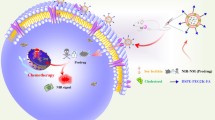
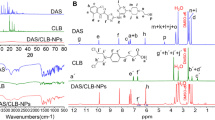
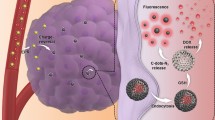
Abstract
Nano-drug delivery systems (nanoDDS) have been extensively investigated clinically to improve the therapeutic effect of anti-cancer drugs. However, the complicated synthesis during the preparation as well as the potential drug leakage during transportation has greatly limited their general application. In this work, a calixarene-integrated nanoDDS (CanD) that achieves tumor-targeted delivery and tracking of anti-cancer drugs in vivo is presented. The hypoxia-responsive calixarene (SAC4A) exhibits high binding affinity to a series of anti-cancer drugs and rhodamine B (RhB) under normoxic condition while decreasing the binding affinity under hypoxic condition, which leads to the drug release and fluorescence recovery simultaneously. Furthermore, the hypoxia-responsiveness of SAC4A conveys CanD with tumor-targeting ability, resulting in the enrichment of the drug in tumors and enhancement in tumor suppression in mice. Moreover, CanD could become a general platform allowing the delivery of a wide scope of anti-cancer drugs that have strong host-guest interaction with SAC4A.

Similar content being viewed by others
References
Ma, X.; Zhao, Y. L. Biomedical applications of supramolecular systems based on host-guest interactions. Chem. Rev. 2015, 115, 7794–7839.
Sun, T. M.; Zhang, Y. S.; Pang, B.; Hyun, D. C.; Yang, M. X.; Xia, Y. N. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem., Int. Ed. 2014, 53, 12320–12364.
Shi, J. J.; Votruba, A. R.; Farokhzad, O. C.; Langer, R. Nanotechnology in drug delivery and tissue engineering: From discovery to applications. Nano Lett. 2010, 10, 3223–3230.
Yang, Y.; Liu, J. J.; Sun, X. Q.; Feng, L. Z.; Zhu, W. W.; Liu, Z.; Chen, M. W. Near-infrared light-activated cancer cell targeting and drug delivery with aptamer-modified nanostructures. Nano Res. 2016, 9, 139–148.
Mei, L.; Zhu, G. Z.; Qiu, L. P.; Wu, C. C.; Chen, H. P.; Liang, H.; Cansiz, S.; Lv, Y. F.; Zhang, X. B.; Tan, W. H. Self-assembled multifunctional DNA nanoflowers for the circumvention of multidrug resistance in targeted anticancer drug delivery. Nano Res. 2015, 8, 3447–3460.
Bae, Y.; Fukushima, S.; Harada, A.; Kataoka, K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: Polymeric micelles that are responsive to intracellular pH change. Angew. Chem., Int. Ed. 2003, 42, 4640–4643.
Chen, F.; Li, Y.; Lin, X. J.; Qiu, H. Y.; Yin, S. C. Polymeric systems containing supramolecular coordination complexes for drug delivery. Polymers (Basel) 2021, 13, 370.
Qiu, L. P.; Chen, T.; Öçsoy, I.; Yasun, E.; Wu, C. C.; Zhu, G. Z.; You, M. X.; Han, D.; Jiang, J. H.; Yu, R. Q. et al. A cell-targeted, size-photocontrollable, nuclear-uptake nanodrug delivery system for drug-resistant cancer therapy. Nano Lett. 2015, 15, 457–463.
Schlich, M.; Longhena, F.; Faustini, G.; O’Driscoll, C. M.; Sinico, C.; Fadda, A. M.; Bellucci, A.; Lai, F. Anionic liposomes for small interfering ribonucleic acid (siRNA) delivery to primary neuronal cells: Evaluation of alpha-synuclein knockdown efficacy. Nano Res. 2017, 10, 3496–3508.
