Abstract
As a consequence of anthropogenic activities and climate change, accelerated terrestrial sediment runoff is causing the gradual mudification of soft sediment estuarine habitats worldwide. Increased sediment mud content (< 63 µm) has been recognised to alter seagrass morphology and cause declines in primary production in unvegetated habitats. However, the effect of increased mud content on primary production in seagrass meadows remains largely unknown. To address this, primary production in intertidal seagrass meadows (Zostera muelleri) and adjacent unvegetated habitats was measured in situ using benthic incubation chambers across an existing sedimentary gradient (nine sites spanning 5–33% mud content). An additional two unvegetated mudflat sites (39–49% mud content) were also sampled to expand the gradient. Seagrass net (NPP) and gross primary production (GPP) was greater than in the adjacent unvegetated habitat and did not vary with mud content, even after standardising GPP by photosynthesising biomass (i.e. photosynthetic efficiency). In contrast, in the adjacent unvegetated habitat, photosynthetic efficiency declined with increasing mud content. Inclusion of the additional mudflat sites negatively impacted NPP, GPP, and photosynthetic efficiency in the unvegetated habitat. Thus, while primary production in seagrass meadows may have some resilience to future increases in mud content (up to ~33%), further degradation and loss of seagrass habitats could result in the expansion of unvegetated habitats and ultimately lead to production losses, likely to be most acute in areas with high mud content (≥ 39%).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Worldwide intertidal seagrass meadows are renowned as a highly productive coastal vegetation type that supports a broad array of ecosystem services (Duarte et al. 2010; Nordlund et al. 2016). Yet, in many areas, unvegetated tidal flats dominated by microphytobenthos (i.e. benthic microalgae), although generally less productive than seagrass meadows on a per area basis (Bahlmann et al. 2015; Drylie et al. 2018; Gustafsson and Norkko 2016), may make even greater contributions to coastal benthic primary production due to their wider extent. Due to multiple anthropogenic stressors, seagrass meadows are declining in many areas around the world, with an estimated global net loss of 19% (5602 km2) of surveyed meadow area since 1880 (Dunic et al. 2021; Waycott et al. 2009). As seagrass decline generally results in a subsequent shift to unvegetated sediment habitats, microphytobenthos are likely taking on an increasingly important role in supporting estuarine and coastal production. It is therefore important that we understand the relative contributions of both seagrass and unvegetated habitats to estuarine primary production and how they will respond to future environmental stressors.
While natural levels of terrestrial runoff provide coastal environments with an important source of sediment, organic matter, and nutrients, terrestrial sediment is now entering many coastal environments at accelerated rates. This is recognised as a widespread environmental problem affecting nearshore ecosystems (GESAMP 1993; Thrush et al. 2004), and is particularly prevalent throughout the Pacific Rim where a combination of geography, land use intensification and, more recently, climate change have increased coastal sediment loading (Lohrer et al. 2006; Milliman and Mei-e 1995; Rodriguez et al. 2020; Seneviratne et al. 2012; Thrush et al. 2004). For example, in many New Zealand estuaries, sediment deposition rates have increased by over an order of a magnitude over the past 200 years since European colonisation (e.g. Pakuranga Estuary, Auckland where rates have increased from ≤ 0.5 to 0.8–33 mm y−1) (Hunt 2019; Morrison et al. 2009; Swales et al. 2002). Terrestrial sediment inputs can contain a high proportion of fine silts and clays (< 63 µm), which can be continuously deposited and resuspended by currents and wind generated waves (Green and Coco 2014), causing an increase in water-column turbidity and a reduction in seafloor light availability (Anthony et al. 2004; Kirk 1985). In areas of low water flow, these fine sediments settle to the seafloor and can accumulate over time, leading to an increased sediment mud content (hereafter ‘mud content’) stressing benthic communities (Thrush et al. 2004).
Suspended sediments are a major controller of water-column light attenuation (i.e. turbidity; Anthony et al. 2004) and are therefore recognised as an important driver of benthic primary production in coastal environments (Lee et al. 2007; MacIntyre et al. 1996). The highest rates of productivity generally occur above light saturation levels (e.g. > ~200 µmol photons m−2 s−1 for temperate seagrass (calculated from whole plant review by Lee et al. (2007)), and microphytobenthos (reviewed by Mangan et al. (2020a))) and benthic primary producers can respond to changing light regimes through a combination of morphological, physiological and/or behavioural adaptations (Consalvey et al. 2004; Kohlmeier et al. 2014; Park et al. 2016). While the effects of changes in light climate on benthic primary production have received some attention, by comparison, changes in the sedimentary environment, which often accompanies elevated suspended sediments generated from terrestrial inputs, are much less understood.
When fine terrestrially derived sediments are deposited on predominantly sandy estuarine sediments, they alter the sediment physicochemical properties and benthic macrofaunal community composition. With increases in mud content, sediment permeability is reduced, and substrate surface area is increased (Huettel et al. 2014). This can affect the penetration of light into the sediment (Billerbeck et al. 2007), sediment redox potentials (Glud 2008), phytotoxin concentrations (e.g. hydrogen sulphide (Terrados et al. 1999)), and the transport and exchange of solutes across the sediment–water interface (Huettel et al. 2014, 2003). Additionally, changes in macrofaunal community composition with increasing mud content include altering key species size and abundance (e.g. the clam Austrovenus stutchburyi (Pratt et al. 2014a)), reducing macrofaunal diversity (e.g. Anderson 2008; Thrush et al. 2003), and modifying organism behaviour (e.g. feeding rates (McCartain et al. 2017) and larval recruitment (Thrush et al. 1996)). To understand how mud content may affect benthic primary production, it is therefore important to also consider the indirect effects generated by interactions with other ecosystem components (Thrush et al. 2021, 2014). For example, changes in macrofaunal biodiversity (which occurs with changing mud content) alters nutrient availability indirectly affecting primary production (e.g. Lohrer et al. 2004; Pratt et al. 2014a; Rodil et al. 2011). Thus, in situ studies incorporating these real-world interactions are needed.
For seagrass, changes in mud content have been recognised to alter their morphology and distribution. As mud content increases, the depth limits at which seagrass is found becomes shallower (Ferguson et al. 2016; Krause-Jensen et al. 2011). Moreover, mud content has been correlated with changes in biomass, increases in the ratio of above- to below-ground biomass, and increases in leaf length and area index (Ferguson et al. 2016; Halun et al. 2002; Terrados et al. 1998; Zabarte-Maeztu et al. 2021). Changes in seagrass growth with changing sediment physico-chemistry also appear to be species-specific (e.g. Livingston et al. 1998; Terrados et al. 1999). For the Zostera genus, higher mud content has been shown in Z. muelleri to reduce rhizome growth in laboratory mesocosms (Zabarte-Maeztu et al. 2021), and field manipulations have demonstrated that prolonged sediment anoxia (known to accompany increased mud content) can reduce Z. marina leaf growth (Terrados et al. 1999). However, the effect of mud content and changes in morphology on seagrass meadow production is unknown.
