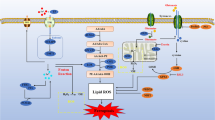
Abstract
Ischemic heart disease, which results from plaque formation in the coronary arteries, hinders the flow of oxygenated blood to the heart, leading to ischemia. Reperfusion injury remains a significant challenge for researchers, and the mechanisms underlying myocardial ischemia–reperfusion injury (MIRI) are not entirely understood. The review directs future research into potential targets in clinical treatment based on our present understanding of the pathophysiological mechanisms of MIRI. The study provides insights into the mechanisms underlying MIRI and offers direction for future research in this area. The use of targeted therapies may hold promise in improving cardiac function in the elderly and minimizing the adverse effects of revascularization therapies. The purpose of this review is to analyze the role of activated protein C (APC) in the pathogenesis of ischemic heart disease, heart failure, and myocardial ischemia–reperfusion injury, and discuss the potential of APC-based therapeutics.
Graphical Abstract




Similar content being viewed by others
Data Availability
NA.
References
Nowbar AN, Gitto M, Howard JP, Francis D, Al-Lamee R. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes. 2019;12(6):e005375.
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update From the GBD 2019 Study. J Am Coll Cardiol [Internet]. 2020;76(25):2982–3021.
Adnan G, Singh DP, Mahajan K. Coronary artery thrombus. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
Chen S, Li A, Wu J, Huang Y, Zou T, Tailaiti T, et al. Dexmedetomidine reduces myocardial ischemia-reperfusion injury in young mice through MIF/AMPK/GLUT4 axis. BMC Anesthesiol. 2022;22(1):289.
Sarangi PP, Lee HW, Kim M. Activated protein C action in inflammation. Br J Haematol. 2010;148(6):817–33.
Ren D, Giri H, Li J, Rezaie AR. The cardioprotective signaling activity of activated protein C in heart failure and ischemic heart diseases. Int J Mol Sci. 2019;20(7):1762.
Neyrinck AP, Liu KD, Howard JP, Matthay MA. Protective mechanisms of activated protein C in severe inflammatory disorders. Br J Pharmacol. 2009;158(4):1034–47.
Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res. 2016;119(1):91–112.
Ahmad M, Mehta P, Reddivari AKR, et al. Percutaneous coronary intervention. [Updated 2022 Sep 30]. In: StatPearls [Internet].
Jenča D, Melenovský V, Stehlik J, Staněk V, Kettner J, Kautzner J, Adámková V, Wohlfahrt P. Heart failure after myocardial infarction: incidence and predictors. ESC Heart Fail. 2021;8(1):222–37.
Soares ROS, Losada DM, Jordani MC, Évora P, Castro-E-Silva O. Ischemia/reperfusion injury revisited: an overview of the latest pharmacological strategies. Int J Mol Sci. 2019;20(20):5034.
Zhang Q, Wang L, Wang S, Cheng H, Xu L, Pei G, Wang Y, Fu C, Jiang Y, He C, Wei Q. Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct Target Ther. 2022;7(1):78.
Ojha N, Dhamoon AS. Myocardial infarction. [Updated 2022 Aug 8]. In: StatPearls [Internet].
Pluijmert NJ, Atsma DE, Quax PHA. Post-ischemic myocardial inflammatory response: a complex and dynamic process susceptible to immunomodulatory therapies. Front Cardiovasc Med. 2021;28(8):647785.
Hannoodee S, Nasuruddin DN. Acute inflammatory response. [Updated 2022 Nov 14].
Theofilis P, Sagris M, Oikonomou E, Antonopoulos AS, Siasos G, Tsioufis C, Tousoulis D. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines. 2021;9(7):781.
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017;9(6):7204–18.
Rapoport BL, Steel HC, Theron AJ, Heyman L, Smit T, Ramdas Y, Anderson R. High mobility group box 1 in human cancer. Cells. 2020;9(7):1664.
Ong SB, Hernández-Reséndiz S, Crespo-Avilan GE, Mukhametshina RT, Kwek XY, Cabrera-Fuentes HA, Hausenloy DJ. Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther. 2018;186:73–87.
Suresh R, Mosser DM. Pattern recognition receptors in innate immunity, host defense, and immunopathology. Adv Physiol Educ. 2013;37(4):284–91.
Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11(5):255–65.
