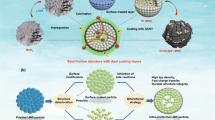
Abstract
Despite the high energy density of lithium-rich (Li-rich) cathodes, their implementation is hampered by the unsatisfied rate capacity and poor cycling performance accompanied with substantial voltage decay. To address these issues, the hierarchical yolk-shell structured Li1.2Mn0.54Ni0.13Co0.13O2 cathodes (YK-LMNCO) was proposed and synthesized through a facile glycerol assisted solvothermal approach and the following lithiation process. Benefitting from the shortened lithium diffusion lengths and the enhanced tolerance to the large volume variation upon lithium ions intercalation/de-intercalation, the unique structure reciprocates an initial coulombic efficiency of 85.8%, an outstanding capacity retention rate of 89.1% after cycling at 2.0 C for 200 cycles with a minor voltage drop, and a capacity retention rate of 93.8% after cycling at 10.0 C for 500 cycles, 85.2% for 1,000 cycles. When assembled with graphite as anode, the YK-LMNCO//graphite full cell shows a remarkable capacity retention rate of 87.2% after cycling at 5.0 C for 50 cycles. Our facile strategy for constructing the yolk-shell structured Li-rich cathodes with high capacity and voltage stability sheds light on synthesizing other lithium storage materials.

Similar content being viewed by others
References
Cui, B. H.; Hu, Z.; Liu, C.; Liu, S. L.; Chen, F. S.; Hu, S.; Zhang, J. F.; Zhou, W.; Deng, Y. D.; Qin, Z. B. et al. Heterogeneous lamellar-edged Fe-Ni(OH)2/Ni3S2 nanoarray for efficient and stable seawater oxidation. Nano Res. 2021, 14, 1149–1155.
Yang, C.; Lv, F.; Zhang, Y. L.; Wen, J.; Dong, K.; Su, H.; Lai, F. L.; Qian, G. Y.; Wang, W.; Hilger, A. et al. Confined Fe2VO4⊂nitrogen-doped carbon nanowires with internal void space for high-rate and ultrastable potassium-ion storage. Adv. Energy Mater. 2019, 9, 1902674.
Yang, C.; Lv, F.; Dong, K.; Lai, F. L.; Zhao, K. N.; Sun, F.; Dou, S. M.; Wang, Q.; Xu, J.; Zhang, P. P. et al. Carbon-coated ultrathin metallic V5Se8 nanosheet for high-energy-density and robust potassium storage. Energy Storage Mater. 2021, 35, 1–11.
Hou, P. Y.; Zhang, H. Z.; Zi, Z. Y.; Zhang, L. Q.; Xu, X. J. Core-shell and concentration-gradient cathodes prepared via co-precipitation reaction for advanced lithium-ion batteries. J. Mater. Chem. A 2017, 5, 4254–4279.
Li, Q.; Dang, R. B.; Chen, M. M.; Lee, Y. L.; Hu, Z. B.; Xiao, X. L. Synthesis method for long cycle life lithium-ion cathode material: Nickel-rich core-shell LiNi0.8Co0.1Mn0.1O2. ACS Appl. Mater. Interfaces 2018, 10, 17850–17860.
Nan, C. Y.; Lu, J.; Li, L. H.; Li, L. L.; Peng, Q.; Li, Y. D. Size and shape control of LiFePO4 nanocrystals for better lithium ion battery cathode materials. Nano Res. 2013, 6, 469–477.
Zhao, S. Q.; Yan, K.; Zhang, J. Q.; Sun, B.; Wang, G. Q. Enhanced electrochemical performance in lithium ion batteries of a hollow spherical lithium-rich cathode material synthesized by a molten salt method. Angew. Chem., Int. Ed. 2020, 6, 2208–2210.
Boulineau, A.; Croguennec, L.; Delmas, C.; Weill, F. Reinvestigation of Li2MnO3 structure: Electron diffraction and high resolution TEM. Chem. Mater. 2009, 21, 4216–4222.