In unvegetated habitats dominated by microphytobenthos, increases in mud content are recognised to cause a decline in primary production (Billerbeck et al. 2007; Douglas et al. 2018; Pratt et al. 2014a). Reductions in photosynthetic efficiency (i.e. gross primary production (GPP) standardised by sediment chlorophyll a content) with increasing mud content were also observed in a study that spanned nine estuaries (Pratt et al. 2014a). However, most field studies examining the effect of mud content on microphytobenthic production have been restricted in terms of number of sites or treatments (e.g. Billerbeck et al. 2007; Haro et al. 2020; Kristensen et al. 1997). This small-scale approach limits the ability to determine rates of change as mud content increases. Additionally, previous studies have not incorporated multiple habitat types. As the sensitivity to anthropogenic stressors is likely to vary between primary producers, research investigating how different habitats may respond to accelerated terrestrial sediment inputs will be vital in informing future estuarine management decisions.
This study aims to investigate how changes in mud content may affect submerged primary production in intertidal seagrass meadows and adjacent unvegetated habitats. To assess this, we used an in situ correlative study design sampling across an existing spatial gradient in mud content within a single large estuary. Performing correlative studies in situ over environmental gradients can incorporate natural variability in environmental properties enhancing the generality of findings and the ability to extrapolate trends to larger scales (Hewitt et al. 2007). We measured whole community primary production using large-scale benthic incubation chambers, incorporating the real-world complexities of estuarine ecosystems. In response to changing sediment physicochemical conditions, based on the literature, we predict that increases in mud content will drive changes in seagrass morphology including increased leaf size and changes in the ratio of above- to below-ground biomass which may help maintain rates of primary production. We also predict, consistent with previous studies, that increasing mud content will cause declines in unvegetated primary production. By working across a gradient in mud content, our study will enable us to identify threshold responses in benthic primary production to mud content.
Methods
Study Site
Tauranga Harbour, located within the Bay of Plenty region of New Zealand, is a large (200 km2) barrier enclosed estuary that is predominantly intertidal (66% (de Lange and Healy 1990)). The estuary has a mean water depth of 2.1 m and experiences semi-diurnal tides with a spring-neap range of 1.24–1.62 m (Heath 1975). Within the intertidal regions of the estuary, two dominant habitat types include seagrass meadows (Z. muelleri, New Zealand’s sole seagrass species) and unvegetated habitats (i.e. sand-/mud-flats). Across New Zealand, seagrass meadows are mainly restricted to intertidal areas (Turner and Schwarz 2006), and in Tauranga Harbour have not been reported in sediments with > 35% mud content (Crawshaw 2020). From 1959 to 2019, seagrass distribution in Tauranga Harbour has declined by 73% (Ha et al. 2021; Park 2016). Long-term monitoring has additionally reported an increase in sedimentation in Tauranga Harbour at 59% of monitored sites (n = 65; Lawton and Conroy 2019), consistent with increases in mud content measured across multiple New Zealand North Island estuaries (Mills and Allen 2021).
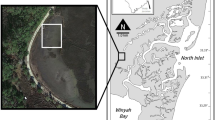
In Tauranga Harbour, nine mid-intertidal sites with seagrass meadows (> 200 m2) and nearby adjacent unvegetated flats were selected (Fig. 1). At these sites, a study area of approximately 50 m2 was established > 0.5 m either side of the seagrass fringe. Because the seagrass distribution was restricted to sites with ≤ 35% mud content, a further two sites were added to expand the unvegetated sand-mud gradient (Fig. 1). Within these sites, two study areas > 25 m apart (referred to as plots 1 and 2) with visually different sediment properties were chosen. All sites were located in the mid-intertidal with emersion periods of ~4–6 h and mean high tide water depths of 0.8–1.3 m (see Supplementary Table S1).
Location of Tauranga Harbour, New Zealand (insert), and the 11 intertidal sites. At nine sites, seagrass and adjacent unvegetated habitats were sampled, whereas URE and WMA (‘Mud’ sites) had no seagrass, so two unvegetated habitat plots (> 25 m apart) were sampled. See Supplementary Table S1 for site-specific GPS coordinates
Field Sampling
Field sampling was undertaken from January to mid-March 2019 (austral summer; see Supplementary Table S1), during mid-day high tides to ensure high levels of natural irradiance. Site habitats/plots were sampled on the same day, with the exception of TUA, where habitats were sampled on consecutive days due to equipment restrictions. Sampling was undertaken in generally sunny conditions with minimal cloud cover.
Within each habitat/plot, five pairs of light and dark benthic incubation chambers (0.25 m2; ~37 L) were positioned parallel to the seagrass fringe and/or the nearest channel. Chambers were deployed at low tide in undisturbed areas with a similar high seagrass percentage cover. Light and dark chambers were separated by ~ 0.5 m, with > 1 m between chamber pairs. Chamber bases (L:W:D = 0.5 × 0.5 × 0.15 m) were pushed 5 cm into the sediment and contained a PME miniDOT dissolved oxygen and temperature logger (1 min sampling frequency), a HOBO pendant light (Lux) and temperature logger (5 min sampling frequency), and a Sea-Bird Electronics pump (on for 5 s every 40–50 s; to provide consistent intermittent non-directional stirring of chamber water). Perspex transparent dome chamber lids were clamped onto the bases at ~0.3 m water depth, following complete air removal of the chamber. Each lid had a nylon tubing sampling port, with an open inlet on the opposing side to ensure the chamber volume remained constant upon sampling. For dark chambers, shade cloth clamped over the lid ensured full darkness. To account for water-column fluxes, three pairs of light and dark bottles (~1.3 L) were also filled with seawater and positioned near the seabed. For initial sampling, following flushing of sampling hoses, a 60 mL sample was extracted from each chamber using Leur-lock syringes. Three 60 mL ambient water-column samples were also collected from the surrounding area. After a ~4–5 h incubation period, another 60 mL sample was extracted from each chamber, alongside a sample collected from each light and dark bottle. The salinity and dissolved oxygen concentrations (mg/L) of the samples were measured immediately onshore using a YSI ProSolo ODO/CT probe.
Site Characteristics
To provide an indication of water-column light attenuation during incubations, light measurements (specifically photosynthetically active radiation (PAR)) were collected above the high tide mark and at the seafloor (referred to as PAR-Site and PAR-Seafloor, respectively) using calibrated Odyssey PAR loggers (integrated 5 min sampling frequency). A HOBO pendant light (Lux) and temperature logger (5 min sampling frequency) was additionally positioned on the seafloor and on shore.
On the day of chamber sampling, five sediment cores (2.6 cm diameter, 2 cm depth) were collected around each chamber, amalgamated into a single replicate per chamber, frozen and stored in the dark until further analysis. To assess macrofaunal community composition, a large core (13 cm diameter, 15 cm depth) was taken within 0.5 m of each chamber. The contents of each core were sieved on site over a 500 µm mesh with all material preserved in 70% isopropyl alcohol. Following chamber incubations, two replicates of an additional four sediment cores (2.6 cm diameter, 2 cm depth) were collected around each chamber pair to measure porewater dissolved inorganic nutrient concentrations. For an estimation of seagrass percentage cover, a plan view photograph of each seagrass chamber was taken. To analyse seagrass biomass and leaf characteristics, a large core (13 cm diameter, 15 cm depth) was also collected from within each chamber with the contents sieved over a 1 mm mesh. All seagrass material was stored flat in tin foil and frozen.