Sun, W. X.; Jiang, H. T.; Wu, X.; Xu, Z. Y.; Yao, C.; Wang, J.; Qin, M.; Jiang, Q.; Wang, W.; Shi, D. Q. et al. Strong dual-crosslinked hydrogels for ultrasound-triggered drug delivery. Nano Res. 2019, 12, 115–119.
Webber, M. J.; Langer, R. Drug delivery by supramolecular design. Chem. Soc. Rev. 2017, 46, 6600–6620.
Yu, G. C.; Chen, X. Y. Host-guest chemistry in supramolecular theranostics. Theranostics 2019, 9, 3041–3074.
Leroux, J. C. Editorial: Drug delivery: Too much complexity, not enough reproducibility. Angew. Chem., Int. Ed. 2017, 56, 15170–15171.
Wang, L.; Li, L. L.; Fan, Y. S.; Wang, H. Host-guest supramolecular nanosystems for cancer diagnostics and therapeutics. Adv. Mater. 2013, 25, 3888–3898.
Cao, S. P.; Pei, Z. C.; Xu, Y. Q.; Pei, Y. X. Glyco-nanovesicles with activatable near-infrared probes for real-time monitoring of drug release and targeted delivery. Chem. Mater. 2016, 28, 4501–4506.
Wu, X. M.; Sun, X. R.; Guo, Z. Q.; Tang, J. B.; Shen, Y. Q.; James, T. D.; Tian, H.; Zhu, W. H. In vivo and in situ tracking cancer chemotherapy by highly photostable NIR fluorescent theranostic prodrug. J. Am. Chem. Soc. 2014, 136, 3579–3588.
Yan, C. X.; Guo, Z. Q.; Liu, Y. J.; Shi, P.; Tian, H.; Zhu, W. H. A sequence-activated AND logic dual-channel fluorescent probe for tracking programmable drug release. Chem. Sci. 2018, 9, 6176–6182.
Zhang, Y. F.; Yin, Q.; Yen, J.; Li, J. Ying, H. Z.; Wang, H.; Hua, Y. Y.; Chaney, E. J.; Boppart, S. A.; Cheng, J. J. Non-invasive, real-time reporting drug release in vitro and in vivo. Chem. Commun. 2015, 51, 6948–6951.
Gao, D.; Xu, H.; Philbert, M. A.; Kopelman, R. Bioeliminable nanohydrogels for drug delivery. Nano Lett. 2008, 8, 3320–3324.
Cheng, J. J.; Khin, K. T.; Jensen, G. S.; Liu, A. J.; Davis, M. E. Synthesis of linear, β-cyclodextrin-based polymers and their camptothecin conjugates. Bioconjug. Chem. 2003, 14, 1007–1017.
Eliasof, S.; Lazarus, D.; Peters, C. G.; Case, R. I.; Cole, R. O.; Hwang, J.; Schluep, T.; Chao, J.; Lin, J.; Yen, Y. et al. Correlating preclinical animal studies and human clinical trials of a multifunctional, polymeric nanoparticle. Proc. Natl. Acad. Sci. USA 2013, 110, 15127–15132.
Schluep, T.; Hwang, J.; Hildebrandt, I. J.; Czernin, J.; Choi, C. H. J.; Alabi, C. A.; Mack, B. C.; Davis, M. E. Pharmacokinetics and tumor dynamics of the nanoparticle IT-101 from PET imaging and tumor histological measurements. Proc. Natl. Acad. Sci. USA 2009, 106, 11394–11399.
Guo, S. W.; Song, Y. S.; He, Y. L.; Hu, X. Y.; Wang, L. Y. Highly efficient artificial light-harvesting systems constructed in aqueous solution based on supramolecular self-assembly. Angew. Chem., Int. Ed. 2018, 57, 3163–3167.
Li, B.; Meng, Z.; Li, Q. Q.; Huang, X. Y.; Kang, Z. Y.; Dong, H. J.; Chen, J. Y.; Sun, J.; Dong, Y. S.; Li, J. et al. A pH responsive complexation-based drug delivery system for oxaliplatin. Chem. Sci. 2017, 8, 4458–4464.
