Abstract
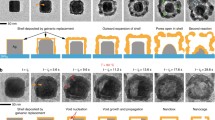
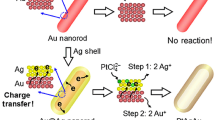
The sacrificial templates used in galvanic replacement reactions dictate the properties of the hollow metal nanostructures formed. Here, we demonstrate that substrate-based Au-Ag nanoshells with radically altered properties are obtained by merely coating silver templates with an ultrathin layer of gold prior to their insertion into the reaction vessel. The so-formed nanoshells exhibit much smoother surfaces, a higher degree of crystallinity and are far more robust. Dealloying the nanoshells results in the first demonstration of substrate-based nanocages. Such cages exhibit a well-defined pattern of geometric openings in directions corresponding to the {111}-facets of the starting template material. The ability to engineer the cage geometry through adjustments to the orientational relationship between the crystal structure of the starting template and that of underlying substrate is demonstrated. Together these discoveries provide the framework to advance our understanding of the mechanisms governing substratebased galvanic replacement reactions.

Similar content being viewed by others
References
Xia, X. H.; Wang, Y.; Ruditskiy, A.; Xia, Y. N. Galvanic replacement: A simple and versatile route to hollow nanostructures with tunable and well-controlled properties. Adv. Mater. 2013, 25, 6313–6333.
Cobley, C. M.; Xia, Y. N. Engineering the properties of metal nanostructures via galvanic replacement reactions. Mater. Sci. Eng. R 2010, 70, 44–62.
Yin, Y. D.; Erdonmez, C.; Aloni, S.; Alivisatos, A. P. Faceting of nanocrystals during chemical transformation: From solid silver spheres to hollow gold octahedra. J. Am. Chem. Soc. 2006, 128, 12671–12673.
Zhang, Q. B.; Xie, J. P.; Lee, J. Y.; Zhang, J. X.; Boothroyd, C. Synthesis of Ag@AgAu metal core/alloy shell bimetallic nanoparticles with tunable shell compositions by a galvanic replacement reaction. Small 2008, 4, 1067–1071.
Yu, Y.; Zhang, Q. B.; Xie, J. P.; Lee, J. Y. Engineering the architectural diversity of heterogeneous metallic nanocrystals. Nat. Commun. 2013, 4, 1454.
Skrabalak, S. E.; Au, L.; Li, X. D.; Xia, Y. N. Facile synthesis of Ag nanocubes and Au nanocages. Nat. Protoc. 2007, 2, 2182–2190.
Chen, J. Y.; Yang, M. X.; Zhang, Q.; Cho, E. C.; Cobley, C. M.; Kim, C.; Glaus, C.; Wang, L. H. V.; Welch, M. J.; Xia, Y. N. Gold nanocages: A novel class of multifunctional nanomaterials for theranostic applications. Adv. Funct. Mater. 2010, 20, 3684–3694.
Xia, Y. N.; Halas, N. J. Shape-controlled synthesis and surface plasmonic properties of metallic nanostructures. MRS Bull. 2005, 30, 338–348.
Sun, Y. G.; Xia, Y. N. Increased sensitivity of surface plasmon resonance of gold nanoshells compared to that of gold solid colloids in response to environmental changes. Anal. Chem. 2002, 74, 5297–5305.
Sun, Y. G.; Mayers, B.; Xia, Y. N. Metal nanostructures with hollow interiors. Adv. Mater. 2003, 15, 641–646.
Fu, E.; Ramsey, S. A.; Chen, J. Y.; Chinowsky, T. M.; Wiley, B.; Xia, Y. N.; Yager, P. Resonance wavelength-dependent signal of absorptive particles in surface plasmon resonance-based detection. Sens. Actuators 2007, 123, 606–613.
Kim, S. W.; Kim, M.; Lee, W. Y.; Hyeon, T. Fabrication of hollow palladium spheres and their successful application to the recyclable heterogeneous catalyst for Suzuki coupling reactions. J. Am. Chem. Soc. 2002, 124, 7642–7643.
Yu, X. F.; Wang, D. S.; Peng, Q.; Li, Y. D. High performance electrocatalyst: Pt-Cu hollow nanocrystals. Chem. Commun. 2011, 47, 8094–8096.