Laboratory Analysis
For the analysis of sediment properties (median grain size, and mud, organic, water, chlorophyll a, and phaeopigment content), sediment samples were thawed, homogenised, and divided into three subsamples. Median grain size and mud content (% < 63 µm) were measured using a Malvern Mastersizer 3000 (particle size range 0.05–2000 µm) following digestion of organic matter using 10% hydrogen peroxide. Water content was measured from samples dried to a constant weight at 60 °C, with organic content subsequently measured by weight loss on ignition from samples held at 550 °C for 4 h (Heiri et al. 2001). For chlorophyll a (measured as a proxy for microphytobenthos biomass) and phaeopigment content, freeze-dried sediment was steeped in 90% buffered acetone prior to fluorometric measurement (Turner 10-AU fluorometer) before and after acidification by hydrochloric acid (Arar and Collins 1997). Median grain size, and mud, water, and organic content were measured from dark chamber samples only (n = 5 per habitat/plot).
To process sediment porewater for nutrients, 4 mL of de-ionised water was added to each porewater sediment sample. Samples were then vortexed and centrifuged, with the supernatant porewater filtered (1.2 µm Whatman GF/C glass fibre filter) and stored at −20 °C. Sediment samples were dried at 60 °C for determination of porosity. Dissolved inorganic nutrient concentrations (NH4+-N, NO3−-N, NO2–N, and PO43−-P) of sediment porewater were measured using flow injection analysis following standard operating procedures (Lachat QuickChem 8500 series 2 FIA +). As NO3− & NO2− concentrations were close to detection limits, NOx concentrations were used for statistical analyses.
The adult abundance (shell length ≥ 10 mm) of two key bivalve species, Austrovenus stutchburyi and Macomona liliana (Sandwell et al. 2009; Thrush et al. 2006; Woodin et al. 2016), was recorded from each macrofaunal core. To provide a general plot assessment of macrofaunal community composition, three dark chamber macrofaunal cores (first, third, and fifth chamber pairs) from each plot were stained with Rose of Bengal, sorted and identified to the lowest taxonomic level practicable (usually species).
Seagrass percent cover was analysed using 100 point random count analysis (CPCe v4.1) using the classifications of live blades, dead blades, and unvegetated (with undefinable points removed; Kohler and Gill 2006). To assess seagrass morphological characteristics, thawed seagrass samples were separated into above- (leaves) and below-ground (sheath, rhizomes, roots) material. All seagrass leaves with visible sheath attachment were counted, and the length and width (mm) of 10 leaves selected at random (unbroken where possible) were measured. Above- and below-ground biomass was measured from samples dried at 60 °C to a constant weight. Seagrass carbon and nitrogen content (C:N ratio) was measured on an Elementar Vario EL cube CHN analyser using a subsample of the dried above- and below-ground biomass from the light chambers (n = 5 per plot).
Additional Environmental Data
To understand the spatial drivers of seagrass morphology, we investigated a suite of environmental variables including integrated measures of summer light availability and wind-wave exposure. In February 2020 (1 year after chamber sampling), as part of another study (J. Green, unpublished data), an Odyssey PAR logger (integrated 10 min sampling frequency) was positioned at each of the seagrass sites for 5 weeks to provide a relative measure of integrated light availability. Median seafloor light intensity during submergence and emergence was calculated using data 2 h either side of high and low tide during daylight hours; providing a measure of the ‘normal’ availability of light each seagrass meadow was exposed to. Due to equipment malfunction, an integrated measure of seafloor light data from the ATH site was not acquired; so for statistical analyses, the average of the median high and low tide light value from the remaining seagrass sites was used. This approach is consistent with the fact that the light level measured at this site during chamber sampling was similar to the median of all the other sites (Table 1). To determine if wind-wave exposure influenced seagrass morphology, a dimensionless wave exposure variable was calculated for each site (Eq. 1) using mean wind speed, wind direction percentage frequency, and fetch (i.e. distance from each site to nearest land) (Keddy 1982; Turner et al. 1999):
Wind speed records (2007 to 2017; 10 min sampling interval) were provided by the Bay of Plenty Regional Council from an environmental monitoring station in Mount Maunganui (sourced from: https://envdata.boprc.govt.nz/) and separated into 30° compass wind direction bins.
Data Analysis
Macrofaunal community composition was analysed using a number of univariate descriptors including the abundances of adult A. stutchburyi and M. liliana, number of organisms (excluding adult bivalves), number of species, and Shannon-Weiner diversity index (H’). Shannon-Weiner index was selected as a measure of species diversity as it accounts for both the abundance and evenness of taxa (Heip and Engels 1974) and was calculated using the ‘vegan’ package (Oksanen et al. 2022) in R Studio (R 4.2.1 (R Core Team 2023)).
Chamber fluxes of dissolved oxygen (µmol O2 m−2 h−1) were calculated from a 10 min average of dissolved oxygen readings from the PME miniDOT logger at the start and end of the incubation period. Water-column fluxes were generally < 5% of the chamber fluxes, so were ignored. Dark chambers provided a measure of sediment community respiration, with light chambers providing a measure net primary production (NPP). For each chamber pair, a measure of gross primary production (GPP) was calculated by correcting NPP for the sediment community respiration measured in the adjacent dark chamber. If a dark chamber failed (e.g. due to shade cloth lifting; n = 22/110)—indicated by a positive dissolved oxygen flux or detectable chamber light reading—an average of the remaining plot dark chambers was used to the calculate GPP. Due to the use of benthic chambers, NPP and GPP represent a whole community measure of primary production. Photosynthetic efficiency was estimated by standardising GPP by per unit of seagrass above-ground biomass or sediment chlorophyll a content (Drylie et al. 2018; Pratt et al. 2014a).
Statistical Analysis
Pearson correlation matrices between environmental variables were constructed using the ‘psych’ package (Revelle 2024) in R Studio (R 4.2.1 (R Core Team 2023)). Principal coordinates analysis (PCO) of seagrass traits (leaf count, length, width and surface area, total percentage cover, and above-, below-ground, and total biomass) was used to evaluate differences in seagrass morphological condition as a function of site. Vectors of seagrass morphological traits and site environmental variables (Pearson correlation coefficient ≥ 0.3) were overlain to illustrate which variables were correlated with the multivariate distribution of the sites.
Bivariate plots of measures of primary production, with sediment mud content as the dependent variable, were created and analysed with linear regressions using the ‘tidyverse’ package (Wickham et al. 2019) in R Studio (R 4.2.1 (R Core Team 2023)). To determine if measures of NPP, GPP, and sediment community respiration across the mud content gradient differed between adjacent habitats (seagrass and unvegetated), a one-way fixed factor PERMANCOVA was performed (9999 permutations; covariate = mud content). Where mud × habitat interactions were not significant, reduced models (i.e. without the interaction term) were run.
