Abstract
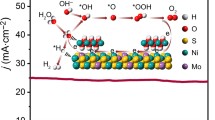
Designing high-performance electrocatalysts toward hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) is essential to reduce the activation barrier and optimize free adsorption energy of reactive intermediates. Herein, we report that incorporating high-valence Cr into NiSe2 (CrxNi1−xSe2) kinetically and thermodynamically expedites elementary steps of both HER and OER. The as-prepared Cr0.05Ni0.95Se2 catalyst displays excellent HER and OER activities, with low overpotentials of 89 and 272 mV at the current density of 10 mA·cm−2 (j10), respectively, and remains stable during operation for 30 h. A low cell voltage of only 1.59 V is required to drive j10 in alkaline media. In situ Raman spectroscopy reveals that Cr incorporation facilitates the formation of NiOOH active species during the OER process. Meanwhile, theoretical explorations demonstrate that high-valence Cr incorporation efficiently accelerates water dissociation kinetics and improves H* adsorption during HER process, lowering the activation barrier of OER and optimizing the adsorption energy of oxygen-based intermediate, thus kinetically and thermodynamically enhancing the intrinsic performance of NiSe2 for over water splitting. This strategy provides a new horizon to design transition metal based electrocatalysts in the clean energy field.

Similar content being viewed by others
References
Li, Y. J.; Sun, Y. J.; Qin, Y. N.; Zhang, W. Y.; Wang, L.; Luo, M. C.; Yang, H.; Guo, S. J. Recent advances on water-splitting electrocatalysis mediated by noble-metal-based nanostructured materials. Adv. Energy Mater. 2020, 10, 1903120.
Guo, L. L.; Yu, Q. P.; Zhai, X. J.; Chi, J. Q.; Cui, T.; Zhang, Y.; Lai, J. P.; Wang, L. Reduction-induced interface reconstruction to fabricate MoNi4-based hollow nanorods for hydrazine oxidation assisted energy-saving hydrogen production in seawater. Nano Res. 2022, 15, 8846–8856.
Wang, Q. Q.; Li, J. Q.; Li, Y. J.; Shao, G. M.; Jia, Z.; Shen, B. L. Non-noble metal-based amorphous high-entropy oxides as efficient and reliable electrocatalysts for oxygen evolution reaction. Nano Res. 2022, 15, 8751–8759.
Wu, L. B.; Zhang, F. H.; Song, S. W.; Ning, M. H.; Zhu, Q.; Zhou, J. Q.; Gao, G. H.; Chen, Z. Y.; Zhou, Q. C.; Xing, X. X. et al. Efficient alkaline water/seawater hydrogen evolution by a nanorod-nanoparticle-structured Ni-MoN catalyst with fast water-dissociation kinetics. Adv. Mater. 2022, 34, 2201774.
Xu, W. J.; Chang, J. F.; Cheng, Y. G.; Liu, H. Q.; Li, J. F.; Ai, Y. J.; Hu, Z. N.; Zhang, X. Y.; Wang, Y. M.; Liang, Q. L. et al. A multi-step induced strategy to fabricate core–shell Pt-Ni alloy as symmetric electrocatalysts for overall water splitting. Nano Res. 2022, 15, 965–971.
Wu, J. D.; Fan, J. C.; Zhao, X.; Wang, Y.; Wang, D. W.; Liu, H. T.; Gu, L.; Zhang, Q. H.; Zheng, L. R.; Singh, D. J. et al. Atomically dispersed MoOx on rhodium metallene boosts electrocatalyzed alkaline hydrogen evolution. Angew. Chem., Int. Ed. 2022, 61, e202207512.
Yang, Q. F.; Zhu, B. T.; Wang, F.; Zhang, C. J.; Cai, J. H.; Jin, P.; Feng, L. Ru/NC heterointerfaces boost energy-efficient production of green H2 over a wide pH range. Nano Res. 2022, 15, 5134–5142.
Xu, Y. C.; Wei, S. T.; Gan, L. F.; Zhang, L.; Wang, F.; Wu, Q.; Cui, X. Q.; Zheng, W. T. Amorphous carbon interconnected ultrafine CoMnP with enhanced Co electron delocalization yields Pt-like activity for alkaline water electrolysis. Adv. Funct. Mater. 2022, 32, 2112623.