Sun, Y. G.; Xia, Y. N. Mechanistic study on the replacement reaction between silver nanostructures and chloroauric acid in aqueous medium. J. Am. Chem. Soc. 2004, 126, 3892–3901.
Lu, X. M.; Tuan, H. Y.; Chen, J. Y.; Li, Z. Y.; Korgel, B. A.; Xia, Y. Mechanistic studies on the galvanic replacement between multiply twinned particles of Ag and HAuCl4 in an organic medium. J. Am. Chem. Soc. 2007, 129, 1733–1742.
Sun, Y. G.; Wang, Y. X. Monitoring galvanic replacement reaction between silver nanowires and HAuCl4 by in situ transmission X-ray microscopy. Nano Lett. 2011, 11, 4386–4392.
Ott, A.; Bhargava, S. K.; O’Mullane, A. P. A study of the galvanic replacement reaction at surfaces and the role of lateral charge propagation. Surf. Sci. 2012, 606, L5–L9.
Cobley, C. M.; Zhang, Q.; Song, W.; Xia, Y. N. The role of surface nonuniformity in controlling the initiation of a galvanic replacement reaction. Chem. Asian J. 2011, 6, 1479–1484.
Sun, Y. G.; Mayers, B. T.; Xia, Y. N. Template-engaged replacement reaction: A one-step approach to the large-scale synthesis of metal nanostructures with hollow interiors. Nano Lett. 2002, 2, 481–485.
Skrabalak, S. E.; Chen, J. Y.; Sun, Y. G.; Lu, X. M.; Au, L.; Cobley, C. M.; Xia, Y. N. Gold nanocages: Synthesis, properties, and applications, Acc. Chem. Res. 2008, 41, 1587–1595.
Kim, M. H.; Lu, X. M.; Wiley, B.; Lee, E. P.; Xia, Y. N. Morphological evolution of single-crystal Ag nanospheres during the galvanic replacement reaction with HAuCl4. J. Phys. Chem. C 2008, 112, 7872–7876.
Camargo, P. H. C.; Xiong, Y. J.; Ji, L.; Zuo, J. M.; Xia, Y. N. Facile synthesis of tadpole-like nanostructures consisting of Au heads and Pd tails. J. Am. Chem. Soc. 2007, 129, 15452–15453.
Henry, C. R. Morphology of supported nanoparticles. Prog. Surf. Sci. 2005, 80, 92–116.
Gilroy, K. D.; Farzinpour, P.; Sundar, A.; Tan, T.; Hughes, R. A.; Neretina, S. Substrate-based galvanic replacement reactions carried out on heteroepitaxially formed silver templates. Nano Res. 2013, 6, 418–428.
Farzinpour, P.; Sundar, A.; Gilroy, K. D.; Eskin, Z. E.; Hughes, R. A.; Neretina, S. Dynamic templating: A large area processing route for the assembly of periodic arrays of sub-micrometer and nanoscale structures. Nanoscale 2013, 5, 1929–1938.
Ridelman, Y.; Singh, G.; Popovitz-Biro, R.; Wolf, S. G.; Das, S.; Klajn, R. Metallic nanobowls by galvanic replacement reaction on heterodimeric nanoparticles. Small 2012, 8, 654–660.
Rao, Y. Y.; Tao, Q.; An, M.; Rong, C. H.; Dong, J.; Dai, Y. R.; Qian, W. P. Novel and simple route to fabricate 2D ordered gold nanobowl arrays based on 3D colloidal crystals. Langmuir 2011, 27, 13308–13313.
Li, X. L.; Zhang, Y. Z.; Shen, Z. X.; Fan, H. J. Highly ordered arrays of particle-in-bowl plasmonic nanostructures for surface enhanced Raman scattering. Small 2012, 8, 2548–2554.
Ye, J.; Van Dorpe, P.; Van Roy, W.; Borghs, G.; Maes, G. Fabrication, characterization, and optical properties of gold nanobowl submonolayer structures. Langmuir 2009, 25, 1822–1827.
Vitos, L.; Ruban, A. V.; Skriver, H. L.; Kollár, J. The surface energy of metals. Surf. Sci. 1998, 411, 186–202.