Wu, X.; Li, Y.; Lin, C.; Hu, X. Y.; Wang, L. Y. GSH- and pH-responsive drug delivery system constructed by water-soluble pillar[5]arene and lysine derivative for controllable drug release. Chem. Commun. 2015, 51, 6832–6835.
Xu, Z.; Jia, S. R.; Wang, W.; Yuan, Z.; Jan Ravoo, B.; Guo, D. S. Heteromultivalent peptide recognition by co-assembly of cyclodextrin and calixarene amphiphiles enables inhibition of amyloid fibrillation. Nat. Chem. 2019, 11, 86–93.
Zheng, Z.; Yu, H. J.; Geng, W. C.; Hu, X. Y.; Wang, Y. Y.; Li, Z. H.; Wang, Y. F.; Guo, D. S. Guanidinocalix[5]arene for sensitive fluorescence detection and magnetic removal of perfluorinated pollutants. Nat. Commun. 2019, 10, 5762.
Chen, H.; Chen, Y. Y.; Wu, H.; Xu, J. F.; Sun, Z. W.; Zhang, X. Supramolecular polymeric chemotherapy based on cucurbit[7]uril-PEG copolymer. Biomaterials 2018, 178, 697–705.
Chen, Y. Y.; Huang, Z. H.; Zhao, H. Y.; Xu, J. F.; Sun, Z. W.; Zhang, X. Supramolecular chemotherapy: Cooperative enhancement of antitumor activity by combining controlled release of oxaliplatin and consuming of spermine by cucurbit[7]uril. Acs Appl. Mater. Interfaces 2017, 9, 8602–8608.
Sun, C.; Zhang, H. P.; Li, S. K.; Zhang, X. J.; Cheng, Q.; Ding, Y. F.; Wang, L. H.; Wang, R. B. Polymeric nanomedicine with “Lego” surface allowing modular functionalization and drug encapsulation. ACS Appl. Mater. Interfaces 2018, 10, 25090–25098.
Karim, A. A.; Dou, Q. Q.; Li, Z. B.; Loh, X. J. Emerging supramolecular therapeutic carriers based on host-guest interactions. Chem. Asian J. 2016, 11, 1300–1321.
Li, J.; Fang, Y.; Zhang, Y. F.; Wang, H. M.; Yang, Z. M.; Ding, D. Supramolecular self-assembly-facilitated aggregation of tumor-specific transmembrane receptors for signaling activation and converting immunologically cold to hot tumors. Adv. Mater. 2021, 33, 2008518.
Li, X. S.; Bai, H. T.; Yang, Y. C.; Yoon, J.; Wang, S.; Zhang, X. Supramolecular antibacterial materials for combatting antibiotic resistance. Adv. Mater. 2019, 31, 1805092.
Webber, M. J.; Appel, E. A.; Meijer, E. W.; Langer, R. Supramolecular biomaterials. Nat. Mater. 2016, 15, 13–26.
Chen, J. Y.; Zhang, Y. D.; Meng, Z.; Guo, L.; Yuan, X. Y.; Zhang, Y. H.; Chai, Y.; Sessler, J. L.; Meng, Q. B.; Li, C. J. Supramolecular combination chemotherapy: A pH-responsive co-encapsulation drug delivery system. Chem. Sci. 2020, 11, 6275–6282.
Hu, X. Y.; Gao, J.; Chen, F. Y.; Guo, D. S. A host—guest drug delivery nanosystem for supramolecular chemotherapy. J. Control. Release 2020, 324, 124–133.
Wang, Q.; Tian, L.; Xu, J. Z.; Xia, B.; Li, J.; Lu, F.; Lu, X. M.; Wang, W. J.; Huang, W.; Fan, Q. L. Multifunctional supramolecular vesicles for combined photothermal/photodynamic/hypoxia-activated chemotherapy. Chem. Commun. 2018, 54, 10328–10331.