Fan, H. F.; Jiao, D. X.; Fan, J. C.; Wang, D. W.; Zaman, B.; Zhang, W.; Zhang, L.; Zheng, W. T.; Cui, X. Q. Enhancing water-dissociation kinetics and optimizing intermediates adsorption free energy of cobalt phosphide via high-valence Zr incorporating for alkaline water electrolysis. J. Energy Chem. 2023, 83, 119–127.
Zheng, Y. Y.; Tian, Y. R.; Sarwar, S.; Luo, J. J.; Zhang, X. Y. Carbon nanotubes decorated NiSe2 nanosheets for high-performance supercapacitors. J. Power Sources 2020, 452, 227793.
Zheng, X. Y.; Sun, S. C.; Liu, Y.; Li, D.; Tian, D.; Zhu, J. J.; Jiang, D. L. Synergistic modulation of NiSe2 by doping with chromium and nitrogen for high-efficiency overall water splitting. Appl. Surf. Sci. 2023, 609, 155406.
Sun, Y. Q.; Xu, K.; Wei, Z. X.; Li, H. L.; Zhang, T.; Li, X. Y.; Cai, W. P.; Ma, J. M.; Fan, H. J.; Li, Y. Strong electronic interaction in dual-cation-incorporated NiSe2 nanosheets with lattice distortion for highly efficient overall water splitting. Adv. Mater. 2018, 30, 1802121.
Wang, T. T.; Gao, D. Q.; Xiao, W.; Xi, P. X.; Xue, D. S.; Wang, J. Transition-metal-doped NiSe2 nanosheets towards efficient hydrogen evolution reactions. Nano Res. 2018, 11, 6051–6061.
Zhou, J.; Yuan, L. W.; Wang, J. W.; Song, L. L.; You, Y.; Zhou, R.; Zhang, J. J.; Xu, J. Combinational modulations of NiSe2 nanodendrites by phase engineering and iron-doping towards an efficient oxygen evolution reaction. J. Mater. Chem. A 2020, 8, 8113–8120.
Ni, S.; Qu, H. N.; Xu, Z. H.; Zhu, X. Y.; Xing, H. F.; Wang, L.; Yu, J. M.; Liu, H. Z.; Chen, C. M.; Yang, L. R. Interfacial engineering of the NiSe2/FeSe2 p–p heterojunction for promoting oxygen evolution reaction and electrocatalytic urea oxidation. Appl. Catal. B: Environ. 2021, 299, 120638.
Gu, C.; Hu, S. J.; Zheng, X. S.; Gao, M. R.; Zheng, Y. R.; Shi, L.; Gao, Q.; Zheng, X.; Chu, W. S.; Yao, H. B. et al. Synthesis of sub-2 nm iron-doped NiSe2 nanowires and their surface-confined oxidation for oxygen evolution catalysis. Angew. Chem. 2018, 130, 4084–4088.
Xu, W. C.; Fan, G. L.; Zhu, S. L.; Liang, Y. Q.; Cui, Z. D.; Li, Z. Y.; Jiang, H.; Wu, S. L.; Cheng, F. Y. Electronic structure modulation of nanoporous cobalt phosphide by carbon doping for alkaline hydrogen evolution reaction. Adv. Funct. Mater. 2021, 31, 2107333.
Ma, Y. F.; Chen, M.; Geng, H. B.; Dong, H. F.; Wu, P.; Li, X. M.; Guan, G. Q.; Wang, T. J. Synergistically tuning electronic structure of porous β-Mo2C spheres by Co doping and Mo-vacancies defect engineering for optimizing hydrogen evolution reaction activity. Adv. Funct. Mater. 2020, 30, 2000561.
Fan, H. F.; Jia, J. J.; Wang, D. W.; Fan, J. C.; Wu, J. D.; Zhao, J. X.; Cui, X. Q. High-valence Zr-incorporated nickel phosphide boosting reaction kinetics for highly efficient and robust overall water splitting. Chem. Eng. J. 2023, 455, 140908.
Zhou, Y. N.; Hu, W. H.; Zhen, Y. N.; Dong, B.; Dong, Y. W.; Fan, R. Y.; Liu, B.; Liu, D. P.; Chai, Y. M. Metallic MoOx layrr promoting high-valence Mo doping into CoP nanowires with ultrahigh activity for hydrogen evolution at 2000 mA·cm−2. Appl. Catal. B: Environ. 2022, 309, 121230.
