Abstract
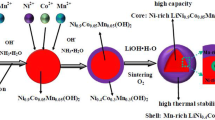
Nickel-rich cathode materials have attracted considerable interest because of their high specific capacities, voltage ranges, and low cost. However, serious capacity attenuation and poor rate performance limit their application. This study proposes a novel strategy to improve the cycle stability of the nickel-rich LiNi0.8Co0.1Mn0.1O2 (NCM811) layer material by designing core-shell LiNi0.8Co0.1Mn0.1O2 (CS-NCM811). CS-NCM811 is designed by the characteristic reaction between dimethylglyoxime (C4H8N2O2) and nickel ion to form Ni(C4H7N2O2)2. The CS-NCM811 is characterized with high nickel content in its core and high manganese content on its surface, leading to a high capacity and excellent cycle stability. The capacity retention of CS-NCM811 was 72.8%, much higher than that of NCM811 (47.1%) after 500 cycles at a rate of 5 C. Not only is this method a novel strategy to design high capacity cathode materials but also provides some new insights into the cycle stability of nickel-rich layered cathode materials.

Similar content being viewed by others
References
Tarascon, J. M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367.
Kim, J.; Manthiram, A. A manganese oxyiodide cathode for rechargeable lithium batteries. Nature 1997, 390, 265–267.
Nakashima, T.; Kimizuka, N. Interfacial synthesis of hollow TiO2 microspheres in ionic liquids. J. Am. Chem. Soc. 2003, 125, 6386–6387.
Hou, Y.; Chang, K.; Li, B.; Tang, H. W.; Wang, Z. Y.; Zou, J. L.; Yuan, H. M.; Lu, Z. G.; Chang, Z. R. Highly [010]-oriented self-assembled LiCoPO4/C nanoflakes as high-performance cathode for lithium ion batteries. Nano Res. 2018, 11, 2424–2435.
Cho, Y.; Oh, P.; Cho, J. A new type of protective surface layer for high-capacity Ni-based cathode materials: Nanoscaled surface pillaring layer. Nano Lett. 2013, 13, 1145–1152.
Li, X. L.; Yan, P. F.; Xiao, X. C.; Woo, J. H.; Wang, C. M.; Liu, J.; Zhang, J. G. Design of porous Si/C-graphite electrodes with long cycle stability and controlled swelling. Energy Environ. Sci. 2017, 10, 1427–1434.
Wang, L. P.; Zhang, H. Q.; Mou, C. X.; Cui, Q. L.; Deng, Q. J.; Xue, J.; Dai, X. Y.; Li, J. Z. Dicarboxylate CaC8H4O4 as a high-performance anode for Li-ion batteries. Nano Res. 2015, 8, 523–532.
Shim, J. H.; Im, J. S.; Kang, H.; Cho, N.; Kim, Y. M.; Lee, S. Implications of cation-disordered grain boundaries on the electrochemical performance of the LiNi0.5Co0.2Mn0.3O2 cathode material for lithium ion batteries. J. Mater. Chem. A 2018, 6, 16111–16120.
Shi, J. L.; Xiao, D. D.; Zhang, X. D.; Yin, Y. X.; Guo, Y. G.; Gu, L.; Wan, L. J. Improving the structural stability of Li-rich cathode materials via reservation of cations in the Li-slab for Li-ion batteries. Nano Res. 2017, 10, 4201–4209.
Wang, J.; He, X.; Paillard, E.; Laszczynski, N.; Li, J.; Passerini, S. Lithium- and manganese-rich oxide cathode materials for high-energy lithium ion batteries. Adv. Energy Mater. 2016, 6, 1600906.
Fu, F.; Xu, G. L.; Wang, Q.; Deng, Y. P.; Li, X.; Li, J. T.; Huang, L.; Sun, S. G. Synthesis of single crystalline hexagonal nanobricks of LiNi1/3Co1/3Mn1/3O2 with high percentage of exposed {010} active facets as high rate performance cathode material for lithium-ion battery. J. Mater. Chem. A 2013, 1, 3860–3864.
Xie, J.; Zhao, J.; Liu, Y. Y.; Wang, H. T.; Liu, C.; Wu, T.; Hsu, P. C.; Lin, D. C.; Jin, Y.; Cui, Y. Engineering the surface of LiCoO2 electrodes using atomic layer deposition for stable high-voltage lithium ion batteries. Nano Res. 2017, 10, 3754–3764.