Anzai A, Ko S, Fukuda K. Immune and inflammatory networks in myocardial infarction: current research and its potential implications for the clinic. Int J Mol Sci. 2022;23(9):5214.
Stavenuiter F, Bouwens EA, Mosnier LO. Down-regulation of the clotting cascade by the protein C pathway. Hematol Educ. 2013;7(1):365–74.
Wojtukiewicz MZ, Hempel D, Sierko E, Tucker SC, Honn KV. Endothelial protein C receptor (EPCR), protease activated receptor-1 (PAR-1) and their interplay in cancer growth and metastatic dissemination. Cancers (Basel). 2019;11(1):51.
Rezaie AR. Regulation of the protein C anticoagulant and antiinflammatory pathways. Curr Med Chem. 2010;17(19):2059–69.
Müller J, Friedrich M, Becher T, Braunstein J, Kupper T, Berdel P, Gravius S, Rohrbach F, Oldenburg J, Mayer G, Pötzsch B. Monitoring of plasma levels of activated protein C using a clinically applicable oligonucleotide-based enzyme capture assay. J Thromb Haemost. 2012;10(3):390–8.
Gupta A, Patibandla S. Protein C deficiency. [Updated 2022 Jul 4]. In: StatPearls [Internet].
Lee GD, Ju S, Kim JY, Kim TH, Yoo JW, Lee SJ, Cho YJ, Jeong YY, Jeon KN, Lee JD, Kim HC. Risk factor and mortality in patients with pulmonary embolism combined with infectious disease. Tuberc Respir Dis (Seoul). 2020;83(2):157–66.
Mutua V, Gershwin LJ. A review of neutrophil extracellular traps (NETs) in disease: potential anti-NETs therapeutics. Clin Rev Allergy Immunol. 2021;61(2):194–211.
Martí-Carvajal AJ, Solà I, Gluud C, Lathyris D, Cardona AF. Human recombinant protein C for severe sepsis and septic shock in adult and paediatric patients. Cochrane Database Syst Rev. 2012;12(12):CD004388.
Han X, Alu A, Liu H, Shi Y, Wei X, Cai L, Wei Y. Biomaterial-assisted biotherapy: a brief review of biomaterials used in drug delivery, vaccine development, gene therapy, and stem cell therapy. Bioact Mater. 2022;19(17):29–48.
Mechanic OJ, Gavin M, Grossman SA. Acute myocardial infarction. [Updated 2022 Aug 8]. In: StatPearls [Internet].
Frank A, Bonney M, Bonney S, Weitzel L, Koeppen M, Eckle T. Myocardial ischemia reperfusion injury: from basic science to clinical bedside. Semin Cardiothorac Vasc Anesth. 2012;16(3):123–32.
He J, Liu D, Zhao L, Zhou D, Rong J, Zhang L, Xia Z. Myocardial ischemia/reperfusion injury: mechanisms of injury and implications for management (Review). Exp Ther Med. 2022;23(6):430.
Hong M, Rong J, Tao X, Xu Y. The emerging role of ferroptosis in cardiovascular diseases. Front Pharmacol. 2022;26(13):822083.
Huang Y, Sun X, Juan Z, Zhang R, Wang R, Meng S, Zhou J, Li Y, Xu K, Xie K. Dexmedetomidine attenuates myocardial ischemia-reperfusion injury in vitro by inhibiting NLRP3 Inflammasome activation. BMC Anesthesiol. 2021;21(1):104.
Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–35.
Eibl C, Hessenberger M, Wenger J, Brandstetter H. Structures of the NLRP14 pyrin domain reveal a conformational switch mechanism regulating its molecular interactions. Acta Crystallogr D Biol Crystallogr. 2014;70(Pt 7):2007–18.
Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20(13):3328.
Zheng Y, Xu L, Dong N, Li F. NLRP3 inflammasome: the rising star in cardiovascular diseases. Front Cardiovasc Med. 2022;20(9):927061.
Van Opdenbosch N, Lamkanfi M. Caspases in cell death, inflammation, and disease. Immunity. 2019;50(6):1352–64.
McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5(4):a008656.
Burdette BE, Esparza AN, Zhu H, Wang S. Gasdermin D in pyroptosis. Acta Pharm Sin B. 2021;11(9):2768–82.
