.018
Barceló-Coblijn G et al (2007) Brain neutral lipids mass is increased in α-synuclein gene-ablated mice. J Neurochem 110(1):132–141. https://doi.org/10.1111/j.1471-4159.2006.04348.x
van Maarschalkerweerd A, Vetri V, Vestergaard B (2015) Cholesterol facilitates interactions between α-synuclein oligomers and charge-neutral membranes. FEBS Lett 589:2661–2667. https://doi.org/10.1016/j.febslet.2015.08.013
Emamzadeh FN, Aojula H, McHugh PC, Allsop D (2016) Effects of different isoforms of apoE on aggregation of the α-synuclein protein implicated in Parkinson’s disease. Neurosci Lett 618:146–151. https://doi.org/10.1016/j.neulet.2016.02.042
Zeppelin T, Ladefoged LK, Sinning S et al (2018) A direct interaction of cholesterol with the dopamine transporter prevents its out-to-inward transition. PLoS Comput Biol 14(1):e1005907. https://doi.org/10.1371/journal.pcbi.1005907
Orłowski A, Grzybek M, Bunker A et al (2012) Strong preferences of dopamine and l-dopa towards lipid head group: importance of lipid composition and implication for neurotransmitter metabolism. J Neurochem 122:681–690. https://doi.org/10.1111/j.1471-4159.2012.07813.x
Jones KT, Zhen J, Reith MEA (2012) Importance of cholesterol in dopamine transporter function. J Neurochem 123:700–715. https://doi.org/10.1111/jnc.12007
Zhuge W, Wen F, Ni Z et al (2019) Dopamine burden triggers cholesterol overload following disruption of synaptogenesis in minimal hepatic encephalopathy. Neuroscience 410:1–15. https://doi.org/10.1016/j.neuroscience.2019.04.056
Raju A, Jaisankar P, Borah A, Mohanakumar KP (2017) 1-Methyl-4-phenylpyridinium-induced death of differentiated SH-SY5Y neurons is potentiated by cholesterol. Ann Neurosci 24:243–251. https://doi.org/10.1159/000481551
Kölsch H, Lütjohann D, Tulke A et al (1999) The neurotoxic effect of 24-hydroxycholesterol on SH-SY5Y human neuroblastoma cells. Brain Res 818:171–175. https://doi.org/10.1016/s0006-8993(98)01274-8
Yamanaka K, Saito Y, Yamamori T et al (2011) 24(S)-hydroxycholesterol induces neuronal cell death through necroptosis, a form of programmed necrosis. J Biol Chem 286:24666–24673. https://doi.org/10.1074/jbc.M111.236273
Yamanaka K, Urano Y, Takabe W et al (2014) Induction of apoptosis and necroptosis by 24(S)-hydroxycholesterol is dependent on activity of acyl-CoA:cholesterol acyltransferase 1. Cell Death Dis 5:e990. https://doi.org/10.1038/cddis.2013.524
Cheng D, Kim WS, Garner B (2008) Regulation of alpha-synuclein expression by liver X receptor ligands in vitro. Neuroreport 19:1685–1689. https://doi.org/10.1097/WNR.0b013e32831578b2
Schommer J, Marwarha G, Schommer T et al (2018) 27-Hydroxycholesterol increases α-synuclein protein levels through proteasomal inhibition in human dopaminergic neurons. BMC Neurosci 19(1):17. https://doi.org/10.1186/s12868-018-0420-5
Marwarha G, Rhen T, Schommer T, Ghribi O (2008) The oxysterol 27-hydroxycholesterol regulates α-synuclein and tyrosine hydroxylase expression levels in human neuroblastoma cells through modulation of liver X receptors and estrogen receptors-relevance to Parkinson’s disease. J Neurochem 107:1722–1729. https://doi.org/10.1111/j.1471-4159.2008.05736.x
Bosco DA, Fowler DM, Zhang Q et al (2006) Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate alpha-synuclein fibrilization. Nat Chem Biol 2:249–253. https://doi.org/10.1038/nchembio782
Bieschke J, Zhang Q, Bosco DA et al (2006) Small molecule oxidation products trigger disease-associated protein misfolding. Acc Chem Res 39:611–619. https://doi.org/10.1021/ar0500766
Yan J, Sun J, Huang L et al (2014) Simvastatin prevents neuroinflammation by inhibiting N-methyl-D-aspartic acid receptor 1 in 6-hydroxydopamine-treated PC12 cells. J Neurosci Res 92:634–640. https://doi.org/10.1002/jnr.23329
