Abstract
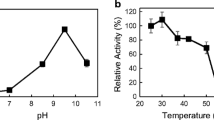
The efficient regeneration of nicotinamide cofactors is an important process for industrial applications because of their high cost and stoichiometric requirements. In this study, the FDH1 β-subunit of NAD-dependent formate dehydrogenase from Methylobacterium extorquens AM1 was heterologously expressed in Escherichia coli. It showed water-forming NADH oxidase (NOX-2) activity in the absence of its α-subunit. The β-subunit oxidized NADH and generated NAD+. The enzyme showed a low NADH oxidation activity (0.28 U/mg enzyme). To accelerate electron transfer from the enzyme to oxygen, four electron mediators were tested; flavin mononucleotide, flavin adenine dinucleotide, benzyl viologen (BV), and methyl viologen. All tested electron mediators increased enzyme activity; addition of 250 μM BV resulted in the largest increase in enzyme activity (9.98 U/mg enzyme; a 35.6-fold increase compared with that in the absence of an electron mediator). Without the aid of an electron mediator, the enzyme had a substrate-binding affinity for NADH (K m) of 5.87 μM, a turnover rate (k cat) of 0.24/sec, and a catalytic efficiency (k cat/K m) of 41.31/mM/sec. The addition of 50 μM BV resulted in a 22.75-fold higher turnover rate (k cat, 5.46/sec) and a 2.64-fold higher catalytic efficiency (k cat/K m, 107.75/mM/sec).
Similar content being viewed by others
References
Li, Z., Z. Shi, X. Li, L. Li, J. Zheng, and Z. Wang (2013) Evaluation of high butanol/acetone ratios in ABE fermentations with cassava by graph theory and NADH regeneration analysis. Biotechnol. Bioproc. Eng. 18: 759–769.
Bommarius, A. S., M. Schwarm, and K. Drauz (1998) Biocatalysis to amino acid-based chiral pharmaceuticals — Examples and perspectives. J. Mol. Catal. B: Enz. 5: 1–11.
Van Der Donk, W. A. and H. Zhao (2003) Recent developments in pyridine nucleotide regeneration. Curr. Opin. Biotechnol. 14: 421–426.
Berríos-Rivera, S. J., G. N. Bennett, and K.-Y. San (2002) Metabolic engineering of Escherichia coli: Increase of NADH availability by overexpressing an NAD+-dependent formate dehydrogenase. Metab. Eng. 4: 217–229.
Tishkov, V. I., A. G. Galkin, V. V. Fedorchuk, P. A. Savitsky, A. M. Rojkova, H. Gieren, and M.-R. Kula (1999) Pilot scale production and isolation of recombinant NAD+- and NADP+- specific formate dehydrogenases. Biotechnol. Bioeng. 64: 187–193.
Walcarius, A., R. Nasraoui, Z. Wang, F. Qu, V. Urbanova, M. Etienne, M. Göllü, A. S. Demir, J. Gajdzik, and R. Hempelmann (2011) Factors affecting the electrochemical regeneration of NADH by (2,2′-bipyridyl) (pentamethylcyclopentadienyl)-rhodium complexes: Impact on their immobilization onto electrode surfaces. Bioelectrochem. 82: 46–54.
Ali, I., B. Soomro, and S. Omanovic (2011) Electrochemical regeneration of NADH on a glassy carbon electrode surface: The influence of electrolysis potential. Electrochem. Commun. 13: 562–565.
Hollmann, F., A. Schmid, and E. Steckhan (2001) The first synthetic application of a monooxygenase employing indirect electrochemical NADH regeneration. Angew. Chem., Int. Ed. 40: 169–171.
Raj, S. M., C. Rathnasingh, W.-C. Jung, E. Selvakumar, and S. Park (2010) A Novel NAD+-dependent aldehyde dehydrogenase encoded by the puuC gene of Klebsiella pneumoniae DSM 2026 that utilizes 3-hydroxypropionaldehyde as a substrate. Biotechnol. Bioproc. Eng. 15: 131–138.
Riebel, B. R., P. R. Gibbs, W. B. Wellborn, and A. S. Bommarius (2003) Cofactor Regeneration of both NAD+ from NADH and NADP+ from NADPH:NADH Oxidase from Lactobacillus sanfranciscensis. Adv. Synth. Catal. 345: 707–712.
Raj, S. M., C. Rathnasingh, J.-E. Jo, and S. Park (2008) Production of 3-hydroxypropionic acid from glycerol by a novel recombinant Escherichia coli BL21 strain. Proc. Biochem. 43: 1440–1446.
Ashok, S., S. Mohan Raj, Y. Ko, M. Sankaranarayanan, S. Zhou, V. Kumar, and S. Park (2013) Effect of puuC overexpression and nitrate addition on glycerol metabolism and anaerobic 3-hydroxypropionic acid production in recombinant Klebsiella pneumoniae ΔglpKΔdhaT. Metab. Eng. 15: 10–24.
Wang, L., H. Chong, and R. Jiang (2012) Comparison of alkyl hydroperoxide reductase and two water-forming NADH oxidases from Bacillus cereus ATCC 14579. Appl. Microbiol. Biotechnol. 96: 1265–1273.
Yang, X. and K. Ma (2007) Characterization of an exceedingly active NADH oxidase from the anaerobic hyperthermophilic bacterium Thermotoga maritima. J. Bacteriol. 189: 3312–3317.