Zalinger ZB, Elliott R, Weiss SR. Role of the inflammasome-related cytokines Il-1 and Il-18 during infection with murine coronavirus. J Neurovirol. 2017;23(6):845–54.
D’Oria R, Schipani R, Leonardini A, Natalicchio A, Perrini S, Cignarelli A, Laviola L, Giorgino F. The role of oxidative stress in cardiac disease: from physiological response to injury factor. Oxid Med Cell Longev. 2020;14(2020):5732956.
Su LJ, Zhang JH, Gomez H, Murugan R, Hong X, Xu D, Jiang F, Peng ZY. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med Cell Longev. 2019;13(2019):5080843.
Zhang H, Ye J, Wang X, Liu Z, Chen T, Gao J. Muscone inhibits the excessive inflammatory response in myocardial infarction by targeting TREM-1. Evid Based Complement Alternat Med. 2022;10(2022):9112479.
Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20(7):1126–67.
Azevedo PS, Polegato BF, Minicucci MF, Paiva SA, Zornoff LA. Cardiac remodeling: concepts, clinical impact, pathophysiological mechanisms and pharmacologic treatment. Arq Bras Cardiol. 2016;106(1):62–9.
Carbone F, Bonaventura A, Montecucco F. Neutrophil-related oxidants drive heart and brain remodeling after ischemia/reperfusion injury. Front Physiol. 2020;4(10):1587.
Liu Y, Zhang J, Zhang D, Yu P, Zhang J, Yu S. Research progress on the role of pyroptosis in myocardial ischemia-reperfusion injury. Cells. 2022;11(20):3271.
Xue T, Xue Y, Fang Y, Lu C, Fu Y, Lai Z, Qin X, Huang F, Zeng Z, Huang J. Exploring myocardial ischemia-reperfusion injury mechanism of cinnamon by network pharmacology, molecular docking, and experiment validation. Comput Math Methods Med. 2023;23(2023):1066057.
Wareham LK, Liddelow SA, Temple S, Benowitz LI, Di Polo A, Wellington C, Goldberg JL, He Z, Duan X, Bu G, Davis AA, Shekhar K, Torre A, Chan DC, Canto-Soler MV, Flanagan JG, Subramanian P, Rossi S, Brunner T, Bovenkamp DE, Calkins DJ. Solving neurodegeneration: common mechanisms and strategies for new treatments. Mol Neurodegener. 2022;17(1):23.
Shvedova M, Anfinogenova Y, Atochina-Vasserman EN, Schepetkin IA, Atochin DN. c-Jun N-terminal kinases (JNKs) in myocardial and cerebral ischemia/reperfusion injury. Front Pharmacol. 2018;5(9):715.
Wang J, Yang L, Rezaie AR, Li J. Activated protein C protects against myocardial ischemic/reperfusion injury through AMP-activated protein kinase signaling. J Thromb Haemost. 2011;9(7):1308–17.
Rovai ES, Alves T, Holzhausen M. Protease-activated receptor 1 as a potential therapeutic target for COVID-19. Exp Biol Med (Maywood). 2021;246(6):688–94.
Li Z, Yin M, Zhang H, Ni W, Pierce RW, Zhou HJ, Min W. BMX represses thrombin-PAR1-mediated endothelial permeability and vascular leakage during early sepsis. Circ Res. 2020;126(4):471–85.
Griffin JH, Zlokovic BV, Mosnier LO. Activated protein C: biased for translation. Blood. 2015;125(19):2898–907.
Heo Y, Jeon H, Namkung W. PAR4-mediated PI3K/Akt and RhoA/ROCK signaling pathways are essential for thrombin-induced morphological changes in MEG-01 cells. Int J Mol Sci. 2022;23(2):776.
Gurevich VV, Gurevich EV. Biased GPCR signaling: possible mechanisms and inherent limitations. Pharmacol Ther. 2020;211:107540.
Zhao P, Metcalf M, Bunnett NW. Biased signaling of protease-activated receptors. Front Endocrinol (Lausanne). 2014;13(5):67.
Raggi C, Diociaiuti M, Caracciolo G, Fratini F, Fantozzi L, Piccaro G, Fecchi K, Pizzi E, Marano G, Ciaffoni F, Bravo E, Fiani ML, Sargiacomo M. Caveolin-1 endows order in cholesterol-rich detergent resistant membranes. Biomolecules. 2019;9(7):287.