Distance-based linear models (DistLM) were used to identify environmental drivers of NPP, GPP, and photosynthetic efficiency for the seagrass and unvegetated habitats (which in this analysis included the additional mudflat sites). Environmental predictor variables (light, temperature, porewater nutrients, sediment properties, macrofaunal properties, seagrass above- and below-ground C:N ratios, and multivariate measures of seagrass morphology (derived from PCO axes 1 and 2)) were included in the model runs, except for where high co-linearity (Pearson’s r > 0.8) between variables occurred (Dormann et al. 2013). This resulted in the removal of the predictor that explained the least variability in the model. Lux measurements from the site and individual light chambers, rather than PAR-Site and PAR-Seafloor, were chosen as predictor variables in the DistLM analyses so that between chamber variability in light intensity could be accounted for. Marginal tests were performed to identify significant environmental predictors (p ≤ 0.1; 9999 permutations). Stepwise procedures (using corrected Akaike information criterion (AICc); Burnham and Anderson 2002) with mud content forced to be included first (regardless of significance in marginal tests) were then used to identify the best combination of predictor variables for the most parsimonious model fit. PCO, PERMANCOVA and DistLM analyses were performed using the PERMANOVA + package on Primer v7 (Anderson et al. 2008; Clarke and Gorley 2015) using normalized data and Euclidean distance-based matrices.
Results
Environmental Variables
Across all study sites, a sediment mud gradient of 5.3–32.8% in seagrass (n = 9) and 5.0–49.0% in unvegetated flats (n = 13) was achieved (Table 1). At most sites (the exception being BRW & TUA), the seagrass habitat was on average 6.3% higher in mud content than the adjacent unvegetated habitat (n = 7). Median grain sizes (MGS) ranged from very fine to medium sand (113–251 µm) in the seagrass and from very fine to fine sand (65–248 µm) in the unvegetated habitat. In both the seagrass and unvegetated habitats, increasing mud content was correlated with decreasing median grain size (r = −0.62 and −0.88, p < 0.0001, n = 45 and 65, respectively) and increasing water and organic content (r = 0.68–0.90, p < 0.0001, n = 45 and 65 for seagrass and unvegetated habitats, respectively; see Supplementary Tables S2 and S3). For the porewater nutrient concentrations, only ammonium in the unvegetated habitat was correlated with increasing mud content (r = 0.91, p < 0.0001, n = 13; see Supplementary Table S4).
At each site, all macrofaunal variables (adult bivalve (A. stutchburyi and M. liliana) counts, number of species, number of organisms, and Shannon diversity index) were generally similar between adjacent seagrass and unvegetated habitats (see Supplementary Table S1). The number of species and number of organisms were however higher (by ≥ 1.6 times) in the adjacent unvegetated habitat sites when compared to the mudflat sites. In the seagrass habitat, increasing mud content was correlated with decreasing A. stutchburyi count and Shannon diversity (r = −0.38 and −0.49, p = 0.01, n = 45 and 27, respectively), and increasing M. liliana count (r = 0.36, p = 0.01, n = 45; see Supplementary Tables S2 and S3). Meanwhile, all macrofaunal variables showed negative correlations with increasing mud content in the unvegetated habitats (r = −0.53 to −0.76; p < 0.0001, n = 39–65).
Site light conditions (daily and those integrated over a 5 week period) were variable due to short-term changes in weather conditions and surrounding site geography (Table 1). Sampling day and integrated high tide seafloor light availability varied between sites (by 563 and 136 µmol photons m−2 s−1, respectively), and showed moderate (but not significant (p > 0.05)) negative correlations with increasing mud content in the seagrass (r = −0.61 and −0.55, p = 0.08 and 0.12, respectively, n = 9) and unvegetated habitat (r = −0.35 and −0.61, p = 0.24 and 0.08, n = 13 and 9, respectively; see Supplementary Table S4). During chamber incubations, the water-column attenuated between 58 and 93% of ambient light (Site vs. Seafloor PAR). In the seagrass habitat, water-column light attenuation was positively correlated with mud content (r = 0.69, p = 0.04, n = 9), but this relationship was not significant in the unvegetated habitat (r = 0.51, p = 0.07, n = 13; see Supplementary Table S4). In both the seagrass and unvegetated habitats, water-column light attenuation was not significantly correlated with mean high tide water depth (r = 0.27 and 0.20, p = 0.48 and 0.51, n = 9 and 13, respectively).
Primary Producer Biomass and Seagrass Morphology
Sediment chlorophyll a (i.e. an estimate of microphytobenthos biomass) and phaeopigment contents were higher in the seagrass compared to the adjacent unvegetated habitat (by 8–41%), except at TPU where the reverse was true (by 27%; Table 1). Both chlorophyll a and phaeopigment content increased with mud content in the seagrass (r = 0.49 and 0.75, p = 0.0006 and < 0.0001, respectively, n = 45) and unvegetated habitats (r = 0.50 and 0.71, respectively, p < 0.0001, n = 65; see Supplementary Tables S2 and S3). Only minor variations in chlorophyll a content (< 2 µg g−1 DW) were evident between the two plots within the mudflat sites.
Variations in seagrass biomass were evident across the sites with a range of 26–90 and 60–192 g DW m−2 for above- and below-ground biomass, respectively (Table 1). Seagrass below-ground biomass decreased with increasing mud content (r = −0.35, p = 0.02, n = 45), but above-ground and total biomass were not correlated with mud content (p = 0.48 and 0.20, respectively, n = 45; see Supplementary Tables S2 and S3). The ratio of above- to below-ground seagrass biomass ranged from 0.4 to 1.0 and increased with mud content (r = 0.58, p < 0.0001, n = 45) as did all metrics of leaf size (width, length, and surface area; r = 0.34–0.53, p = 0.0002–0.02, n = 45).
The principal coordinates analysis (PCO) of multivariate seagrass morphology illustrates overlap across multiple sites (Fig. 2). The first PCO axis explained 46.6% of the variability in seagrass morphology, with biomass metrics (above-, below-ground, and total) and total seagrass cover being the main variables correlated with this axis (Fig. 2a). Comparatively, seagrass leaf dimension parameters (surface area, width, and length) and the ratio of above- to below-ground biomass appear to be correlated with the second PCO axis which explained 33.9% of the variability. When environmental variables were overlaid on the PCO, the first axis was most correlated to the median low tide light (r = 0.41; Fig. 2b). Conversely, sedimentary variables (mud, organic, and phaeopigment content) and exposure were more correlated with the second axis. Notably, mud content was the environmental variable most strongly correlated to the second PCO axis (r = −0.45, n = 45) and appears positively correlated with leaf width and the ratio of above- to below-ground biomass (Fig. 2b). Since together the first two axes of the seagrass morphology PCO explained 80.5% of the total variability, the first and second PCO axis scores were used as metrics for seagrass morphological condition (referred to as SG cond. 1 and 2) in the DistLM analyses.
