Abstract
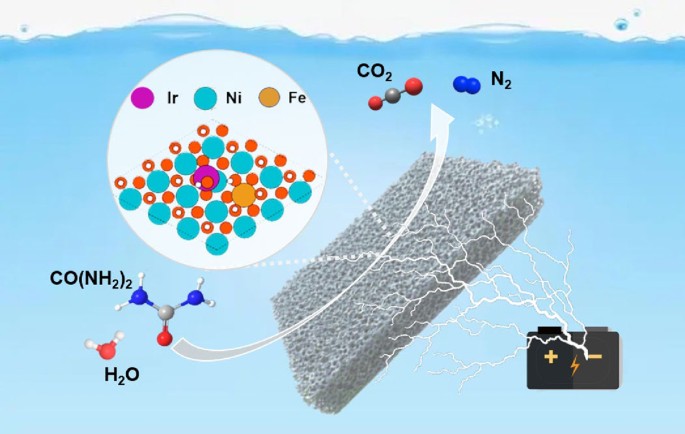
Single-atom catalysts (SACs) with high catalytic activity as well as great stability are demonstrating great promotion in electrocatalytic energy conversion, which is also a big challenge to achieve. Herein, we proposed a facile synthetic strategy to construct nickel-iron bimetallic hydroxide nanoribbon stabilized single-atom iridium catalysts (Ir-NiFe-OH), where the nickel-iron hydroxide nanoribbon not only can serve as good electronic conductor, but also can well stabilize and fully expose single-atom sites. Adopted as catalyst for urea oxidation reaction (UOR), it exhibited excellent UOR performance that it only needed a low operated potential of 1.38 V to achieve the current density of 100 mA·cm−2. In-situ Fourier transform infrared spectroscopy, X-ray absorption spectrum, and density functional theory calculations proved that Ir species are active centers and the existence of both Ni and Fe in the local structure of Ir atom can optimize the d-band center of Ir species, promoting the adsorption of intermediates and desorption of products for UOR. The hydrogen evolution reaction (HER)/UOR electrocatalytic cell demanded voltages of 1.46 and 1.50 V to achieve 50 and 100 mA·cm−2, respectively, which demonstrated a higher activity and better stability than those of conventional catalysts. This work opens a new avenue to develop catalysts for UORs with boosted activity and stability.
Similar content being viewed by others
References
Geng, S. K.; Zheng, Y.; Li, S. Q.; Su, H.; Zhao, X.; Hu, J.; Shu, H. B.; Jaroniec, M.; Chen, P.; Liu, Q. H. et al. Nickel ferrocyanide as a high-performance urea oxidation electrocatalyst. Nat. Energy 2021, 6, 904–912.
Zheng, M.; Wang, J. Regulating the oxygen affinity of single atom catalysts by dual-atom design for enhanced oxygen reduction reaction activity. Chem. Res. Chin. Univ. 2022, 38, 1275–1281.
Gao, Y.; Liu, B. Z.; Wang, D. S. Microenvironment engineering of single/dual-atom catalysts for electrocatalytic application. Adv. Mater. 2023, 35, 2209654.
Wang, T. H.; Fu, X. Z.; Wang, S. Y. Etching oxide overlayers of NiFe phosphide to facilitate surface reconstruction for oxygen evolution reaction. Green Energy Environ. 2022, 7, 365–371.
Han, A. L.; Sun, W. M.; Wan, X.; Cai, D. D.; Wang, X. J.; Li, F.; Shui, J. L.; Wang, D. S. Construction of Co4 atomic clusters to enable Fe−N4 motifs with highly active and durable oxygen reduction performance. Angew. Chem. 2023, 135, e202303185
Qi, Y. X.; Li, T. T.; Hu, Y. J.; Xiang, J. H.; Shao, W. Q.; Chen, W. H.; Mu, X. Q.; Liu, S. L.; Chen, C. Y.; Yu, M. et al. Single-atom Fe embedded Co3S4 for efficient electrocatalytic oxygen evolution reaction. Chem. Res. Chin. Univ. 2022, 38, 1282–1286
Zheng, X. B.; Yang, J. R.; Li, P.; Jiang, Z. L.; Zhu, P.; Wang, Q. S.; Wu, J. B.; Zhang, E. H.; Sun, W. P.; Dou, S. X. et al. Dual-atom support boosts nickel-catalyzed urea electrooxidation. Angew. Chem., Int. Ed. 2023, 62, e202217449.