Zhu, W.; Tai, Z. G.; Shu, C. Y.; Chong, S. K.; Guo, S. W.; Ji, L. J.; Chen, Y. Z.; Liu, Y. N. The superior electrochemical performance of a Li-rich layered cathode material with Li-rich spinel Li4Mn5O12 and MgF2 double surface modifications. J. Mater. Chem. A 2020, 8, 7991–8001.
Zhang, L. J.; Wu, B. R.; Li, N.; Wu, F. Hierarchically porous microrod lithium-rich cathode material Li1.2Ni0.13Mn0.54Co0.13O2 for high performance lithium-ion batteries. Electrochim. Acta 2014, 118, 67–74.
Zhang, Y.; Zhang, W. S.; Shen, S. Y.; Yan, X. H.; Wu, A. M.; Yin, J. W.; Zhang, J. L. Hollow porous bowl-shaped lithium-rich cathode material for lithium-ion batteries with exceptional rate capability and stability. J. Power Sources 2018, 380, 164–173.
Liu, Y. C.; Wang, J.; Wu, J. W.; Ding, Z. Y.; Yao, P. H.; Zhang, S. L.; Chen, Y. N. 3D cube-maze-like Li-rich layered cathodes assembled from 2D porous nanosheets for enhanced cycle stability and rate capability of lithium-ion batteries. Adv. Energy Mater. 2020, 10, 1903139.
Wang, Z.; Lin, X. Y.; Zhang, J. T.; Wang, D.; Ding, C. Y.; Zhu, Y. M.; Gao, P.; Huang, X. X.; Wen, G. W. Spherical layered Li-rich cathode material: Unraveling the role of oxygen vacancies on improving lithium ion conductivity. J. Power Sources 2020, 462, 228171.
He, Z. J.; Wang, Z. X.; Huang, Z. M.; Chen, H.; Li, X. H.; Guo, H. J. A novel architecture designed for lithium rich layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 oxides for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 16817–16823.
Li, Y.; Bai, Y.; Wu, C.; Ji, Q.; Chen, G. H.; Liu, L.; Wang, H.; Zhou, X. Z.; Wu, F. Three-dimensional fusiform hierarchical micro/nano Li1.2Ni0.2Mn0.6O2 with a preferred orientation (110) plane as a high energy cathode material for lithium-ion batteries. J. Mater. Chem. A 2016, 4, 5942–5951.
de Biasi, L.; Schwarz, B.; Brezesinski, T.; Hartmann, P.; Janek, J.; Ehrenberg, H. Chemical, structural, and electronic aspects of formation and degradation behavior on different length scales of Ni-Rich NCM and Li-Rich HE-NCM cathode materials in Li-ion batteries. Adv. Mater. 2019, 31, 1900985.
Gent, W. E.; Li, Y. Y.; Ahn, S.; Lim, J.; Liu, Y. J.; Wise, A. M.; Gopal, C. B.; Mueller, D. N.; Davis, R.; Weker, J. N. et al. Persistent state-of-charge heterogeneity in relaxed, partially charged Li1−xNi1/3Co1/3Mn1/3O2 secondary particles. Adv. Mater. 2016, 28, 6631–6638.
Lei, Y. K.; Ni, J.; Hu, Z. J.; Wang, Z. M.; Gui, F. K.; Li, B.; Ming, P. W.; Zhang, C. M.; Elias, Y.; Aurbach, D. et al. Surface modification of Li-rich Mn-based layered oxide cathodes: Challenges, materials, methods, and characterization. Adv. Energy Mater. 2020, 10, 2002506.
Zhang, X.; Zhou, Y, P.; Luo, B.; Zhu, H. C.; Chu, W.; Huang, K. Microwave-assisted synthesis of NiCo2O4 double-shelled hollow spheres for high-performance sodium ion batteries. Nano-Micro Lett. 2018, 10, 13.
Yu, X. Y.; Yao, X. Z.; Luo, T.; Jia, Y.; Liu, J. H.; Huang, X. J. Facile synthesis of urchin-like NiCo2O4 hollow microspheres with enhanced electrochemical properties in energy and environmentally related applications. ACS Appl. Mater. Interfaces 2014, 6, 3689–3695.