Yan J, Xu Y, Zhu C et al (2011) Simvastatin prevents dopaminergic neurodegeneration in experimental parkinsonian models: the association with anti-inflammatory responses. PLoS One 6:6. https://doi.org/10.1371/journal.pone.0020945
Xu Y, Long L, Yan J et al (2012) Simvastatin induces neuroprotection in 6-OHDA-lesioned PC12 via the PI3K/AKT/caspase 3 pathway and anti-inflammatory responses. CNS Neurosci Ther 19:170–177. https://doi.org/10.1111/cns.12053
Selley ML (2005) Simvastatin prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced striatal dopamine depletion and protein tyrosine nitration in mice. Brain Res 1037:1–6. https://doi.org/10.1016/j.brainres.2004.02.083
Kumar A, Sharma N, Gupta A et al (2012) Neuroprotective potential of atorvastatin and simvastatin (HMG-CoA reductase inhibitors) against 6-hydroxydopamine (6-OHDA) induced Parkinson-like symptoms. Brain Res 1471:13–22. https://doi.org/10.1016/j.brainres.2012.06.050
Yan J-Q, Ma Y-J, Sun J-C et al (2014) Neuroprotective effect of lovastatin by inhibiting NMDA receptor1 in 6-hydroxydopamine treated PC12 cells. Int J Clin Exp Med 7:3313–3319
Koob AO, Ubhi K, Paulsson JF et al (2010) Lovastatin ameliorates α-synuclein accumulation and oxidation in transgenic mouse models of α-synucleinopathies. Exp Neurol 221:267–274. https://doi.org/10.1016/j.expneurol.2009.11.015
Lee Y-C, Lin C-H, Wu R-M et al (2013) Discontinuation of statin therapy associates with Parkinson disease: a population-based study. Neurology 81:410–416. https://doi.org/10.1212/WNL.0b013e31829d873c
Huang X, Alonso A, Guo X et al (2015) Statins, plasma cholesterol and risk of Parkinson’s disease: a prospective study. Mov Disord Off J Mov Disord Soc 30:552–559. https://doi.org/10.1002/mds.26152
Liu G, Sterling NW, Kong L et al (2017) Statins may facilitate Parkinson’s disease: insight gained from a large, national claims database. Mov Disord Off J Mov Disord Soc 32:913–917. https://doi.org/10.1002/mds.27006
Rozani V, Giladi N, El-Ad B et al (2017) Statin adherence and the risk of Parkinson’s disease: a population-based cohort study. PLoS One 12:e0175054. https://doi.org/10.1371/journal.pone.0175054
Yan J, Qiao L, Tian J et al (2019) Effect of statins on Parkinson’s disease: a systematic review and meta-analysis. Medicine (Baltimore) 98:e14852. https://doi.org/10.1097/MD.0000000000014852
Bai S, Song Y, Huang X et al (2016) Statin use and the risk of Parkinson’s disease: an updated meta-analysis. PLoS One 11:3. https://doi.org/10.1371/journal.pone.0152564
Sheng Z, Jia X, Kang M (2016) Statin use and risk of Parkinson’s disease: a meta-analysis. Behav Brain Res 309:29–34. https://doi.org/10.1016/j.bbr.2016.04.046
Sierra S, Ramos MC, Molina P et al (2011) Statins as neuroprotectants: a comparative in vitro study of lipophilicity, blood-brain-barrier penetration, lowering of brain cholesterol, and decrease of neuron cell death. J Alzheimers Dis JAD 23:307–318. https://doi.org/10.3233/JAD-2010-101179
van der Most PJ, Dolga AM, Nijholt IM et al (2009) Statins: mechanisms of neuroprotection. Prog Neurobiol 88:64–75. https://doi.org/10.1016/j.pneurobio.2009.02.002
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81822016 and 81771382) and the Natural Science Foundation of Hubei Province (No. 2018CFA036) to Z. Zhang.
Author information
Authors and Affiliations
Contributions
LJD conceived and drafted the manuscript. LZ helped to draw the schematics. The final manuscript was read and approved by all authors.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dai, L., Zou, L., Meng, L. et al. Cholesterol Metabolism in Neurodegenerative Diseases: Molecular Mechanisms and Therapeutic Targets. Mol Neurobiol 58, 2183–2201 (2021). https://doi.org/10.1007/s12035-020-02232-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02232-6