Principal coordinate analysis (PCO; Euclidean distance) of multivariate seagrass morphology as a function of site (n = 10 per site). Symbols indicative of site locations presented in Fig. 1. Vector overlay presents a seagrass morphological traits and b environmental variables (n = 1–10 per site; only variables with Pearson’s correlation (r) ≥ 0.3 with either axis presented (circle limits r = 1)). Vector abbreviations are AGB- seagrass above-ground biomass (g DW m−2); BGB, seagrass below-ground biomass (g DW m−2); TB, seagrass total biomass (g DW m−2); Cover, total seagrass percentage cover (%); Count, blade count (# core−1); Width, blade width (mm); Length, blade length (mm); SA, blade surface area (mm2); Mud, sediment mud content (% < 63 µm); WC, sediment water content (%); OC, sediment organic content (%); Chl-a, sediment chlorophyll a content (µg g−1 DW); Phaeo, sediment phaeopigment content (µg g−1 DW); LT, median low tide PAR integrated over a 5 week period (µmol photons m−2 s−1); Exposure, mean wind-wave exposure
Variations in Primary Production
Net (NPP) and gross primary production (GPP) in both the seagrass and adjacent unvegetated habitats varied across the nine sites, but there was no significant interaction between mud content and habitat type (p-perm ≥ 0.66, n = 45; Fig. 3a and b, Table 2). These variables also had no significant relationship with mud content (p-perm ≥ 0.59, n = 45). Overall, NPP and GPP was higher in the seagrass compared to the adjacent unvegetated habitat (p-perm = 0.0001, n = 45). Specifically, on average, GPP was two times higher in the seagrass compared to the adjacent unvegetated habitat (6482 vs. 3209 μmol O2 m−2−h−1). For the unvegetated habitat, with the inclusion of mudflat sites/plots (extending the sedimentary gradient past the point where seagrass can thrive), NPP and GPP both decreased with increasing mud content (r = −0.71 and −0.70, respectively, p = 0.007, n = 13; Fig. 3a and b). On average, during chamber incubations, all seagrass sites exhibited positive NPP (i.e. net production of O2 in light chambers), while two of the adjacent unvegetated sites (BRW & ONG) and three mudflat plots (URE-plot 1 and WMA-plot 1 and 2) exhibited negative NPP (i.e. net consumption of O2 in light chambers; Fig. 3a).
Variation in sediment mud content (% < 0.63 µm) and measures of primary production in seagrass (black) and unvegetated sediment (grey) habitats: a net primary production (NPP); b gross primary production (GPP); and c photosynthetic efficiency (GPPSG- seagrass above-ground biomass standardised GPP (seagrass habitat); GPPChl-a- chlorophyll a biomass standardised GPP (unvegetated habitat)). Data represent the site average (n = 5) ± 1 SE, and symbols are indicative of site locations presented in Fig. 1. Trendlines fitted represent significant linear correlations (Pearson’s r = −0.70 to −0.83, p = 0.0005–0.007, n = 9–13). There were no significant relationships in the seagrass habitat nor in the unvegetated habitat when the ‘mud’ only sites were excluded (p = 0.34–0.65, n = 9), except for chlorophyll a biomass standardised productivity (c). See Supplementary Fig. S1 for sediment community respiration
In the seagrass habitat, site averages of photosynthetic efficiency (i.e. GPP standardised for seagrass above-ground biomass) did not significantly vary with mud content (p = 0.34, n = 9; Fig. 3c). Conversely, in the unvegetated habitat, photosynthetic efficiency (i.e. GPP standardised for chlorophyll a content) decreased with increasing mud content for both the adjacent and extended (i.e. mudflat plots included) unvegetated habitat datasets (r = −0.83, p = 0.006 and 0.0005, n = 9 and 13, respectively).
Environmental Predictors of Primary Production
Aside from mud content in the unvegetated habitat, in marginal tests, several environmental variables were independently correlated with NPP, GPP, and photosynthetic efficiency both in the seagrass (n = 9) and unvegetated habitats (n = 13; see Supplementary Table S6). Marginal tests indicated that chamber light (Lux) was a strong predictor of NPP and GPP for both seagrass and unvegetated habitats, as well as for photosynthetic efficiency in the unvegetated habitat (explaining 37–61% of the variance; p = 0.0001, n = 45–65). For the seagrass habitat, the first seagrass morphology PCO axis (Fig. 2) was also reasonably well correlated with NPP and GPP (explaining 22 and 30%; p = 0.001 and 0.0001, respectively, n = 45). Meanwhile, the first and second seagrass morphology PCO axes explained, respectively, 8 and 26% (p = 0.06 and 0.0006, n = 45) of the variability in photosynthetic efficiency in the seagrass habitat.
In stepwise models, 54–80% of the variability in primary production (NPP, GPP, and photosynthetic efficiency) in the seagrass and unvegetated habitats was explained by environmental predictors (Table 3). The most influential predictor of each primary production response variable differed between habitat types. In the unvegetated habitat, mud content was the environmental variable explaining the greatest amount of variability for NPP, GPP, and photosynthetic efficiency (36–54%). In contrast, porewater phosphate concentration was the single greatest contributor to the total explained variance in the seagrass habitat NPP (44%), whereas for GPP, chamber light was the greatest contributor (42%; chamber light also contributed 25% to the unvegetated habitat GPP). Temperature contributed an additional 12% to the variability in seagrass GPP. In comparison, seagrass morphology PCO axis 2 (Fig. 2) explained 21% of the variability in photosynthetic efficiency in the seagrass habitat with M. liliana count contributing a further 18%.
Discussion
Anthropogenic activities and climate change are accelerating the input of terrestrial sediment into coastal waterways, causing estuarine soft sediment habitats to become muddier (Seneviratne et al. 2012; Thrush et al. 2004). While this process occurs gradually over years to decades, by sampling across an existing gradient in sediment mud content, this study provides a comparable in situ assessment of how primary production in two key soft sediment habitats (seagrass meadows and unvegetated sediments) may alter with future increases in sedimentation. Overall, we found that seagrass meadows per unit area were more productive than the adjacent unvegetated habitats (likely owing to a greater photosynthesising biomass); consistent with the findings of previous studies (Bahlmann et al. 2015; Drylie et al. 2018; Flowers et al. 2023; Gustafsson and Norkko 2016). However, if seagrass meadow degradation and losses continue to occur (Dunic et al. 2021; Waycott et al. 2009), our results indicate that the shift to a greater estuary-wide area of unvegetated habitat could result in a reduction of spatially integrated summertime intertidal primary production. Furthermore, the significant reduction in unvegetated primary production as mud content increased indicates that this impact may be exacerbated in muddier conditions.
Unlike seagrass, which had higher rates of primary production and thereby net primary production (NPP) remained positive during incubations, five of the unvegetated plots had negative NPP. In order to evaluate net trophic states over a daily period (assuming 12 h of light/darkness and constant submersion), we calculated photosynthesis to respiration ratios in the two habitats (Eyre and Ferguson 2002). More than half (5/9) of our seagrass sites exhibited net autotrophy (i.e. photosynthesis to respiration ratio > 1), while all unvegetated sites exhibited net heterotrophy (i.e. photosynthesis to respiration ratio < 1; see Supplementary Fig. S2). While this calculation overestimates gross primary production (GPP; as it does not account for production during emergence (Drylie et al. 2018; Flowers et al. 2023; Migné et al. 2018; Ouisse et al. 2011) or potential reduced light levels at different times of the day), these photosynthesis to respiration ratios highlight the potential shift to a more heterotrophic system if the areal extent of seagrass meadows in Tauranga Harbour continue to decline. As a system shifts from autotrophic to heterotrophic, rather than remineralised inorganic nutrients being assimilated by primary producers at the sediment–water interface, they can be released in greater amounts into the overlying water-column (Eyre and Ferguson 2002). A shift in pelagic nutrient availability with increased seafloor heterotrophy is likely to have cascading consequences for coastal ecosystems through increasing water-column algae concentrations (Vitousek et al. 1997), ultimately heightening the risk of an estuary shifting to a more eutrophic state (e.g. Cooper and Brush 1993; Munkes 2005).