Lopez de Felipe, F. and J. Hugenholtz (2001) Purification and characterisation of the water forming NADH-oxidase from Lactococcus lactis. Int. Dairy. J. 11: 37–44.
Laukel, M., L. Chistoserdova, M. E. Lidstrom, and J. A. Vorholt (2003) The tungsten-containing formate dehydrogenase from Methylobacterium extorquens AM1: Purification and properties. Eur. J. Biochem. 270: 325–333.
Oh, J.-I. and B. Bowien (1998) Structural analysis of the fds operon encoding the NAD+-linked formate dehydrogenase of Ralstonia eutropha. J. Biol. Chem. 273: 26349–26360.
Deckert, G., P. V. Warren, T. Gaasterland, W. G. Young, A. L. Lenox, D. E. Graham, R. Overbeek, M. A. Snead, M. Keller, M. Aujay, R. Huber, R. A. Feldman, J. M. Short, G. J. Olsen, and R. V. Swanson (1998) The complete genome of the hyperthermophilic bacterium Aquifex aeolicus. Nature 392: 353–358.
Riebel, B. R., P. R. Gibbs, W. B. Wellborn, and A. S. Bommarius (2002) Cofactor regeneration of NAD+ from NADH: Novel water-forming NADH oxidases. Adv. Synth. Catal. 344: 1156–1168.
Xia, B., H. Cheng, V. Bandarian, G. H. Reed, and J. L. Markley (1996) Human ferredoxin: Overproduction in Escherichia coli, reconstitution in vitro, and spectroscopic studies of iron-sulfur cluster ligand cysteine-to-serine mutants. Biochem. 35: 9488–9495.
Wu, X., H. Kobori, I. Orita, C. Zhang, T. Imanaka, X.-H. Xing, and T. Fukui (2012) Application of a novel thermostable NAD(P)H oxidase from hyperthermophilic archaeon for the regeneration of both NAD+ and NADP+. Biotechnol. Bioeng. 109: 53–62.
Geueke, B., B. Riebel, and W. Hummel (2003) NADH oxidase from Lactobacillus brevis: A new catalyst for the regeneration of NAD. Enz. Microb. Technol. 32: 205–211.
Hummel, W. and B. Riebel (2003) Isolation and biochemical characterization of a new NADH oxidase from Lactobacillus brevis. Biotechnol. Lett. 25: 51–54.
Lowry, O. H., J. V. Passonneau, and M. K. Rock (1961) The stability of pyridine nucleotides. J. Biol. Chem. 236: 2756–2759.
Wong, C.-H. and G. M. Whitesides (1981) Enzyme-catalyzed organic synthesis: NAD(P)H cofactor regeneration by using glucose 6-phosphate and the glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides. J. Am. Chem. Soc. 103: 4890–4899.
Farrington, J. A., M. Ebert, and E. J. Land (1978) Bipyridylium quaternary salts and related compounds. Part 6. — Pulse radiolysis studies of the reaction of paraquat radical analogues with oxygen. J. Chem. Soc. Faraday Trans. 74: 655–675.
Monk, P. M. S., F. Delage, and S. M. Costa Vieira (2001) Electrochromic paper: Utility of electrochromes incorporated in paper. Electrochim. Acta 46: 2195–2202.
Hoogvliet, J. C., L. C. Lievense, C. Van Dijk, and C. Veeger (1988) Electron transfer between the hydrogenase from Desulfovibrio vulgaris (Hildenborough) and viologens. 1. Investigations by cyclic voltammetry. Eur. J. Biochem. 174: 273–280.
Frey, P. A. and A. D. Hegeman (2007) Enzymatic reaction mechanisms. pp. 158–162. Oxford University Press, New York, NY, USA.
Gao, H., M. K. Tiwari, Y. C. Kang, and J.-K. Lee (2012) Characterization of H2O-forming NADH oxidase from Streptococcus pyogenes and its application in l-rare sugar production. Bioorg. Med. Chem. Lett. 22: 1931–1935.
Zhang, Y.-W., M. K. Tiwari, H. Gao, S. S. Dhiman, M. Jeya, and J.-K. Lee (2012) Cloning and characterization of a thermostable H2O-forming NADH oxidase from Lactobacillus rhamnosus. Enz. Microb. Technol. 50: 255–262.
Rocha-Martín, J., D. Vega, J. M. Bolivar, C. A. Godoy, A. Hidalgo, J. Berenguer, J. M. Guisán, and F. López-Gallego (2011) New biotechnological perspectives of a NADH oxidase variant from Thermus thermophilus HB27 as NAD+-recycling enzyme. BMC Biotechnol. 11: 101.
Reda, T., C. M. Plugge, N. J. Abram, and J. Hirst (2008) Reversible interconversion of carbon dioxide and formate by an electroactive enzyme. Proc. Natl. Acad. Sci. U. S. A. 105: 10654–10658.
Axley, M. J., A. Bock, and T. C. Stadtman (1991) Catalytic properties of an Escherichia coli formate dehydrogenase mutant in which sulfur replaces selenium. Proc. Natl. Acad. Sci. U. S. A. 88: 8450–8454.
Schwede, T., J. Kopp, N. Guex, and M. C. Peitsch (2003) SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 31: 3381–3385.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choe, H., Lee, S., Hwang, H. et al. Expression of the NAD-dependent FDH1 β-subunit from Methylobacterium extorquens AM1 in Escherichia coli and its characterization. Biotechnol Bioproc E 19, 613–620 (2014). https://doi.org/10.1007/s12257-014-0126-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-014-0126-1