Li, M. Y.; Cai, B. H.; Tian, R. M.; Yu, X. J.; Breese, M. B. H.; Chu, X. Z.; Han, Z. J.; Li, S. A.; Joshi, R.; Vinu, A. et al. Vanadium doped 1T MoS2 nanosheets for highly efficient electrocatalytic hydrogen evolution in both acidic and alkaline solutions. Chem. Eng. J. 2021, 409, 128158.
Wang, M. H.; Lou, Z. X.; Wu, X. F.; Liu, Y. W.; Zhao, J. Y.; Sun, K. Z.; Li, W. X.; Chen, J. C.; Yuan, H. Y.; Zhu, M. H. et al. Operando high-valence Cr-modified NiFe hydroxides for water oxidation. Small 2022, 18, 2200303.
Zhang, L. P.; Zhang, J. T.; Fang, J. J.; Wang, X. Y.; Yin, L. K.; Zhu, W.; Zhuang, Z. B. Cr-doped CoP nanorod arrays as high-performance hydrogen evolution reaction catalysts at high current density. Small 2021, 17, 2100832.
Li, W. F.; Jiang, Y.; Li, Y. R.; Gao, Q.; Shen, W.; Jiang, Y. M.; He, R. X.; Li, M. Electronic modulation of CoP nanoarrays by Cr-doping for efficient overall water splitting. Chem. Eng. J. 2021, 425, 130651.
Shi, Y. M.; Du, W.; Zhou, W.; Wang, C. H.; Lu, S. S.; Lu, S. Y.; Zhang, B. Unveiling the promotion of surface-adsorbed chalcogenate on the electrocatalytic oxygen evolution reaction. Angew. Chem., Int. Ed. 2020, 59, 22470–22474.
Zhong, H. Y.; Wang, X. P.; Sun, G. X.; Tang, Y. X.; Tan, S. D.; He, Q.; Zhang, J.; Xiong, T.; Diao, C. Z.; Yu, Z. G. et al. Optimization of oxygen evolution activity by tuning \({{\rm{e}}^ \star }_{\rm{g}}\) band broadening in nickel oxyhydroxide. Energy Environ. Sci. 2023, 16, 641–652.
Zhang, X. R.; Sun, C. Y.; Xu, S. S.; Huang, M. R.; Wen, Y.; Shi, X. R. DFT-assisted rational design of CoMx/CC (M = Fe, Mn, and Ni) as efficient electrocatalyst for wide pH range hydrogen evolution and oxygen evolution. Nano Res. 2022, 15, 8897–8907.
Vydrov, O. A.; Heyd, J.; Krukau, A. V.; Scuseria, G. E. Importance of short-range versus long-range Hartree–Fock exchange for the performance of hybrid density functionals. J. Chem. Phys. 2006, 125, 074106.
Zhou, Y. N.; Chen, L. L.; Sheng, L.; Luo, Q. Q.; Zhang, W. H.; Yang, J. L. Dual-metal atoms embedded into two-dimensional covalent organic framework as efficient electrocatalysts for oxygen evolution reaction: A DFT study. Nano Res. 2022, 15, 7994–8000.
Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799.
Zhou, Y. N.; Liu, H. J.; Shi, Z. N.; Zhou, J. C.; Dong, B.; Zhao, H. Y.; Wang, F. G.; Yu, J. F.; Chai, Y. M. Microwave-assisted molybdenum-nickel alloy for efficient water electrolysis under large current density through spillover and Fe doping. Nano Res. 2022, 15, 5873–5883.
Wang, Y.; Li, X. P.; Zhang, M. M.; Zhang, J. F.; Chen, Z. L.; Zheng, X. R.; Tian, Z. L.; Zhao, N. Q.; Han, X. P.; Zaghib, K. et al. Highly active and durable single-atom tungsten-doped NiS0.5Se0.5 nanosheet@NiS0.5Se0.5 nanorod heterostructures for water splitting. Adv. Mater. 2022, 34, 2107053.
Kim, J.; Jung, H.; Jung, S. M.; Hwang, J.; Kim, D. Y.; Lee, N.; Kim, K. S.; Kwon, H.; Kim, Y. T.; Han, J. W. et al. Tailoring binding abilities by incorporating oxophilic transition metals on 3D nanostructured Ni arrays for accelerated alkaline hydrogen evolution reaction. J. Am. Chem. Soc. 2021, 143, 1399–1408.
