Abstract
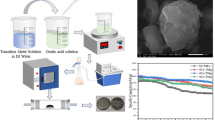
A high voltage layered Li1.2Ni0.16Co0.08Mn0.56O2 cathode material with a hollow spherical structure has been synthesized by molten-salt method in a NaCl flux. Characterization by X-ray diffraction and scanning electron microscopy confirmed its structure and proved that the as-prepared powder is constituted of small, homogenously sized hollow spheres (1–1.5 μm). The material exhibited enhanced rate capability and high first cycle efficiency due to the good dispersion of secondary particles. Galvanostatic cycling at different temperatures (20, 40, and 60 °C) and a current rate of 2 C (500 mA·g−1) showed no significant capacity fade.

Similar content being viewed by others
References
Tarascon, J. M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367.
Besenhard, J. O.; Winter, M. Insertion reactions in advanced electrochemical energy storage. Pure Appl. Chem. 1998, 70, 603–608.
Winter, M.; Besenhard, J. O. Wiederaufladbare batterien. Chem. Unserer Zeit 1999, 33, 252–266.
Wagner, R.; Preschitschek, N.; Passerini, S.; Leker, J.; Winter, M. Current research trends and prospects among the various materials and designs used in lithium-based batteries. J. Appl. Electrochem. 2013, 43, 481–496.
Ohzuku, T.; Brodd, R. J. An overview of positive-electrode materials for advanced lithium-ion batteries. J. Power Sources 2007, 174, 449–456.
Chen, Z. H.; Dahn, J. R. Reducing carbon in LiFePO4/C composite electrodes to maximize specific energy, volumetric energy, and tap density. J. Electrochem. Soc. 2002, 149, A1184–A1189.
Lu, Z. H.; MacNeil, D. D.; Dahn, J. R. Layered cathode materials Li[NixLi(1/3-2x/3)Mn(2/3−x/3)]O2 for lithium-ion batteries. Electrochem. Solid-State Lett. 2001, 4, A191–A194.
Shin, S. S.; Sun, Y. K.; Amine, K. Synthesis and electrochemical properties of Li[Li(1−2x)/3NixMn(2−x)/3]O2 as cathode materials for lithium secondary batteries. J. Power Sources 2002, 112, 634–638.
Johnson, C. S.; Kim, J. S.; Lefief, C.; Li, N.; Vaughey, J. T.; Thackeray, M. M. The significance of the Li2MnO3 component in “composite” xLi2MnO3·(1−x)LiMn0.5Ni0.5O2 electrodes. Electrochem. Comm. 2004, 6, 1085–1091.
Wu, Y.; Manthiram, A. High capacity, surface-modified layered Li[Li(1−x)/3Mn(2−x)/3Ni x/3Co x/3]O2 cathodes with low irreversible capacity loss. Electrochem. Solid-State Lett. 2006, 9, A221–A224.
Zheng, J. M.; Zhang, Z. R.; Wu, X. B.; Dong, Z. X.; Zhu, Z.; Yang, Y. The effects of AlF3 coating on the performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 positive electrode material for lithium-ion battery. J. Electrochem. Soc. 2008, 155, A775–A782.
Wang, J.; He, X.; Paillard, E.; Liu, H. D.; Passerini, S.; Winter, M.; Li, J. Improved rate capability of layered Li-rich cathode for lithium ion battery by electrochemical treatment. ECS Electrochem. Lett. 2013, 2, A78–A80.
Kumai, K.; Miyashiro, H.; Kobayashi, Y.; Takei, K.; Ishikawa, R. Gas generation mechanism due to electrolyte decomposition in commercial lithium-ion cell. J. Power Sources 1999, 81–82, 715–719.
Kong, W. H.; Li, H.; Huang, X. J.; Chen, L. Q. Gas evolution behaviors for several cathode materials in lithium-ion batteries. J. Power Sources 2005, 142, 285–291.
Holzapfel, M.; Würsig, A.; Scheifele, W.; Vetter, J.; Novák, P. Oxygen, hydrogen, ethylene and CO2 development in lithium-ion batteries. J. Power Sources 2007, 174, 1156–1160.
Kang, S. H.; Thackeray, M. M. Enhancing the rate capability of high capacity xLi2MnO3·(1−x)LiMO2 (M = Mn, Ni, Co) electrodes by Li-Ni-PO4 treatment. Electrochem. Comm. 2009, 11, 748–751.
