Abstract
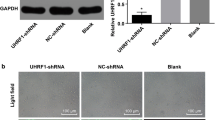
In order to better understand the role of HIF-1α in the proliferation of the retinoblastoma cells, a siRNA knockdown of HIF-1α followed by a proliferation assay was performed. Further sequencing was then carried out in order to assess knockdown efficiency and expression of HIF-1α. Upregulation of HIF-1α gene expression in CoCl2-treated retinoblastoma cells was demonstrated via melting curve analysis from PCR tests and was further analyzed using western blot and densitometry analysis. Reduction of HIF-1α expression in retinoblastoma, post HIF-1α knockdown, was observed after siRNA transfection into Y-79 cells. Knockdown of HIF-1α resulted in a significant decrease in proliferation thereby demonstrating that HIF-1α is involved in promoting survival and proliferation in retinoblastoma cells. Stabilization of HIF-1α in retinoblastoma cells using CoCl2 was unsuccessful.






Similar content being viewed by others
Notes
The MTT Assay
The tetrazolium salt (MTT) is reduced in metabolically active cells by a mitochondrial dehydrogenase to form insoluble purple formazan crystals, which are solubilized by the addition of an acidified isopropanol solution. The color can then be quantified by a spectrophotometer. Because tetrazolium salts are reduced only by metabolically active cells, this assay exclusively detects viable cells. Because proliferating cell are more metabolically active, absorbance values that are higher than the control cells indicate an increase in the rate of cell proliferation. Conversely, a lower absorbance value indicates a decrease in cell proliferation.
Relative and Comparative CT Methods
Relative quantitation (ΔCT) compares transcript abundance across multiple samples, using a co-amplified internal control (in our case, GAPDH) for sample normalization. Comparative quantitation (ΔΔCT) compares the CT values of the samples of interest (in our case, the knockdowns) with a control. The CT value is the cycle number at which fluorescence crosses the threshold (i.e. exceeds background level).
Densitometry
Densitometry analysis was performed using ImageJ, a java based imaging program developed at the National Institute of Health (http://rsbweb.nih.gov/ij/).
References
Arean C, Orellana ME, Abourbih D, Abreu C, Pifano I, Burnier MN Jr (2010) Expression of vascular endothelial growth factor in retinoblastoma. Arch Ophthalmol 128(2):223–229
Boutrid H, Jockovich ME, Murray TG, Pina Y, Feuer WJ, Lampidis TJ, Cebulla CM (2008) Targeting hypoxia, a novel treatment for advanced retinoblastoma. Investig Ophthalmol Vis Sci 49(7):2799–2805
Kunz M, Ibrahim SM (2003) Molecular responses to hypoxia in tumor cells. Mol Cancer 2:23
Mayes PA, Dolloff NG, Daniel CJ, Liu JJ, Hart LS, Kuribayashi K, Allen JE, Jee DI, Dorsey JF, Liu YY et al (2011) Overcoming hypoxia-induced apoptotic resistance through combinatorial inhibition of GSK-3beta and CDK1. Cancer Res 71(15):5265–5275
Hockel M, Vaupel P (2001) Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst 93(4):266–276
Piret JP, Mottet D, Raes M, Michiels C (2002) CoCl2, a chemical inducer of hypoxia-inducible factor-1, and hypoxia reduce apoptotic cell death in hepatoma cell line HepG2. Ann N Y Acad Sci 973:443–447
Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3(10):721–732
Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meir EG (2005) Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro-Oncology 7(2):134–153
Loboda A, Jozkowicz A, Dulak J (2010) HIF-1 and HIF-2 transcription factors—similar but not identical. Mol Cells 29(5):435–442
Mendez O, Zavadil J, Esencay M, Lukyanov Y, Santovasi D, Wang SC, Newcomb EW, Zagzag D (2010) Knock down of HIF-1alpha in glioma cells reduces migration in vitro and invasion in vivo and impairs their ability to form tumor spheres. Mol Cancer 9:133