Our measurements of submerged primary production and differences between intertidal seagrass and unvegetated sediments are within the range previously measured in New Zealand (e.g. Drylie et al. 2018; Lohrer et al. 2016; Mangan et al. 2020b; Pratt et al. 2014a). Although less productive on a per area basis, when scaled across the intertidal regions of Tauranga Harbour, unvegetated habitats are almost five times more extensive in area than seagrass meadows (6609 vs. 1361 ha, respectively (Shao et al. 2024)). When scaling production rates (using an average GPP across all sites), based on the area occupied by each habitat within Tauranga Harbour, the importance of unvegetated production rates becomes clear: owing to the much larger area occupied, these unvegetated intertidal flats contribute two times more GPP per hour during submergence in austral summer than seagrass meadows (~178,400 vs. 88,200 mol O2 h−1, respectively). Although these calculations do not account for temporal variability in intertidal productivity (e.g. Flowers et al. 2023), by taking productivity measurements from ≥ 9 sites across a single estuary, they incorporate variability in production across a wide spatial scale. While unvegetated habitats clearly play a significant role in estuarine productivity, as seagrass are more productive per unit area, these simple estimations highlight the importance of future management and restoration of seagrass meadows. Restoration actions will also increase the additional ecosystem services provided by seagrass meadows including fine sediment deposition (Heiss et al. 2000), nutrient regeneration (Eyre et al. 2011), and high rates of carbon sequestration (Duarte et al. 2005b; Mcleod et al. 2011).
In the seagrass habitats, NPP, GPP, and photosynthetic efficiency (i.e. above-ground biomass standardised GPP) were not affected by changes in mud content. However, variability in Z. muelleri morphology was evident across the seagrass sites. Additionally, seagrass morphological condition (based on PCO2 axis coordinates, Fig. 2) was identified as the main contributor to seagrass photosynthetic efficiency in the stepwise DistLM model. Consistent with the findings of Ferguson et al. (2016), with increasing mud content, the ratio of above- to below-ground seagrass biomass increased. This was driven by a reduction in below-ground biomass which has high respiratory demands. When light availability is not limiting production rates, to optimise sediment oxygen concentrations (which are reduced with increasing mud content (Glud 2008; Huettel et al. 2014)), seagrass can divert some of their oxygen production to be released from their roots (Enríquez et al. 2001; Sand-Jensen et al. 1982). This sub-surface oxygenation may improve the availability of nutrients and reduce the accumulation of phytotoxins (Brodersen et al. 2015; reviewed by Duarte et al. 2005a); factors that could otherwise limit primary production.
Sediment anoxia and phytotoxins such as hydrogen sulphide are known to increase with mud content and have been shown to stunt seagrass growth (Glud 2008; Kilminster et al. 2008; Terrados et al. 1999). In Tauranga Harbour, reports of Z. muelleri distribution (and thereby meadow productivity) are limited to areas ≤ 35% mud content (Crawshaw 2020). This is consistent with previous reports of Zostera distributions (e.g. Moksnes et al. 2018; Short 1987; Wendländer et al. 2020; Zabarte-Maeztu et al. 2020), with only a few exceptions (e.g. up to 72% (Edgar and Shaw 1995)). The trade-off for Z. muelleri to prioritise maintaining above-ground biomass may have enabled sustained rates of productivity in sub-optimal conditions. However, by losing below-ground biomass, this may come at the cost of reducing the aerobic sediment microbiome, in areas where mud content is high. Thus, in sediments > 35% mud content, the ability for sub-surface oxygenation may be limited and could be potentially contributing to the restriction of seagrass distribution.
In the unvegetated habitat, the inclusion of mudflat sites (≥ 39% mud content) drove significant declines in NPP and GPP with increasing mud content. This differed to the findings of Douglas et al. (2018) and Pratt et al. (2014a) who found significant decreases in GPP with increasing mud in gradients ≤ 30% mud content. This disparity could be due to differences in the spatial scales of these previous studies; Douglas et al. (2018) sampled 12 locations at a single site, while Pratt et al. (2014a) incorporated data from 18 sites across nine estuaries. Additionally, the sites of these studies may differ in other environmental and/or ecological characteristics (e.g. macrofaunal community composition (Lohrer et al. 2016; Sandwell et al. 2009) and water-column turbidity (Drylie et al. 2018; Mangan et al. 2020b; Pratt et al. 2014b)), which could contribute to changes in productivity rates. In this study, sediment chlorophyll a content increased with increasing mud content. Previously, microphytobenthos have also been shown to adapt to increased mud content by shifting from a predominantly sedentary (episammic) to vertically migrating (epipelic) diatom species (Consalvey et al. 2004). This shift in community composition and increase in photosynthetic biomass could have enabled rates of primary production to be maintained in sediments with up to 35% mud content (i.e. adjacent unvegetated sites). Although, consistent with previous studies (Douglas et al. 2018; Pratt et al. 2014a; Thomas et al. 2022), photosynthetic efficiency (i.e. GPP standardised by sediment chlorophyll a content) declined with increasing mud across the adjacent unvegetated sites both with and without the inclusion of mudflat sites. This relationship may also have contributed to the significant decline observed in NPP and GPP with the inclusion of mudflat sites.
Light availability was identified as a strong driver of primary production in both of the soft sediment habitats examined. Notably, in the seagrass habitat, chamber light was the greatest contributor to the total variance in GPP in the stepwise models and contributed to stepwise models of NPP, GPP, and photosynthetic efficiency in the unvegetated habitat. Seafloor light availability during chamber incubations for each site was however generally above, or approximately equal to, the submerged photosynthetic saturating irradiance estimated for seagrass and unvegetated habitats at the TUA site (192 and 258 µmol photons m−2 s−1, respectively (Flowers et al. 2023)). Only at WPA was seafloor light availability below (by > 50 µmol photons m−2 s−1) the TUA saturating irradiance in both habitats, but the site still had a net production of oxygen in the light chambers (i.e. positive NPP). However, the median daytime incident light levels collected over a 5 week period was below saturating irradiance for two of the seagrass and five of the adjacent unvegetated habitat sites (by 31–44 and 22–110 µmol photons m−2 s−1, respectively). This indicates that some of our sites may regularly experience light limitations which could impact rates of productivity. Although as benthic primary producers can undertake adaptations to optimise light harvesting (e.g. changes in seagrass pigments (Kohlmeier et al. 2014) and/or microphytobenthos community composition (Consalvey et al. 2004)), these light requirements are site-specific (Lee et al. 2007). Nevertheless, this strong influence of light supports previous findings highlighting that increased water-column turbidity, namely through increased terrestrial sediment inputs (Kirk 1985; Thrush et al. 2004), will negatively affect benthic primary production of soft sediment habitats (Drylie et al. 2018; Mangan et al. 2020b; Pratt et al. 2014b).