Griffin JH, Zlokovic BV, Mosnier LO. Activated protein C, protease activated receptor 1, and neuroprotection. Blood. 2018;132(2):159–69.
Heuberger DM, Schuepbach RA. Protease-activated receptors (PARs): mechanisms of action and potential therapeutic modulators in PAR-driven inflammatory diseases. Thromb J. 2019;29(17):4.
French SL, Hamilton JR. Protease-activated receptor 4: from structure to function and back again. Br J Pharmacol. 2016;173(20):2952–65.
Bouwens EA, Stavenuiter F, Mosnier LO. Mechanisms of anticoagulant and cytoprotective actions of the protein C pathway. J Thromb Haemost. 2013;11 Suppl 1(0 1):242–53.
Dellinger MT, Brekken RA. Phosphorylation of Akt and ERK1/2 is required for VEGF-A/VEGFR2-induced proliferation and migration of lymphatic endothelium. PLoS One. 2011;6(12):e28947.
Rezaie AR. Protease-activated receptor signalling by coagulation proteases in endothelial cells. Thromb Haemost. 2014;112(5):876–82.
Zhang YY, Ning BT. Signaling pathways and intervention therapies in sepsis. Signal Transduct Target Ther. 2021;6(1):407.
Pendurthi UR, Rao LVM. Endothelial cell protein C receptor-dependent signaling. Curr Opin Hematol. 2018;25(3):219–26.
Schuepbach RA, Madon J, Ender M, Galli P, Riewald M. Protease-activated receptor-1 cleaved at R46 mediates cytoprotective effects. J Thromb Haemost. 2012;10(8):1675–84.
Liang HC, Garces de Los Fayos Alonso I, Turner SD, Lagger S, Merkel O, Kenner L. The role of activator protein-1 (AP-1) family members in CD30-positive lymphomas. Cancers (Basel). 2018;10(4):93.
Cook-Mills JM, Marchese ME, Abdala-Valencia H. Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants. Antioxid Redox Signal. 2011;15(6):1607–38.
Nieman MT. Protease-activated receptors in hemostasis. Blood. 2016;128(2):169–77.
Soh UJ, Dores MR, Chen B, Trejo J. Signal transduction by protease-activated receptors. Br J Pharmacol. 2010;160(2):191–203.
Grimsey NJ, Trejo J. Integration of endothelial protease-activated receptor-1 inflammatory signaling by ubiquitin. Curr Opin Hematol. 2016;23(3):274–9.
Burnier L, Mosnier LO. Novel mechanisms for activated protein C cytoprotective activities involving noncanonical activation of protease-activated receptor 3. Blood. 2013;122(5):807–16.
Iriarte A, Figueras A, Cerdà P, Mora JM, Jucglà A, Penín R, Viñals F, Riera-Mestre A. PI3K (phosphatidylinositol 3-kinase) activation and endothelial cell proliferation in patients with hemorrhagic hereditary telangiectasia type 1. Cells. 2019;8(9):971.
Sidhu TS, French SL, Hamilton JR. Differential signaling by protease-activated receptors: implications for therapeutic targeting. Int J Mol Sci. 2014;15(4):6169–83.
Severino P, D’Amato A, Pucci M, Infusino F, Adamo F, Birtolo LI, Netti L, Montefusco G, Chimenti C, Lavalle C, Maestrini V, Mancone M, Chilian WM, Fedele F. Ischemic heart disease pathophysiology paradigms overview: from plaque activation to microvascular dysfunction. Int J Mol Sci. 2020;21(21):8118.
Patil KR, Mahajan UB, Unger BS, Goyal SN, Belemkar S, Surana SJ, Ojha S, Patil CR. Animal models of inflammation for screening of anti-inflammatory drugs: implications for the discovery and development of phytopharmaceuticals. Int J Mol Sci. 2019;20(18):4367.
Rocamora-Reverte L, Melzer FL, Würzner R, Weinberger B. The complex role of regulatory T cells in immunity and aging. Front Immunol. 2021;27(11):616949.
Linton MRF, Yancey PG, Davies SS, et al. The role of lipids and lipoproteins in atherosclerosis. [Updated 2019 Jan 3]. In: Feingold KR, Anawalt B, Blackman MR, et al., editors.