Du, X. Q.; Ding, Y. Y.; Zhang, X. S. MOF-derived Zn−Co−Ni sulfides with hollow nanosword arrays for high-efficiency overall water and urea electrolysis. Green Energy Environ. 2023, 8, 798–811.
Chen, N.; Du, Y. X.; Zhang, G.; Lu, W. T.; Cao, F. F. Amorphous nickel sulfoselenide for efficient electrochemical urea-assisted hydrogen production in alkaline media. Nano Energy 2021, 81, 105605.
Kumar, A.; Liu, X. H.; Lee, J.; Debnath, B.; Jadhav, A. R.; Shao, X. D.; Bui, V. Q.; Hwang, Y.; Liu, Y.; Kim, M. G. et al. Discovering ultrahigh loading of single-metal-atoms via surface tensile-strain for unprecedented urea electrolysis. Energy Environ. Sci. 2021, 14, 6494–6505.
Yang, L. L.; He, R.; Wang, X.; Yang, T. T.; Zhang, T.; Zuo, Y.; Lu, X.; Liang, Z. F.; Li, J. S.; Arbiol, J. et al. Self-supported NiO/CuO electrodes to boost urea oxidation in direct urea fuel cells. Nano Energy 2023, 115, 108714.
Sayed, E. T.; Eisa, T.; Mohamed, H. O.; Abdelkareem, M. A.; Allagui, A.; Alawadhi, H.; Chae, K. J. Direct urea fuel cells: Challenges and opportunities. J. Power Sources 2019, 417, 159–175.
Li, W. H.; Yang, J. R.; Wang, D. S. Long-range interactions in diatomic catalysts boosting electrocatalysis. Angew. Chem., Int. Ed. 2022, 61, e202213318.
Gan, T.; Wang, D. S. Atomically dispersed materials: Ideal catalysts in atomic era. Nano Res., in press, DOI: https://doi.org/10.1007/s12274-023-5700-4.
Wang, L. G.; Liu, H.; Zhuang, J. H.; Wang, D. S. Small-scale big science: From nano- to atomically dispersed catalytic materials. Small Sci. 2022, 2, 2200036.
Wang, Q. S.; Zheng, X. B.; Wu, J. B.; Wang, Y.; Wang, D. S.; Li, Y. D. Recent progress in thermal conversion of CO2 via single-atom site catalysis. Small Struct. 2022, 3, 2200059.
Li, R. Z.; Zhao, J.; Liu, B. Z.; Wang, D. S. Atomic distance engineering in metal catalysts to regulate catalytic performance. Adv. Mater., in press, DOI: https://doi.org/10.1002/adma.202308653.
Yang, J. R.; Li, W. H.; Xu, K. N.; Tan, S. D.; Wang, D. S.; Li, Y. D. Regulating the tip effect on single-atom and cluster catalysts: Forming reversible oxygen species with high efficiency in chlorine evolution reaction. Angew. Chem., Int. Ed. 2022, 61, e202200366.
Shen, J.; Wang, D. S. How to select heterogeneous CO2 reduction electrocatalyst. Nano Res. Energy 2024, 3, e9120096.
Zhu, P.; Xiong, X.; Wang, D. S. Regulations of active moiety in single atom catalysts for electrochemical hydrogen evolution reaction. Nano Res. 2022, 15, 5792–5815.
Li, R. Z.; Wang, D. S. Understanding the structure-performance relationship of active sites at atomic scale. Nano Res. 2022, 15, 6888–6923.
Hunter, B. M.; Hiernger, W.; Winkler, J. R.; Gray, H. B.; Müller, A. M. Effect of interlayer anions on [NiFe]−LDH nanosheet water oxidation activity. Energy Environ. Sci. 2016, 9, 1734–1743.
Yin, H. J.; Tang, Z. Y. Ultrathin two-dimensional layered metal hydroxides: An emerging platform for advanced catalysis, energy conversion and storage. Chem. Soc. Rev. 2016, 45, 4873–4891.
Fu, Y. Y.; Sheng, Q. L.; Zheng, J. B. Au nanoparticles anchored on Ni(OH)2 nanowires with multiple cavities for selective electrochemical detection of dopamine. Anal. Methods 2017, 9, 2812–2820.
Dong, X. L.; Guo, Z. Y.; Song, Y. F.; Hou, M. Y.; Wang, J. Q.; Wang, Y. G.; Xia, Y. Y. Flexible and wire-shaped microsupercapacitor based on Ni(OH)2-nanowire and ordered mesoporous carbon electrodes. Adv. Funct. Mater. 2014, 24, 3405–3412.