Li, Y. X.; Mei, J.; Guo, X. D.; Zhong, B. H.; Liu, H.; Liu, G. B.; Dou, S. X. Hollow Li1.2Mn0.54Ni0.13Co0.13O2 micro-spheres synthesized by a co-precipitation method as a high-performance cathode material for Li-ion batteries. RSC Adv. 2016, 6, 70091–70098.
He, X.; Wang, J.; Kloepsch, R.; Krueger, S.; Jia, H. P.; Liu, H. D.; Vortmann, B.; Jie, L. Enhanced electrochemical performance in lithium ion batteries of a hollow spherical lithium-rich cathode material synthesized by a molten salt method. Nano Res. 2014, 7, 110–118.
Liu, X. P.; Gong, M. X.; Deng, S. F.; Zhao, T. H.; Zhang, J.; Wang, D. L. Recent advances on metal alkoxide-based electrocatalysts for water splitting. J. Mater. Chem. A 2020, 8, 10130–10149.
Shen, L. F.; Yu, L.; Yu, X. Y.; Zhang X. G.; Lou, X. W. Self-templated formation of uniform NiCo2O4 hollow spheres with complex interior structures for lithium-ion batteries and supercapacitors. Angew. Chem., Int. Ed. 2015, 127, 1888–1892.
Zhang, G. Q.; Lou, X. W. General synthesis of multi-shelled mixed metal oxide hollow spheres with superior lithium storage properties. Angew. Chem. 2014, 126, 9187–9190.
Yu, F. D.; Que, L. F.; Wang, Z. B.; Zhang, Y.; Xue, Y.; Liu, B. S.; Gu, D. M. Layered-spinel capped nanotube assembled 3D Li-rich hierarchitectures for high performance Li-ion battery cathodes. J. Mater. Chem. A 2016, 4, 18416–18425.
Zi, Z. Y.; Zhang, Y. T.; Meng, Y. Q.; Gao, G.; Hou, P. Y. Hierarchical Li-rich oxide microspheres assembled from {010} exposed primary grains for high-rate lithium-ion batteries. New J. Chem. 2020, 44, 8486–8493.
Kim, J. H.; Kang, Y. C. Yolk-shell-structured (Fe0.5Ni0.5)9S8 solid-solution powders: Synthesis and application as anode materials for Na-ion batteries. Nano Res. 2017, 10, 3178–3188.
Li, H. L.; Ren, Y. B.; Yang, P. H.; Jian, Z. X.; Wang, W. X.; Xing, Y. L.; Zhang, S. C. Morphology and size controlled synthesis of the hierarchical structured Li1.2Mn0.54Ni0.13Co0.13O2 cathode materials for lithium ion batteries. Electrochim. Acta 2019, 297, 406–416.
Xu, M.; Fei, L. F.; Zhang, W. B.; Li, T.; Lu, W.; Zhang, N.; Lai, Y. Q.; Zhang, Z. A.; Fang, J.; Zhang, K. et al. Tailoring anisotropic Li-ion transport tunnels on orthogonally arranged Li-rich layered oxide nanoplates toward high-performance Li-ion batteries. Nano Lett. 2017, 17, 1670–1677.
Guo, F. F.; Fan, M. H.; Jin, P. P.; Chen, H.; Wu, Y. Y.; Li, G. D.; Zou, X. X. Precursor-mediated synthesis of double-shelled V2O5 hollow nanospheres as cathode material for lithium-ion batteries. CrystEngComm 2016, 18, 4068–4073.
Nayak, P. K.; Erickson, E. M.; Schipper, F.; Penki, T. R.; Munichandraiah, N.; Adelhelm, P.; Sclar, H.; Amalraj, F.; Markovsky; B.; Aurbach, D. Review on challenges and recent advances in the electrochemical performance of high capacity Li- and Mn-rich cathode materials for Li-Ion batteries. Adv. Energy Mater. 2018, 8, 1702397.