Vassiliou AG, Kotanidou A, Dimopoulou I, Orfanos SE. Endothelial damage in acute respiratory distress syndrome. Int J Mol Sci. 2020;21(22):8793.
Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. 2015;19(15):130.
Ayombil F, Petrillo T, Kim H, Camire RM. Regulation of factor V by the anticoagulant protease activated protein C: influence of the B-domain and TFPIα. J Biol Chem. 2022;298(11):102558.
Kheradmand E, Haghjooy-Javanmard S, Dehghani L, Saadatnia M. Polymorphisms at activated protein C cleavage sites of factor V: are they important in the absence of factor V Leiden? Iran J Neurol. 2017;16(1):30–3.
Schreuder M, Reitsma PH, Bos MHA. Blood coagulation factor Va’s key interactive residues and regions for prothrombinase assembly and prothrombin binding. J Thromb Haemost. 2019;17(8):1229–39.
Del Carmen S, Hapak SM, Ghosh S, Rothlin CV. Coagulopathies and inflammatory diseases: ‘…glimpse of a Snark.’ Curr Opin Immunol. 2018;55:44–53.
Cates C, Rousselle T, Wang J, Quan N, Wang L, Chen X, Yang L, Rezaie AR, Li J. Activated protein C protects against pressure overload-induced hypertrophy through AMPK signaling. Biochem Biophys Res Commun. 2018;495(4):2584–94.
Griffin JH, Fernández JA, Lyden PD, Zlokovic BV. Activated protein C promotes neuroprotection: mechanisms and translation to the clinic. Thromb Res. 2016;141 Suppl 2(Suppl 2):S62-4.
Abudureyimu M, Luo X, Wang X, Sowers JR, Wang W, Ge J, Ren J, Zhang Y. Heart failure with preserved ejection fraction (HFpEF) in type 2 diabetes mellitus: from pathophysiology to therapeutics. J Mol Cell Biol. 2022;14(5):mjac028.
Palevski D, Ben-David G, Weinberger Y, Haj Daood R, Fernández JA, Budnik I, Levy-Mendelovich S, Kenet G, Nisgav Y, Weinberger D, Griffin JH, Livnat T. 3K3A-activated protein C prevents microglia activation, inhibits NLRP3 inflammasome and limits ocular inflammation. Int J Mol Sci. 2022;23(22):14196.
Wu S, Zou MH. AMPK, Mitochondrial function, and cardiovascular disease. Int J Mol Sci. 2020;21(14):4987.
Zhang J, Huang L, Shi X, Yang L, Hua F, Ma J, Zhu W, Liu X, Xuan R, Shen Y, Liu J, Lai X, Yu P. Metformin protects against myocardial ischemia-reperfusion injury and cell pyroptosis via AMPK/NLRP3 inflammasome pathway. Aging (Albany NY). 2020;12(23):24270–87.
Toldo S, Mauro AG, Cutter Z, Abbate A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2018;315(6):H1553–68.
Nazir S, Gadi I, Al-Dabet MM, Elwakiel A, Kohli S, Ghosh S, et al. Cytoprotective activated protein C averts Nlrp3 inflammasome–induced ischemia-reperfusion injury via mTORC1 inhibition. Blood. 2017;130(24):2664–77.
Wu X, Iroegbu CD, Peng J, Guo J, Yang J, Fan C. Cell death and exosomes regulation after myocardial infarction and ischemia-reperfusion. Front Cell Dev Biol. 2021;9.
Schirone L, Forte M, D’Ambrosio L, Valenti V, Vecchio D, Schiavon S, et al. An overview of the molecular mechanisms associated with myocardial ischemic injury: state of the art and translational perspectives. Cells. 2022;11.
Suetomi T, Willeford A, Brand CS, Cho Y, Ross RS, Miyamoto S, et al. Inflammation and NLRP3 inflammasome activation initiated in response to pressure overload by Ca(2+)/calmodulin-dependent protein kinase II δ signaling in cardiomyocytes are essential for adverse cardiac remodeling. Circulation. 2018;138(22):2530–44.
Lafuse WP, Wozniak DJ, Rajaram MVS. Role of cardiac macrophages on cardiac inflammation, fibrosis and tissue repair. Cells. 2020;10(1):51.
Van Linthout S, Tschöpe C. Inflammation – cause or consequence of heart failure or both? Curr Heart Fail Rep. 2017;14(4):251–65.
Tschöpe C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18(3):169–93.