Vaupel P, Kelleher DK, Hockel M (2001) Oxygen status of malignant tumors: pathogenesis of hypoxia and significance for tumor therapy. Semin Oncol 28(2 Suppl 8):29–35
Pina Y, Decatur C, Murray T, Houston S, Gologorsky D, Cavalcante M, Cavalcante L, Hernandez E, Celdran M, Feuer W et al (2011) Advanced retinoblastoma treatment: targeting hypoxia by inhibition of the mammalian target of rapamycin (mTOR) in LH(BETA)T(AG) retinal tumors. Clin Ophthalmol 5:337–343
Yuan Y, Hilliard G, Ferguson T, Millhorn DE (2003) Cobalt inhibits the interaction between hypoxia-inducible factor-alpha and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-alpha. J Biol Chem 278(18):15911–15916
Grasselli F, Basini G, Bussolati S, Bianco F (2005) Cobalt chloride, a hypoxia-mimicking agent, modulates redox status and functional parameters of cultured swine granulosa cells. Reprod Fertil Dev 17(7):715–720
Cikos S, Bukovska A, Koppel J (2007) Relative quantification of mRNA: comparison of methods currently used for real-time PCR data analysis. BMC Mol Biol 8
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25(4):402–408
Chang CI, Kim HA, Dua P, Kim S, Li CJ, Lee DK (2011) Structural diversity repertoire of gene silencing small interfering RNAs. Nucleic Acid Ther 21(3):125–131
Burr DB, Molina SA, Banerjee D, Low DM, Takemoto DJ (2011) Treatment with connexin 46 siRNA suppresses the growth of human Y79 retinoblastoma cell xenografts in vivo. Exp Eye Res 92(4):251–259
Manohar SM, Padgaonkar AA, Jalota-Badhwar A, Sonawane V, Rathos MJ, Kumar S, Joshi KS (2011) A novel inhibitor of hypoxia-inducible factor-1 alpha P3155 also modulates PI3K pathway and inhibits growth of prostate cancer cells. BMC Cancer 11
Kaelin WG (1999) Functions of the retinoblastoma protein. Bioessays 21(11):950–958
Wang GL, Semenza GL (1993) Desferrioxamine induces erythropoietin gene-expression and hypoxia-inducible factor-I DNA-binding activity—implications for models of hypoxia signal-transduction. Blood 82(12):3610–3615
Jantsch J, Wiese M, Schodel J, Castiglione K, Glasner J, Kolbe S, Mole D, Schleicher U, Eckardt KU, Hensel M et al (2011) Toll-like receptor activation and hypoxia use distinct signaling pathways to stabilize hypoxia-inducible factor 1 alpha (HIF1A) and result in differential HIF1A-dependent gene expression. J Leukoc Biol 90(3):551–562
Fletcher CDM (1995) Diagnostic histopathology of tumors. Churchill Livingstone, Edinburgh
Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, Ang KK, Story M, Le QT, Giaccia AJ (2009) Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell 35(6):856–867
Berra E, Milanini J, Richard DE, Le Gall M, Vinals F, Gothie E, Roux D, Pages G, Pouyssegur J (2000) Signaling angiogenesis via p42/p44 MAP kinase and hypoxia. Biochem Pharmacol 60(8):1171–1178
Mahon PC, Hirota K, Semenza GL (2001) FIH-1: a novel protein that interacts with HIF-1 alpha and VHL to mediate repression of HIF-1 transcriptional activity. Gene Dev 15(20):2675–2686
Wang GL, Semenza GL (1993) General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A 90(9):4304–4308
Berra E, Pages G, Pouyssegur J (2000) MAP kinases and hypoxia in the control of VEGF expression. Cancer Metastasis Rev 19(1–2):139–145
de Souza N (2007) Too much of a good thing. Nat Methods 4(5):386
Competing Interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernandes, B.F., Coates, J., Odashiro, A.N. et al. Hypoxia-Inducible Factor-1α and its Role in the Proliferation of Retinoblastoma Cells. Pathol. Oncol. Res. 20, 557–563 (2014). https://doi.org/10.1007/s12253-013-9728-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-013-9728-8