Wu, K.; Li, Q.; Dang, R. B.; Deng, X.; Chen, M. M.; Lee, Y. L.; Xiao, X. L.; Hu, Z. B. A novel synthesis strategy to improve cycle stability of LiNi0.8Mn0.1Co0.1O2 at high cut-off voltages through core-shell structuring. Nano Res. 2019, 12, 2460–2467.
Amine, K.; Tukamoto, H.; Yasuda, H.; Fujita, Y. A new three-volt spinel Li1+xMn1.5Ni0.5O4 for secondary lithium batteries. J. Electrochem. Soc. 1996, 143, 1607–1613.
Hu, W. H.; Zhang, Y. X.; Zan, L.; Cong, H. J. Mitigation of voltage decay in Li-rich layered oxides as cathode materials for lithium-ion batteries. Nano Res. 2020, 13, 151–159.
Li, H. Practical evaluation of Li-ion batteries. Joule 2019, 3, 911–914.
Ma, Y. T.; Liu, P. F.; Xie, Q. S.; Zhang, G. B.; Zheng, H. F.; Cai, Y. X.; Li, Z.; Wang, L. S.; Zhu, Z. Z.; Mai, L. Q. et al. Double-shell Lirich layered oxide hollow microspheres with sandwich-like carbon@spinel@layered@spinel@carbon shells as high-rate lithium ion battery cathode. Nano Energy 2019, 59, 184–196.
Li, L. Y.; Liu, Y. C.; Yu, H. B.; Sun, R. T.; Ding, Z. Y.; Li, K. K.; Yuan, Q. H.; Wu, J. W.; Liu, X. J. Hollow spherical 0. 5Li2MnO3·0.5LiMn1/3Ni1/3Co1/3O2 prepared by facile molten salt method for enhanced long-cycle and rate capability of lithium-ion batteries. J. Alloys Compd. 2021, 855, 157376.
Shi, S. J.; Lou, Z. R.; Xia, T. F.; Wang, X. L.; Gu, C. D.; Tu, J. P. Hollow Li1.2Mn0.5Co0.25Ni0.05O2 microcube prepared by binary template as a cathode material for lithium ion batteries. J. Power Sources 2014, 257, 198–204.
Lou, M.; Zhong, H.; Yu, H. T.; Fan, S. S.; Xie, Y.; Yi, T. F. Li1.2Mn0.54Ni0.13Co0.13O2 hollow hierarchical microspheres with enhanced electrochemical performances as cathode material for lithium-ion battery application. Electrochim. Acta 2017, 237, 217–226.
Yu, X. Q.; Lyu, Y. C.; Gu, L.; Wu, H. M.; Bak, S. M.; Zhou, Y. N.; Amine, K.; Ehrlich, S. N.; Li, H.; Nam, K. W. et al. Understanding the rate capability of high-energy-density Li-rich layered Li1.2Ni0.15Co0.1Mn0.55O2 cathode materials. Adv. Energy Mater. 2014, 4, 1300950.
Heubner, C.; Schneider, M.; Michaelis, A. Heat generation rates of NaFePO4 electrodes for sodium-ion batteries and LiFePO4 electrodes for lithium-ion batteries: A comparative study. J. Solid State Electrochem. 2018, 22, 1099–1108.
Sun, J. P.; Tang, K.; Yu, X. Q.; Hu, J.; Li, H.; Huang, X. J. Overpotential and electrochemical impedance analysis on Cr2O3 thin film and powder electrode in rechargeable lithium batteries. Solid State Ionics 2008, 179, 2390–2395.
Zheng, J. M.; Shi, W.; Gu, M.; Xiao, J.; Zuo, P. J.; Wang, C. M.; Zhang, J-G. Electrochemical kinetics and performance of performance of layered composite cathode material Li[Li0.2Ni0.2Mn0.6]O2. J. Electrochem. Soc. 2013, 160, A2212–A2219.
Yu, F. D.; Que, L. F.; Wang, Z. B.; Xue, Y.; Zhang, Y.; Liu, B. S.; Gu, D. M. Controllable synthesis of hierarchical ball-in-ball hollow microspheres for a high performance layered Li-rich oxide cathode material. J. Mater. Chem. A 2017, 5, 9365–9376.