Yang F, Ye XJ, Chen MY, Li HC, Wang YF, Zhong MY, Zhong CS, Zeng B, Xu LH, He XH, Ouyang DY. Inhibition of NLRP3 inflammasome activation and pyroptosis in macrophages by taraxasterol is associated with its regulation on mTOR signaling. Front Immunol. 2021;12:632606.
Mosnier LO. Activated protein C in neuroprotection and malaria. Curr Opin Hematol. 2019;26(5):320–30.
Zhang Y, Ning B. Signaling pathways and intervention therapies in sepsis. Signal Transduct Target Ther. 2021;6(1):407.
Gandhi DM, Rosas R, Greve E, Kentala K, Diby NGD-R, Snyder VA, et al. The parmodulin NRD-21 is an allosteric inhibitor of PAR1 Gq signaling with improved anti-inflammatory activity and stability. Bioorganic Med Chem. 2019;27(17):3788–96.
De Ceunynck K, Peters CG, Jain A, Higgins SJ, Aisiku O, Fitch-Tewfik JL, Chaudhry SA, Dockendorff C, Parikh SM, Ingber DE, Flaumenhaft R. PAR1 agonists stimulate APC-like endothelial cytoprotection and confer resistance to thromboinflammatory injury. Proc Natl Acad Sci U S A. 2018;115(5):E982–91.
Bouyahya A, El Allam A, Aboulaghras S, Bakrim S, El Menyiy N, Alshahrani MM, Al Awadh AA, Benali T, Lee LH, El Omari N, Goh KW, Ming LC, Mubarak MS. Targeting mTOR as a cancer therapy: recent advances in natural bioactive compounds and immunotherapy. Cancers (Basel). 2022;14(22):5520.
Ruf W. New players in the sepsis-protective activated protein C pathway. J Clin Invest. 2010;120(9):3084–7.
Ikezoe T. Thrombomodulin/activated protein C system in septic disseminated intravascular coagulation. J Intensive Care. 2015;3(1):1.
Eliwan H, Omer M, McKenna E, Kelly LA, Nolan B, Regan I, Molloy EJ. Protein C pathway in paediatric and neonatal sepsis . In Frontiers in Pediatrics (Vol. 9), 2022.
Sinha RK, Wang Y, Zhao Z, Xu X, Burnier L, Gupta N, Fernández JA, Martin G, Kupriyanov S, Mosnier LO, Zlokovic BV, Griffin JH. PAR1 biased signaling is required for activated protein C in vivo benefits in sepsis and stroke. Blood. 2018;131(11):1163–71.
Bock F, Shahzad K, Vergnolle N, Isermann B. Activated protein C based therapeutic strategies in chronic diseases. Thromb Haemost. 2014;111(4):610–7.
Furugohri T, Sugiyama N, Morishima Y, et al. Antithrombin-independent thrombin inhibitors, but not direct factor Xa inhibitors, enhance thrombin generation in plasma through inhibition of thrombin-thrombomodulin-protein C system. Thromb Haemost. 2011;106:1076–83.
Furugohri T, Shiozaki Y, Muramatsu S, et al. Different antithrombotic properties of factor Xa inhibitor and thrombin inhibitor in rat thrombosis models. Eur J Pharmacol. 2005;514(35–42):73.
Lattenist L, Jansen MP, Teske G, Claessen N, Meijers JC, Rezaie AR, Esmon CT, Florquin S, Roelofs JJ. Activated protein C protects against renal ischaemia/reperfusion injury, independent of its anticoagulant properties. Thromb Haemost. 2016;116:124–33.
Liu C, Li Y. Propofol relieves inflammation in MIRI rats by inhibiting Rho/Rock signaling pathway. Eur Rev Med Pharmacol Sci. 2021;25(2):976–84.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
NA
Consent for Publication
NA.
Competing Interests
The authors declare no competing interests.
Additional information
Associate Editor Guoping Li oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Johri, N., Matreja, P.S., Agarwal, S. et al. Unraveling the Molecular Mechanisms of Activated Protein C (APC) in Mitigating Reperfusion Injury and Cardiac Ischemia: a Promising Avenue for Novel Therapeutic Interventions. J. of Cardiovasc. Trans. Res. 17, 345–355 (2024). https://doi.org/10.1007/s12265-023-10445-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-023-10445-y