Chen, J. T.; Ci, S. Q.; Wang, G. X.; Senthilkumar, N.; Zhang, M. T.; Xu, Q. H.; Wen, Z. H. Ni(OH)2 nanosheet electrocatalyst toward alkaline urea electrolysis for energy-saving acidic hydrogen production. ChemElectroChem 2019, 6, 5313–5320.
Wang, M.; Wang, J. Q.; Xi, C.; Cheng, C. Q.; Kuai, C. G.; Zheng, X. L.; Zhang, R.; Xie, Y. M.; Dong, C. K.; Chen, Y. J. et al. Valence-state effect of iridium dopant in NiFe(OH)2 catalyst for hydrogen evolution reaction. Small 2021, 17, 2100203.
Wang, L. P.; Zhu, Y. J.; Wen, Y. Z.; Li, S. Y.; Cui, C. Y.; Ni, F. L.; Liu, Y. X.; Lin, H. P.; Li, Y. Y.; Peng, H. S. et al. Regulating the local charge distribution of Ni active sites for the urea oxidation reaction. Angew. Chem., Int. Ed. 2021, 60, 10577–10582.
Jiang, H.; Sun, M. Z.; Wu, S. L.; Huang, B. L.; Lee, C. S.; Zhang, W. J. Oxygen-incorporated NiMoP nanotube arrays as efficient bifunctional electrocatalysts for urea-assisted energy-saving hydrogen production in alkaline electrolyte. Adv. Funct. Mater. 2021, 31, 2104951.
Zhang, L. S.; Wang, L. P.; Lin, H. P.; Liu, Y. X.; Ye, J. Y.; Wen, Y. Z.; Chen, A.; Wang, L.; Ni, F. L.; Zhou, Z. Y. et al. A lattice-oxygen-involved reaction pathway to boost urea oxidation. Angew. Chem., Int. Ed. 2019, 58, 16820–16825.
Singh, R. K.; Schechter, A. Electrochemical investigation of urea oxidation reaction on β Ni(OH)2 and Ni/Ni(OH)2. Electrochim. Acta 2018, 278, 405–411.
Wang, Q. L.; Xu, C. Q.; Liu, W.; Hung, S. F.; Yang, H. B.; Gao, J. J.; Cai, W. Z.; Chen, H. M.; Li, J.; Liu, B. Coordination engineering of iridium nanocluster bifunctional electrocatalyst for highly efficient and pH-universal overall water splitting. Nat. Commun. 2020, 11, 4246.
Zhang, K.; Liu, C. L.; Graham, N.; Zhang, G.; Yu, W. Z. Modulation of dual centers on cobalt-molybdenum oxides featuring synergistic effect of intermediate activation and radical mediator for electrocatalytic urea splitting. Nano Energy 2021, 87, 106217.
Zhang, Q. Z.; Bao, N.; Wang, X. Q.; Hu, X. D.; Miao, X. H.; Chaker, M.; Ma, D. L. Advanced fabrication of chemically bonded graphene/TiO2 continuous fibers with enhanced broadband photocatalytic properties and involved mechanisms exploration. Sci. Rep. 2016, 6, 38066.
Zhu, X. J.; Dou, X. Y.; Dai, J.; An, X. D.; Guo, Y. Q.; Zhang, L. D.; Tao, S.; Zhao, J. Y.; Chu, W. S.; Zeng, X. C. et al. Metallic nickel hydroxide nanosheets give superior electrocatalytic oxidation of urea for fuel cells. Angew. Chem., Int. Ed. 2016, 55, 12465–12469.
Acknowledgments
We acknowledge financial support from the National Natural Science Foundation of China (Nos. 51932001, 51872024, 52022097, and 22293043), the National Key Research and Development Program of China (No. 2018YFA0703503), the Foundation of the Youth Innovation Promotion Association of Chinese Academy of Sciences (No. 2020048). We thank the BL1W1B in BSRF, BL14W1 and BL11B in SSRF for XAS measurement.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2023_6388_MOESM1_ESM.pdf
Nickel-iron in the second coordination shell boost single-atomic-site iridium catalysts for high-performance urea electrooxidation
Rights and permissions
About this article
Cite this article
Chen, X., Wan, J., Chai, J. et al. Nickel-iron in the second coordination shell boost single-atomic-site iridium catalysts for high-performance urea electrooxidation. Nano Res. 17, 3919–3926 (2024). https://doi.org/10.1007/s12274-023-6388-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-6388-1