Li, B.; Yan, P. F.; Sui, M. L.; Ma, E. Transmission electron microscopy study of stacking faults and their interaction with pyramidal dislocations in deformed Mg. Acta Mater. 2010, 58, 173–179.
Mehl, M. J.; Papaconstantopoulos, D. A.; Kioussis, N.; Herbranson, M. Tight-binding study of stacking fault energies and the Rice criterion of ductility in the fcc metals. Phys. Rev. B 2000, 61, 4894–4897.
Lee, B. J.; Shim, J. H.; Baskes, M. I. Semiempirical atomic potentials for the fcc metals Cu, Ag, Au, Ni, Pd, Pt, Al, and Pb based on first and second nearest-neighbor modified embedded atom method. Phys. Rev. B 2003, 68, 144112.
Wang, J. G.; Tian, M. L.; Mallouk, T. E.; Chan, M. H. W. Microtwinning in template-synthesized single-crystal metal nanowires. J. Phys. Chem. B 2004, 108, 841–845.
Lofton, C.; Sigmund, W. Mechanisms controlling crystal habits of gold and silver colloids. Adv. Funct. Mater. 2005, 15, 1197–1208.
Barth, S.; Boland, J. J.; Holmes, J. D. Defect transfer from nanoparticles to nanowires. Nano Lett. 2011, 11, 1550–1555.
Moewe, M.; Chuang, L. C.; Dubrovskii, V. G.; Chang-Hasnain, C. Growth mechanisms and crystallographic structure of InP nanowires on lattice-mismatched substrates. J. Appl. Phys. 2008, 104, 044313.
Wang, Y.; Liao, Z.; Xu, H. Y.; Xiu, F. X.; Kou, X. F.; Wang, Y.; Wang, K. L.; Drennan, J.; Zou, J. Structural evolution of GeMn/Ge superlattices grown by molecular epitaxy under different growth conditions. Nanoscale Res. Lett. 2011, 6, 624.
Merz, M. D.; Dahlgren, S. D. Tensile strength and work hardening of ultrafine-grained high-purity copper. J. Appl. Phys. 1975, 46, 3235–3237.
Ohnishi, H.; Kondo, Y.; Takayanagi, K. UHV electron microscope and simultaneous STM observation of gold stepped surfaces. Surf. Sci. 1998, 415, L1061–L1064.
McCue, I.; Snyder, J.; Li, X.; Chen, Q.; Sieradzki, K.; Erlebacher, J. Apparent inverse Gibbs-Thomson effect in dealloyed nanoporous nanoparticles. Phys. Rev. Lett. 2012, 108, 225503.
Sau, T. K.; Rogach, A. L. Nonspherical noble metal nanoparticles: Colloid-chemical synthesis and morphology control. Adv. Mater. 2010, 22, 1781–1804.
Kim, D.; Giermann, A. L.; Thompson, C. V. Solid-state dewetting of patterned thin films. Appl. Phys. Lett. 2009, 95, 251903.
Thompson, C. V. Solid-state dewetting of thin films. Ann. Rev. Mater. Res. 2012, 42, 399–434.
Erlebacher, J.; Aziz, M. J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 2001, 410, 450–453.
Erlebacher, J.; Seshadri, R. Hard materials with tunable porosity. MRS Bull. 2009, 34, 561–568.
Erlebacher, J. An atomistic description of dealloying: Porosity evolution, the critical potential, and rate-limiting behavior. J. Electrochem. Soc. 2004, 151, C614–C626.
Seo, D.; Song, H. Asymmetric hollow nanorod formation through a partial galvanic replacement reaction. J. Am. Chem. Soc. 2009, 131, 18210–18211.
Farzinpour, P.; Sundar, A.; Gilroy, K. D.; Eskin, Z. E.; Hughes, R. A.; Neretina, S. Altering the dewetting characteristics of ultrathin gold and silver films using a sacrificial antimony layer. Nanotechnology 2012, 23, 495604.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Gilroy, K.D., Sundar, A., Farzinpour, P. et al. Mechanistic study of substrate-based galvanic replacement reactions. Nano Res. 7, 365–379 (2014). https://doi.org/10.1007/s12274-013-0402-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-013-0402-y