Li, F.; Kong, L. L.; Sun, Y. Y.; Jin, Y. C.; Hou, P. Y. Micron-sized monocrystalline LiNi1/3Co1/3Mn1/3O2 as high-volumetric-energy-density cathode for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 12344–12352.
Pan, Z. Y.; Ren, J.; Guan, G. Z.; Fang, X.; Wang, B. J; Doo, S. G.; Son, I. H.; Huang, X. L.; Peng, H. S. Synthesizing nitrogen-doped core-sheath carbon nanotube films for flexible lithium ion batteries. Adv. Energy Mater. 2016, 6, 1600271.
Sun, Y. K.; Han, J. M.; Myung, S. T.; Lee, S. W.; Amine, K. Significant improvement of high voltage cycling behavior AlF3-coated LiCoO2 cathode. Electrochem. Commun. 2006, 8, 821–826.
Hu, G. R.; Zhang, M. F.; Liang, L. W.; Peng, Z. D.; Du, K.; Cao, Y. B. Mg-Al-B co-substitution LiNi0.5Co0.2Mn0.3O2 cathode materials with improved cycling performance for lithium-ion battery under high cutoff voltage. Electrochim. Acta 2016, 190, 264–275.
Cong, L. N.; Zhao, Q.; Wang, Z.; Zhang, Y. H.; Wu, X. L.; Zhang, J. P.; Wang, R. S.; Xie, H. M.; Sun, L. Q. (PO4)3− polyanions doped LiNi1/3Co1/3Mn1/3O2: An ultrafast-rate, long-life and high-voltage cathode material for Li-ion rechargeable batteries. Electrochim. Acta 2016, 201, 8–19.
Zhang, J.; Shi, Y.; Ding, Y.; Peng, L. L.; Zhang, W. K.; Yu, G. H. A conductive molecular framework derived Li2S/N,P-codoped carbon cathode for advanced lithium-sulfur batteries. Adv. Energy Mater. 2017, 7, 1602876.
Wang, D.; Li, X. H.; Wang, Z. X.; Guo, H. J.; Xu, Y.; Fan, Y. L. Comodification of LiNi0.5Co0.2Mn0.3O2 cathode materials with zirconium substitution and surface polypyrrole coating: Towards superior high voltage electrochemical performances for lithium ion batteries. Electrochim. Acta 2016, 196, 101–109.
Ohzuku, T.; Brodd, R. J. An overview of positive-electrode materials for advanced lithium-ion batteries. J. Power Sources 2007, 174, 449–456.
Zhao, E. Y.; Chen, M. M; Hu, Z. B.; Chen, D. F.; Yang, L. M.; Xiao, X. L. Improved cycle stability of high-capacity Ni-rich LiNi0.8Mn0.1Co0.1O2 at high cut-off voltage by Li2SiO3 coating. J. Power Sources 2017, 343, 345–353.
Zhang, J. C.; Yang, Z. Z.; Gao, R.; Gu, L.; Hu, Z. B.; Liu, X. F. Suppressing the structure deterioration of Ni-rich LiNi0.8Co0.1Mn0.1O2 through atomscale interfacial integration of self-forming hierarchical spinel layer with Ni gradient concentration. ACS Appl. Mater. Interfaces 2017, 9, 29794–29803.
Cao, Y. B.; Qi, X. Y.; Hu, K. H.; Wang, Y.; Gan, Z. G.; Li, Y.; Hu, G. R.; Peng, Z. D.; Du, K. Conductive polymers encapsulation to enhance electrochemical performance of Ni-rich cathode materials for Li-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 18270–18280.
Liang, H. M.; Wang, Z. X.; Guo, H. J.; Wang, J. X.; Leng, J. Improvement in the electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode material by Li2ZrO3 coating. Appl. Surf. Sci. 2017, 423, 1045–1053.
Chen, M. M.; Zhao, E. Y.; Chen, D. F.; Wu, M. M.; Han, S. B.; Huang, Q. Z.; Yang, L. M.; Xiao, X. L.; Hu, Z. B. Decreasing Li/Ni disorder and improving the electrochemical performances of Ni-rich LiNi0.8Co0.1Mn0.1O2 by Ca doping. Inorg. Chem. 2017, 56, 8355–8362.