Ni, X. L.; Xiao, X.; Cong, H.; Zhu, Q. J.; Xue, S. F.; Tao, Z. Self-assemblies based on the “outer-surface interactions” of cucurbit[n]urils: New opportunities for supramolecular architectures and materials. Acc. Chem. Res. 2014, 47, 1386–1395.
Webb, M. S.; Johnstone, S.; Morris, T. J.; Kennedy, A.; Gallagher, R.; Harasym, N.; Harasym, T.; Shew, C. R.; Tardi, P.; Dragowska, W. H. et al. In vitro and in vivo characterization of a combination chemotherapy formulation consisting of vinorelbine and phosphatidylserine. Eur. J. Pharm. Biopharm. 2007, 65, 289–299.
Yu, G. C.; Jie, K. C.; Huang, F. H. Supramolecular amphiphiles based on host-guest molecular recognition motifs. Chem. Rev. 2015, 115, 7240–7303.
Zhang, J. X.; Ma, P. X. Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv. Drug Deliv. Rev. 2013, 65, 1215–1233.
Zhou, J.; Yu, G. C.; Huang, F. H. Supramolecular chemotherapy based on host-guest molecular recognition: A novel strategy in the battle against cancer with a bright future. Chem. Soc. Rev. 2017, 46, 7021–7053.
Zhang, T. X.; Zhang, Z. Z.; Yue, Y. X.; Hu, X. Y.; Huang, F.; Shi, L. Q.; Liu, Y.; Guo, D. S. A general hypoxia-responsive molecular container for tumor-targeted therapy. Adv. Mater. 2020, 32, 1908435.
Aryal, S.; Hu, C. M. J.; Zhang, L. F. Polymeric nanoparticles with precise ratiometric control over drug loading for combination therapy. Mol. Pharm. 2011, 8, 1401–1407.
Dondoni, A.; Marra, A. Calixarene and calixresorcarene glycosides: Their synthesis and biological applications. Chem. Rev. 2010, 110, 4949–4977.
Zhang, Z. Z.; Yue, Y. X.; Xu, L. N.; Wang, Y.; Geng, W. C.; Li, J. J.; Kong, X. L.; Zhao, X. Z.; Zheng, Y. D.; Zhao, Y. et al. Macrocyclic-amphiphile-based self-assembled nanoparticles for ratiometric delivery of therapeutic combinations to tumors. Adv. Mater. 2021, 33, 2007719.
Zhang, T. X.; Li, J. J.; Li, H. B.; Guo, D. S. Deep cavitand calixarene-solubilized fullerene as a potential photodynamic agent. Front. Chem. 2021, 9, 710808.
Roy, S. G.; Acharya, R.; Chatterji, U.; De, P. RAFT polymerization of methacrylates containing a tryptophan moiety: Controlled synthesis of biocompatible fluorescent cationic chiral polymers with smart pH-responsiveness. Polym. Chem. 2013, 4, 1141–1152.
Rad, M. N. S.; Behrouz, S. The base-free chemoselective ring opening of epoxides with carboxylic acids using [bmim]Br: a rapid entry into 1, 2-diol mono-esters synthesis. Mol. Divers. 2013, 17, 9–18.
Fu, L. X.; Peng, Y. Q. Isocyanate-functionalized starch as biorenewable backbone for the preparation and application of poly(ethylene imine) grafted starch. Monatsh. Chem. 2017, 148, 1547–1554.
Acknowledgements
This work was supported by National Key Research and Development Programs of China (No. 2018YFA0209700), National Natural Science Foundation of China (NSFC, No. 22077073), Frontiers Science Center for New Organic Matter (No. 63181206), and Fundamental Research Funds for the Central Universities (Nankai University, No. 63206015).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Xu, L., Chai, J., Wang, Y. et al. Calixarene-integrated nano-drug delivery system for tumor-targeted delivery and tracking of anti-cancer drugs in vivo. Nano Res. 15, 7295–7303 (2022). https://doi.org/10.1007/s12274-022-4332-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4332-4