Chu, Y. C.; Chang, C. J.; Zhu, Y. P.; Lin, S. C.; Tung, C. W.; Chen, T. L.; Chen, H. M. Anionic effects on metal pair of Se-doped nickel diphosphide for hydrogen evolution reaction. ACS Sustain. Chem. Eng. 2019, 7, 14247–14255.
Chen, Z. S.; Wei, D. L.; Li, Q.; Wang, X. X.; Yu, S. J.; Liu, L.; Liu, B.; Xie, S. Y.; Wang, J.; Chen, D. Y. et al. Macroscopic and microscopic investigation of Cr(VI) immobilization by nanoscaled zero-valent iron supported zeolite MCM-41 via batch, visual, XPS and EXAFS techniques. J. Cleaner Prod. 2018, 181, 745–752.
Yang, S. Y.; Shi, D. R.; Wang, T.; Yue, X. Y.; Zheng, L.; Zhang, Q. H.; Gu, L.; Yang, X. Q.; Shadike, Z.; Li, H. et al. High-rate cathode CrSSe based on anion reactions for lithium-ion batteries. J. Mater. Chem. A 2020, 8, 25739–25745.
Sikora, M.; Kapusta, C.; Maksymowicz, L.; Lubecka, M.; Cięciwa, B.; Szymczak, R.; Welter, E.; Borowiec, M.; Zając, D. EXAFS study of indium doped magnetic semiconductor CdCr2Se4. J. Alloys Compd. 2004, 362, 151–155.
Li, G.; Feng, S. Y.; Wang, C. T.; Deng, P. L.; Li, J. Co-NiSe2/NF nanosheet for efficient hydrogen evolution reaction. Catal. Commun. 2022, 165, 106443.
Zhang, C. Q.; Zhang, Y.; Zhou, S.; Li, C. X. Self-supported iron-doping NiSe2 nanowrinkles as bifunctional electrocatalysts for electrochemical water splitting. J. Alloys Compd. 2020, 818, 152833.
Gao, J. Y.; Ma, H. Q.; Luo, X. Y.; Yu, L. Q.; Gu, X. P.; Liu, J. H. Boosting electrocatalytic activity of NiSe2 nanosheets by anion–cation dual-doping for highly efficient hydrogen evolution reaction. J. Alloys Compd. 2023, 933, 167793.
Xu, H. K.; Lu, K. B.; Jiang, C. H.; Wei, X. F.; Wang, Z. F.; Ouyang, Y. G.; Dai, F. N. Electronic regulation of a core-shell NiSe2 catalyst by Co doping to accelerate hydrogen evolution. CrystEngComm 2022, 24, 8005–8010.
Dang, Y. C.; Wang, G. Q.; Li, X.; Ma, X.; Yue, F.; Wang, C. T.; Gao, L. J.; Fu, F. Enhanced alkaline/seawater hydrogen evolution reaction performance of NiSe2 by ruthenium and tungsten bimetal doping. Int. J. Hydrogen Energy 2023, 48, 17035–17044.
Teng, X.; Guo, L. X.; Ji, L. L.; Wang, J. Y.; Niu, Y. L.; Hu, Z. B.; Chen, Z. F. Self-growing NiFe-based hybrid nanosheet arrays on Ni nanowires for overall water splitting. ACS Appl. Energy Mater. 2019, 2, 5465–5471.
Hu, X. B.; Zhou, Q. W.; Cheng, P. F.; Su, S. Q.; Wang, X.; Gao, X. S.; Zhou, G. F.; Zhang, Z.; Liu, J. M. Nickel-iron selenide polyhedral nanocrystal with optimized surface morphology as a high-performance bifunctional electrocatalyst for overall water splitting. Appl. Surf. Sci. 2019, 488, 326–334.
Chen, L. L.; Jang, H.; Kim, M. G.; Qin, Q.; Liu, X. E.; Cho, J. Fe,Al-co-doped NiSe2 nanoparticles on reduced graphene oxide as an efficient bifunctional electrocatalyst for overall water splitting. Nanoscale 2020, 12, 13680–13687.
Li, Y. X.; Chen, R.; Yan, D. F.; Wang, S. Y. Regulation of morphology and electronic structure of NiSe2 by Fe for high effective oxygen evolution reaction. Chem. Asian J. 2020, 15, 3845–3852.