Ding, W. X.; Cui, X. Y.; Lei, J.; Lin, X. D.; Zhao, S. L.; Wu, Q. H.; Zheng, M. S.; Dong, Q. F. Hollow spherical lithium-rich layered oxide cathode material with suppressed voltage fading. Electrochim. Acta 2018, 264, 260–268.
Xu, X.; Huo, H.; Jian, J. Y.; Wang, L. G.; Zhu, H.; Xu, S.; He, X. S.; Yin, G. P.; Du, C. Y.; Sun, X. L. Radially oriented single-crystal primary nanosheets enable ultrahigh rate and cycling properties of LiNi0.8Co0.1Mn0.1O2 cathode material for lithium-ion batteries. Adv. Energy Mater. 2019, 9, 1803963.
Kondrakov, A. O.; Schmidt, A.; Xu, J.; Geßwein, H.; Mönig, R.; Hartmann, P.; Sommer, H.; Brezesinski, T.; Janek, J. Anisotropic lattice strain and mechanical degradation of high- and low-nickel NCM cathode materials for Li-ion batteries. J. Phys. Chem. C 2017, 121, 3286–3294.
Zhang, K.; Li, B.; Zuo, Y. X.; Song, J.; Shang, H. F.; Ning, F. H.; Xia, D. G. Voltage decay in layered Li-rich Mn-based cathode materials. Electrochem. Energy Rev. 2019, 2, 606–623.
Yan, P. F.; Zheng, J. M.; Gu, M.; Xiao, J.; Zhang, J. G.; Wang, C. M. Intragranular cracking as a critical barrier for high-voltage usage of layer-structured cathode for lithium-ion batteries. Nat. Commun. 2017, 8, 14101.
Zheng, J. M.; Gu, M.; Xiao, J.; Zuo, P. J.; Wang, C. M.; Zhang, J. G. Corrosion/fragmentation of layered composite cathode and related capacity/voltage fading during cycling process. Nano Lett. 2013, 13, 3824–3830.
Qian, D. N.; Xu, B.; Chi, M. F.; Meng, Y. S. Uncovering the roles of oxygen vacancies in cation migration in lithium excess layered oxides. Phys. Chem. Chem. Phys. 2014, 16, 14665–14668.
Hu, E. Y.; Yu, X. Q.; Lin, R. Q.; Bi, X. X.; Lu, J.; Bak, S.; Nam, K. W.; Xin, H. L.; Jaye, C.; Fischer, D. A. et al. Evolution of redox couples in Li- and Mn-rich cathode materials and mitigation of voltage fade by reducing oxygen release. Nat. Energy 2018, 3, 690–698.
Yang, W. L. Oxygen release and oxygen redox. Nat. Energy 2018, 3, 619–620.
Sun, G.; Yu, F. D.; Que, L. F.; Deng, L.; Wang, M. J.; Jiang, Y. S.; Shao, G. J.; Wang, Z. B. Local electronic structure modulation enhances operating voltage in Li-rich cathodes. Nano Energy 2019, 66, 104102.
Liu, S.; Liu, Z. P.; Shen, X.; Li, W. H.; Gao, Y. R.; Banis, M. N.; Li, M. S.; Chen, K.; Zhu, L.; Yu, R. C. et al. Surface doping to enhance structural integrity and performance of Li-rich layered oxide. Adv. Energy Mater. 2018, 8, 1802105.
Acknowledgements
The authors acknowledge the financial support from Natural Science Foundation of Guangdong Province (No. 2018A030313721), the National Key Research and Development Program of China (No. 2018YFB0703500) and the National Natural Science Foundation of China (No. 91963113).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Liu, Y., Chen, Y., Wang, J. et al. Hierarchical yolk-shell structured Li-rich cathode boosting cycling and voltage stabled LIBs. Nano Res. 15, 3178–3186 (2022). https://doi.org/10.1007/s12274-021-3890-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-021-3890-1