Zhang, B.; Li, L. J.; Zheng, J. C. Characterization of multiple metals (Cr, Mg) substituted LiNi0.8Co0.1Mn0.1O2 cathode materials for lithium ion battery. J. Alloys Compd. 2012, 520, 190–194.
Min, K.; Seo, S. W.; Song, Y. Y.; Lee, H. S.; Cho, E. A first-principles study of the preventive effects of Al and Mg doping on the degradation in LiNi0.8Co0.1Mn0.1O2 cathode materials. Phys. Chem. Chem. Phys. 2017, 19, 1762–1769.
Schipper, F.; Bouzaglo, H.; Dixit, M.; Erickson, E. M.; Weigel, T.; Talianker, M.; Grinblat, J.; Burstein, L.; Schmidt, M.; Lampert, J. et al. From surface ZrO2 coating to bulk Zr doping by high temperature annealing of nickel-rich lithiated oxides and their enhanced electrochemical performance in lithium ion batteries. Adv. Energy Mater. 2018, 8, 1701682.
Vu, D. L.; Lee, J. W. Na-doped layered LiNi0.8Co0.1Mn0.1O2 with improved rate capability and cycling stability. J. Solid State Electrochem. 2018, 22, 1165–1173.
Gu, Y. X.; Jian, F. F. Hollow LiNi0.8Co0.1Mn0.1O2-MgO coaxial fibers: Sol-gel method combined with co-electrospun preparation and electrochemical properties. J. Phys. Chem. C 2008, 112, 20176–20180.
Dong, M. X.; Wang, Z. X.; Li, H. K.; Guo, H. J.; Li, X. H.; Shih, K.; Wang, J. X. Metallurgy inspired formation of homogeneous Al2O3 coating layer to improve the electrochemical properties of LiNi0.8Co0.1Mn0.1O2 cathode material. ACS Sustainable Chem. Eng. 2017, 5, 10199–10205.
Chen, S.; He, T.; Su, Y. F.; Lu, Y.; Bao, L. Y; Chen, L.; Zhang, Q. Y.; Wang, J.; Chen, R. J.; Wu, F. Ni-rich LiNi0.8Co0.1Mn0.1O2 oxide coated by dual-conductive layers as high performance cathode material for lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 29732–29743.
Sun, Y. K.; Lee, B. R.; Noh, H. J.; Wu, H. M.; Myung, S. T.; Amine, K. A novel concentration-gradient Li[Ni0.83Co0.07Mn0.10]O2 cathode material for high-energy lithium-ion batteries. J. Mater. Chem. 2011, 21, 10108–10112.
Sun, Y. K.; Myung, S. T.; Kim, M. H.; Prakash, J.; Amine, K. Synthesis and characterization of Li[(Ni0.8Co0.1Mn0.1)0.8(Ni0.5Mn0.5)0.2]O2 with the microscale core-shell structure as the positive electrode material for lithium batteries. J. Am. Chem. Soc. 2005, 127, 13411–13418.
Sun, Y. K.; Myung, S. T.; Park, B. C.; Prakash, J.; Belharouak, I.; Amine, K. High-energy cathode material for long-life and safe lithium batteries. Nat. Mater. 2009, 8, 320–324.
Li, Q.; Dang, R. B.; Chen, M. M.; Lee, Y. L.; Hu, Z. B.; Xiao, X. L. Synthesis method for long cycle life lithium-ion cathode material: Nickel-rich core-shell LiNi0.8Co0.1Mn0.1O2. ACS Appl. Mater. Interfaces 2018, 10, 17850–17860.
Zhang, J. C.; Gao, R.; Sun, L. M.; Zhang, H.; Hu, Z. B.; Liu, X. F. Unraveling the multiple effects of Li2ZrO3 coating on the structural and electrochemical performances of LiCoO2 as high-voltage cathode materials. Electrochim. Acta 2016, 209, 102–110.
Chen, S.; He, T.; Su, Y. F.; Lu, Y.; Bao, L. Y.; Chen, L.; Zhang, Q. Y.; Wang, J.; Chen, R. J.; Wu, F. Ni-rich LiNi0.8Co0.1Mn0.1O2 oxide coated by dual-conductive layers as high performance cathode material for lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 29732–29743.
Fu, C. C.; Li, G. S.; Luo, D.; Li, Q.; Fan, J. M.; Li, L. P. Nickel-rich layered microspheres cathodes: Lithium/nickel disordering and electrochemical performance. ACS Appl. Mater. Interfaces 2014, 6, 15822–15831.