Chi, J. Q.; Shang, X.; Liang, F.; Dong, B.; Li, X.; Liu, Y. R.; Yan, K. L.; Gao, W. K.; Chai, Y. M.; Liu, C. G. Facile synthesis of pyrite-type binary nickel iron diselenides as efficient electrocatalyst for oxygen evolution reaction. Appl. Surf. Sci. 2017, 401, 17–24.
Zhang, C.; Li, T.; Wei, Q. Z.; Cheng, Z. H.; Wu, J.; Ma, X. X.; Chen, Z. H.; Liu, K. Y.; Zhang, T.; Liu, J. H. Fe-doped NiSe2 nanoparticles as efficient and stable electrocatalysts for oxygen evolution reaction. Chem. Phys. Lett. 2022, 808, 140126.
Zhuang, W. C.; Du, M. L.; Lu, X. H.; Chen, Z. Y.; Huang, Z. J.; Liu, D. S.; Cheng, W. J.; Tian, L. Fe doping modifying electronic structure of NiSe2 for boosting electrocatalytic oxygen evolution reaction. Ionics 2023, 29, 1069–1076.
Zhu, M.; Yan, Q.; Lu, Q.; Xue, Y. Q.; Yan, Y. D.; Yin, J. L.; Zhu, K.; Cheng, K.; Ye, K.; Yan, J. et al. Iron-doped NiSe2in-situ grown on graphene as an efficient electrocatalyst for oxygen evolution reaction. J. Electroanal. Chem. 2020, 866, 114134.
Lv, L.; Li, Z. S.; Ruan, Y. J.; Chang, Y. X.; Ao, X.; Li, J. G.; Yang, Z. X.; Wang, C. D. Nickel-iron diselenide hollow nanoparticles with strongly hydrophilic surface for enhanced oxygen evolution reaction activity. Electrochim. Acta 2018, 286, 172–178.
Zheng, X. R.; Han, X. P.; Cao, Y. H.; Zhang, Y.; Nordlund, D.; Wang, J. H.; Chou, S. L.; Liu, H.; Li, L. L.; Zhong, C. et al. Identifying dense NiSe2/CoSe2 heterointerfaces coupled with surface high-valence bimetallic sites for synergistically enhanced oxygen electrocatalysis. Adv. Mater. 2020, 32, 2000607.
Yang, S. J.; Qin, L. S.; Zhang, W.; Cao, R. The mechanism of water oxidation from Mn-based heterogeneous electrocatalysts. Chin. J. Struct. Chem. 2022, 41, 2204022–2204033.
Zhang, X. P.; Chandra, A.; Lee, Y. M.; Cao, R.; Ray, K.; Nam, W. Transition metal-mediated O–O bond formation and activation in chemistry and biology. Chem. Soc. Rev. 2021, 50, 4804–4811.
Zhang, X. P.; Wang, H. Y.; Zheng, H. Q.; Zhang, W.; Cao, R. O–O bond formation mechanisms during the oxygen evolution reaction over synthetic molecular catalysts. Chin. J. Catal. 2021, 42, 1253–1268.
Exner, K. S. Why the optimum thermodynamic free-energy landscape of the oxygen evolution reaction reveals an asymmetric shape? Mater. Today Energy 2021, 21, 100831.
Li, X. L.; Zhang, X. P.; Guo, M.; Lv, B.; Guo, K.; Jin, X. T.; Zhang, W.; Lee, Y. M.; Fukuzumi, S.; Nam, W. et al. Identifying intermediates in electrocatalytic water oxidation with a manganese corrole complex. J. Am. Chem. Soc. 2021, 143, 14613–14621.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 12034002, 22279044, and 22202080), Jilin Province Science and Technology Development Program (No. 20210301009GX), and the fellowship of China Postdoctoral Science Foundation (No. 2022M711296). The work was carried out at LvLiang Cloud Computing Center of China.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2023_5992_MOESM1_ESM.pdf
Kinetically and thermodynamically expediting elementary steps via high-valence Cr-incorporated of nickel selenide for water electrolysis
Rights and permissions
About this article
Cite this article
Fan, H., Jiao, D., Fan, J. et al. Kinetically and thermodynamically expediting elementary steps via high-valence Cr-incorporated of nickel selenide for water electrolysis. Nano Res. 17, 1199–1208 (2024). https://doi.org/10.1007/s12274-023-5992-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-5992-4