Song, B. H.; Liu, Z. W.; Lai, M. O.; Lu, L. Structural evolution and the capacity fade mechanism upon long-term cycling in Li-rich cathode material. Phys. Chem. Chem. Phys. 2012, 14, 12875–12883.
Wei, G. Z.; Lu, X.; Ke, F. S.; Huang, L.; Li, J. T.; Wang, Z. X.; Zhou, Z. Y.; Sun, S. G. Crystal habit-tuned nanoplate material of Li[Li1/3-2x/3NixMn2/3−x/3]O2 for high-rate performance lithium-ion batteries. Adv. Mater. 2010, 22, 4364–4367.
Shaju, K. M.; Bruce, P. G. A stoichiometric nano-LiMn2O4 spinel electrode exhibiting high power and stable cycling. Chem. Mater. 2008, 20, 5557–5562.
Guo, Y. G.; Hu, J. S.; Wan, L. J. Nanostructured materials for electrochemical energy conversion and storage devices. Adv. Mater. 2008, 20, 2878–2887.
Zhou, L.; Zhao, D. Y.; Lou, X. W. Double-shelled CoMn2O4 hollow microcubes as high-capacity anodes for lithium-ion batteries. Adv. Mater. 2012, 24, 745–748.
Ding, S. J.; Chen, J. S.; Wang, Z. Y.; Cheah, Y. L.; Madhavi, S.; Hu, X.; Lou, X. W. TiO2 hollow spheres with large amount of exposed (001) facets for fast reversible lithium storage. J. Mater. Chem. 2011, 21, 1677–1680.
Jiang, Y.; Yang, Z.; Luo, W.; Hu, X.; Huang, Y. Hollow 0.3Li2MnO3·0.7LiNi0.5Mn0.5O2 microspheres as a high-performance cathode material for lithium-ion batteries. Phys. Chem. Chem. Phys. 2013, 15, 2954–2960.
Qiao, Y.; Li, S. R.; Yu, Y.; Chen, C. H. Synthesis and electrochemical properties of high performance yolk-structured LiMn2O4 microspheres for lithium ion batteries. J. Mater. Chem. A 2013, 1, 860–867.
Zhou, L.; Zhao, D. Y.; Lou, X. W. LiNi0.5Mn1.5O4 hollow structures as high-performance cathodes for lithium-ion batteries. Angew. Chem. Int. Ed. 2012, 51, 239–241.
Bareño, J.; Lei, C. H.; Wen, J. G.; Kang, S. H.; Petrov, I.; Abraham, D. P. Local structure of layered oxide electrode materials for lithium-ion batteries. Adv. Mater. 2010, 22, 1122–1127.
Liu, J. L.; Chen, L.; Hou, M. Y.; Wang, F.; Che, R. C.; Xia, Y. Y. General synthesis of xLi2MnO3·(1−x)LiMn1/3Ni1/3Co1/3O2 nanomaterials by a molten-salt method: Towards a high capacity and high power cathode for rechargeable lithium batteries. J. Mater. Chem. 2012, 22, 25380–25387.
Lutterotti, L.; Matthies, S.; Chateigner, D.; Ferrari, S.; Ricote. J. Rietveld texture and stress analysis of thin films by X-ray diffraction. Mater. Sci. Forum 2002, 408–412, 1603–1608.
Li, J.; Klöpsch, R.; Stan, M. C.; Nowak, S.; Kunze, M.; Winter, M.; Passerini, S. Synthesis and electrochemical performance of the high voltage cathode material Li[Li0.2Mn0.56Ni0.16Co0.08]O2 with improved rate capability. J. Power Sources 2011, 196, 4821–4825.
Zhang, X. Y.; Jiang, W. J.; Mauger, A.; Qilu, R.; Gendron, F.; Julien, C. M. Minimization of the cation mixing in Li1+x (NMC)1−x O2 as cathode material. J. Power Sources 2010, 195, 1292–1301.
Kawamura, T.; Okada, S.; Yamaki, J. I. Decomposition reaction of LiPF6-based electrolytes for lithium ion cells. J. Power Sources 2006, 156, 547–554.
Li, J.; Klöpsch, R.; Nowak, S.; Kunze, M.; Winter, M.; Passerini, S. Investigations on cellulose-based high voltage composite cathodes for lithium ion batteries. J. Power Sources 2011, 196, 7687–7691.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, X., Wang, J., Kloepsch, R. et al. Enhanced electrochemical performance in lithium ion batteries of a hollow spherical lithium-rich cathode material synthesized by a molten salt method. Nano Res. 7, 110–118 (2014). https://doi.org/10.1007/s12274-013-0378-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-013-0378-7