Water-column light attenuation (i.e. Site vs. Seafloor PAR) increased with increasing mud content in the seagrass habitat, although this was not statistically significant in the unvegetated habitat. Similarly, while not significant, moderate negative correlations were also found between seafloor light (sampling day and integrated long-term) and mud content in both habitats. These correlations indicate that we are unable to completely separate the effects of mud content and light availability in this study. Additionally, the coupled effect of mud content and light availability appears to alter seagrass morphology and distribution. With increasing mud content, we found an increase in seagrass leaf size (length, width, and surface area). A similar response was identified in Z. muelleri by Ferguson et al. (2016) and Cymodocea rotundata (a tropical seagrass species) by Halun et al. (2002), whereby greater leaf lengths were associated with sediments with higher mud content. As increases in leaf size has also been correlated with reduced light availability (Ralph et al. 2007), this morphological adaptation is suggested to increase the light harvesting area for photosynthesis. Additionally, in muddier sediments, Ferguson et al. (2016) and Krause-Jensen et al. (2011) identified reductions in the depth limits of Z. marina and Z. muelleri, suggesting that higher mud content increases the minimum light required for seagrass. As sea level is predicted to rise by 1.4 m by 2100 (Rahmstorf 2007), future increases in sedimentation could therefore have substantial effects on the distribution of coastal seagrass meadows.
Rising sea level and an increase in the frequency of severe weather events predicted with climate change will further increase terrestrial runoff and sediment resuspension (Seneviratne et al. 2012; Thrush et al. 2004). In addition to sediment, terrestrial runoff can also increase estuarine inputs of heavy metals and nutrients (driving estuarine eutrophication), which have been recognised to influence benthic macrofaunal community composition (e.g. Sánchez-Moyano et al. 2010; Schmidt et al. 2017) and/or reduce benthic primary production (e.g. via increased pelagic production (Krause-Jensen et al. 2012; Stutes et al. 2006)). As this suggests, environmental stressors often occur simultaneously, and the interaction of multiple stressors may have antagonistic, additive or synergistic effects on coastal primary production (Folt et al. 1999). In this study, we were able to incorporate a gradient in mud content across two soft sediment habitats, but this was not coupled with a gradient in eutrophication. Across a mud content gradient in an unvegetated habitat, sediment nutrient enrichment was identified by Douglas et al. (2018) to counteract the negative effect of mud content on GPP. Additionally, the biomass of seagrass has been found to influence their resilience to the effects of nutrient enrichment (Gladstone-Gallagher et al. 2018). These examples highlight the complex ways in which anthropogenic stressors may influence soft sediment ecosystems and warrants future research.
Increases in the mud content of soft sediment habitats have been reported in many coastal areas, particularly throughout the Pacific Rim (Lohrer et al. 2006; Milliman and Mei-e 1995; Rodriguez et al. 2020; Thrush et al. 2004), and without changes in land-use practices to reduce coastal inputs of terrestrial sediments, this trend is likely to continue. In order to ensure effective management of anthropogenic stressors, future research investigating how changes in mud content can influence the mechanisms and processes that drive soft sediment ecosystem functions will be crucial. Our in situ study highlights the potential resilience of primary production in seagrass meadows and unvegetated habitats within 0–35% mud content range. However, in Tauranga Harbour, intertidal flats with mud contents exceeding 35% appear uninhabited by Z. muelleri, and in the unvegetated habitat, declines in primary production were driven by sites with ≥ 39% mud content. Thus, the results of this study indicate that, if anthropogenic stressors continue to drive seagrass decline, the loss of production due to habitat shifts is likely to be exacerbated in areas with high mud content. Regional management of terrestrial sediment runoff is therefore going to be vital to reduce future declines of estuarine production via habitat degradation and/or loss, especially in the face of global climate change.
Data Availability
Data is available in article Supplementary Information and raw data is available on request from the authors.
References
Anderson, M.J. 2008. Animal-sediment relationships re-visited: Characterising species’ distributions along an environmental gradient using canonical analysis and quantile regression splines. Journal of Experimental Marine Biology and Ecology 366: 16–27. https://doi.org/10.1016/j.jembe.2008.07.006.
Anderson, M.J., R.N. Gorley, and K.R. Clarke. 2008. PERMANOVA+ for PRIMER: Guide to software and statistical methods. Plymouth, UK: PRIMER-E.
Anthony, K.R.N., P.V. Ridd, A.R. Orpin, P. Larcombe, and J. Lough. 2004. Temporal variation of light availability in coastal benthic habitats: Effects of clouds, turbidity, and tides. Limnology and Oceanography 49: 2201–2211. https://doi.org/10.4319/lo.2004.49.6.2201.
Arar, E. J., and G. B. Collins. 1997. Method 445.0: In vitro determination of chlorophyll a and pheophytin a in marine and freshwater algae by fluorescence. Cincinnati, OH: U.S. Environmental Protection Agency.
Bahlmann, E., I. Weinberg, J.V. Lavrič, T. Eckhardt, W. Michaelis, R. Santos, and R. Seifert. 2015. Tidal controls on trace gas dynamics in a seagrass meadow of the Ria Formosa lagoon (southern Portugal). Biogeosciences 12: 1683–1696. https://doi.org/10.5194/bg-12-1683-2015.
Billerbeck, M., H. Røy, K. Bosselmann, and M. Huettel. 2007. Benthic photosynthesis in submerged Wadden Sea intertidal flats. Estuarine, Coastal and Shelf Science 71: 704–716. https://doi.org/10.1016/j.ecss.2006.09.019.
Brodersen, K.E., D.A. Nielsen, P.J. Ralph, and M. Kühl. 2015. Oxic microshield and local pH enhancement protects Zostera muelleri from sediment derived hydrogen sulphide. New Phytologist 205: 1264–1276. https://doi.org/10.1111/nph.13124.
Burnham, K.P., and D.R. Anderson. 2002. A practical information-theoretic approach. New York: Springer.
Clarke, K. R., and R. N. Gorley. 2015. PRIMER v7: user manual/tutorial. Plymouth: PRIMER-E.
Consalvey, M., D.M. Paterson, and G.J.C. Underwood. 2004. The ups and downs of life in a benthic biofilm: Migration of benthic diatoms. Diatom Research 19: 181–202. https://doi.org/10.1080/0269249X.2004.9705870.
Cooper, S.R., and G.S. Brush. 1993. A 2,500-year history of anoxia and eutrophication in Chesapeake Bay. Estuaries 16: 617–626. https://doi.org/10.2307/1352799.
Crawshaw, J. 2020. Seagrass health monitoring in Tauranga Harbour. Environmental Publication 2020/08. Tauranga, New Zealand: Bay of Plenty Regional Council. https://atlas.boprc.govt.nz/api/v1/edms/document/A3701639/content
de Lange, W., and T. Healy. 1990. Wave spectra for a shallow meso-tidal estuarine lagoon: Bay of Plenty, New Zealand. Journal of Coastal Research 6: 189–199.
Dormann, C.F., J. Elith, S. Bacher, C. Buchmann, G. Carl, G. Carré, J.R. García Marquéz, B. Gruber, B. Lafourcade, P.J. Leitão, T. Münkemüller, C. McClean, P.E. Osborne, B. Reineking, B. Schröder, A.K. Skidmore, D. Zurell, and S. Lautenbach. 2013. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 36: 27–46. https://doi.org/10.1111/j.1600-0587.2012.07348.x.