Noh, M.; Cho, J. Optimized synthetic conditions of LiNi0.5Co0.2Mn0.3O2 cathode materials for high rate lithium batteries via Co-precipitation method. J. Electrochem. Soc. 2013, 160, A105–A111.
Yoon, C. S.; Choi, M. J.; Jun, D. W.; Zhang, Q.; Kaghazchi, P.; Kim, K. H.; Sun, Y. K. Cation ordering of Zr-doped LiNiO2 cathode for lithium-ion batteries. Chem. Mater. 2018, 30, 1808–1814.
Saadoune, I.; Delmas, C. On the LixNi0.8Co0.2O2 system. J. Solid State Chem. 1998, 136, 8–15.
Xu, Y. D.; Xiang, W.; Wu, Z. G.; Xu, C. L.; Li, Y. C.; Guo, X. D.; Lv, G. P.; Peng, X.; Zhong, B. H. Improving cycling performance and rate capability of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode materials by Li4Ti5O12 coating. Electrochim. Acta 2018, 268, 358–365.
Huang, Y.; Jin, F. M.; Chen, F. J.; Chen, L. Improved cycle stability and high-rate capability of Li3VO4-coated Li[Ni0.5Co0.2Mn0.3]O2 cathode material under different voltages. J. Power Sources 2014, 256, 1–7.
Huang, Z. J.; Wang, Z. X.; Jing, Q.; Guo, H. J.; Li, X. H.; Yang, Z. H. Investigation on the effect of Na doping on structure and Li-ion kinetics of layered LiNi0.6Co0.2Mn0.2O2 cathode material. Electrochim. Acta 2016, 192, 120–126.
Tao, F.; Yan, X. X.; Liu, J. J.; Zhang, H. L.; Chen, L. Effects of PVP-assisted Co3O4 coating on the electrochemical and storage properties of LiNi0.6Co0.2Mn0.2O2 at high cut-off voltage. Electrochim. Acta 2016, 210, 548–556.
Li, Q.; Wu, K.; Chen, M. M; Lee, Y. L.; Chen, D. F.; Wu, M. M.; Li, F. Q.; Xiao, X. L.; Hu, Z. B. Designing high-voltage and high-rate Li1−xNaxCoO2 by enlarging Li layer spacing. Electrochim. Acta 2018, 273, 145–153.
Li, J. H.; Yang, F. Q.; Xiao, X. C.; Verbrugge, M. W.; Cheng, Y. T. Potentiostatic intermittent titration technique (PITT) for spherical particles with finite interfacial kinetics. Electrochim. Acta 2012, 75, 56–61.
Wang, M.; Luo, M.; Chen, Y. B.; Su, Y. F.; Chen, L.; Zhang, R. Electrochemical deintercalation kinetics of 0.5Li2MnO3·0.5LiNi1/3Mn1/3Co1/3O2 studied by EIS and PITT. J. Alloys Compd. 2017, 696, 907–913.
Wang, Q. S.; Sun, J. H.; Yao, X. L.; Chen, C. H. Thermal stability of LiPF6/EC + DEC electrolyte with charged electrodes for lithium ion batteries. Thermochim. Acta 2005, 437, 12–16.
Wang, Z. G.; Wang, Z. X.; Guo, H. J.; Peng, W. J.; Li, X. H.; Yan, G. C.; Wang, J. X. Mg doping and zirconium oxyfluoride coating co-modification to enhance the high-voltage performance of LiCoO2 for lithium ion battery. J. Alloys Compd. 2015, 621, 212–219.
Acknowledgements
This work was supported by the Youth Innovation Promotion Association CAS (No. 2016152), the University of Chinese Academy of Sciences, and the Scientific Instrument Developing Project of the Chinese Academy of Sciences (No. ZDKYYQ20170001).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2019_2469_MOESM1_ESM.pdf
A novel synthesis strategy to improve cycle stability of LiNi0.8Mn0.1Co0.1O2 at high cut-off voltages through core-shell structuring
Rights and permissions
About this article
Cite this article
Wu, K., Li, Q., Dang, R. et al. A novel synthesis strategy to improve cycle stability of LiNi0.8Mn0.1Co0.1O2 at high cut-off voltages through core-shell structuring. Nano Res. 12, 2460–2467 (2019). https://doi.org/10.1007/s12274-019-2469-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-019-2469-6