Douglas, E.J., C.A. Pilditch, A.M. Lohrer, C. Savage, L.A. Schipper, and S.F. Thrush. 2018. Sedimentary environment influences ecosystem response to nutrient enrichment. Estuaries and Coasts 41: 1994–2008. https://doi.org/10.1007/s12237-018-0416-5.
Drylie, T.P., A.M. Lohrer, H.R. Needham, R.H. Bulmer, and C.A. Pilditch. 2018. Benthic primary production in emerged intertidal habitats provides resilience to high water column turbidity. Journal of Sea Research 142: 101–112. https://doi.org/10.1016/j.seares.2018.09.015.
Duarte, C.M., J.J. Middelburg, and N. Caraco. 2005b. Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2: 1–8. https://doi.org/10.5194/bg-2-1-2005.
Duarte, C. M., M. Holmer, and N. Marbà. 2005a. Plant–microbe interactions in seagrass meadows. In Interactions between macro‐ and microorganisms in marine sediments. Coastal and Estuarine Studies, eds. E. Kristensen, R. R. Haese and J. E. Kostka, 31–60. Washington DC: American Geophysical Union. https://doi.org/10.1029/CE060p0031
Duarte, C.M., N. Marbà, E. Gacia, J.W. Fourqurean, J. Beggins, C. Barrón, and E.T. Apostolaki. 2010. Seagrass community metabolism: assessing the carbon sink capacity of seagrass meadows. Global Biogeochemical Cycles 24: GB4032. https://doi.org/10.1029/2010GB003793
Dunic, J.C., C.J. Brown, R.M. Connolly, M.P. Turschwell, and I.M. Côté. 2021. Long-term declines and recovery of meadow area across the world’s seagrass bioregions. Global Change Biology 27: 4096–4109. https://doi.org/10.1111/gcb.15684.
Edgar, G.J., and C. Shaw. 1995. The production and trophic ecology of shallow-water fish assemblages in southern Australia III. General relationships between sediments, seagrasses, invertebrates and fishes. Journal of Experimental Marine Biology and Ecology 194: 107–131. https://doi.org/10.1016/0022-0981(95)00085-2.
Enríquez, S., N. Marbà, C.M. Duarte, B.I. van Tussenbroek, and G. Reyes-Zavala. 2001. Effects of seagrass Thalassia testudinum on sediment redox. Marine Ecology Progress Series 219: 149–158. https://doi.org/10.3354/meps219149.
Eyre, B.D., and A.J.P. Ferguson. 2002. Comparison of carbon production and decomposition, benthic nutrient fluxes and denitrification in seagrass, phytoplankton, benthic microalgae- and macroalgae-dominated warm-temperate Australian lagoons. Marine Ecology Progress Series 229: 43–59. https://doi.org/10.3354/meps229043.
Eyre, B.D., D. Maher, J.M. Oakes, D.V. Erler, and T.M. Glasby. 2011. Differences in benthic metabolism, nutrient fluxes, and denitrification in Caulerpa taxifolia communities compared to uninvaded bare sediment and seagrass (Zostera capricorni) habitats. Limnology and Oceanography 56: 1737–1750. https://doi.org/10.4319/lo.2011.56.5.1737.
Ferguson, A.J.P., R.K. Gruber, M. Orr, and P. Scanes. 2016. Morphological plasticity in Zostera muelleri across light, sediment, and nutrient gradients in Australian temperate coastal lakes. Marine Ecology Progress Series 556: 91–104. https://doi.org/10.3354/meps11830.
Flowers, G.J.L., H.R. Needham, R.H. Bulmer, A.M. Lohrer, and C.A. Pilditch. 2023. Going under: The implications of sea-level rise and reduced light availability on intertidal primary production. Limnology and Oceanography 68: 1301–1315. https://doi.org/10.1002/lno.12347.
Folt, C.L., C.Y. Chen, M.V. Moore, and J. Burnaford. 1999. Synergism and antagonism among multiple stressors. Limnology and Oceanography 44: 864–877. https://doi.org/10.4319/lo.1999.44.3_part_2.0864.
GESAMP. 1993. Anthropogenic influexes on sediment discharge to the coastal zone and environmental consequences. UNESCO-IOC. GESAMP Journal Serices Reports and Studies 52: 1–67.
Gladstone-Gallagher, R.V., R.W. Hughes, E.J. Douglas, and C.A. Pilditch. 2018. Biomass-dependent seagrass resilience to sediment eutrophication. Journal of Experimental Marine Biology and Ecology 501: 54–64. https://doi.org/10.1016/j.jembe.2018.01.002.
Glud, R.N. 2008. Oxygen dynamics of marine sediments. Marine Biology Research 4: 243–289. https://doi.org/10.1080/17451000801888726.
Green, M.O., and G. Coco. 2014. Review of wave-driven sediment resuspension and transport in estuaries. Reviews of Geophysics 52: 77–117. https://doi.org/10.1002/2013RG000437.
Gustafsson, C., and A. Norkko. 2016. Not all plants are the same: Exploring metabolism and nitrogen fluxes in a benthic community composed of different aquatic plant species. Limnology and Oceanography 61: 1787–1799. https://doi.org/10.1002/lno.10334.
Ha, N.-T., M. Manley-Harris, T.-D. Pham, and I. Hawes. 2021. Detecting multi-decadal changes in seagrass cover in Tauranga Harbour, New Zealand, using Landsat imagery and boosting ensemble classification techniques. ISPRS International Journal of Geo-Information 10: 371. https://doi.org/10.3390/ijgi10060371.
Halun, Z., J. Terrados, J. Borum, L. Kamp-Nielsen, C.M. Duarte, and M.D. Fortes. 2002. Experimental evaluation of the effects of siltation-derived changes in sediment conditions on the Philippine seagrass Cymodocea rotundata. Journal of Experimental Marine Biology and Ecology 279: 73–87. https://doi.org/10.1016/S0022-0981(02)00366-0.
Haro, S., M. Lara, I. Laiz, C.J. González, J. Bohórquez, E. Garcia-Robledo, A. Corzo, and S. Papaspyrou. 2020. Microbenthic net metabolism along intertidal gradients (Cadiz Bay, SW Spain): Spatio-temporal patterns and environmental factors. Frontiers in Marine Science 7: 1–22. https://doi.org/10.3389/fmars.2020.00039.
Heath, R.A. 1975. Stability of some New Zealand coastal inlets. New Zealand Journal of Marine and Freshwater Research 9: 449–457. https://doi.org/10.1080/00288330.1975.9515580.
Heip, C., and P. Engels. 1974. Comparing species diversity and evenness indices. Journal of the Marine Biological Association of the United Kingdom 54: 559–563. https://doi.org/10.1017/S0025315400022748.
Heiri, O., A.F. Lotter, and G. Lemcke. 2001. Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results. Journal of Paleolimnology 25: 101–110. https://doi.org/10.1023/A:1008119611481.
Heiss, W.M., A.M. Smith, and P.K. Probert. 2000. Influence of the small intertidal seagrass Zostera novazelandica on linear water flow and sediment texture. New Zealand Journal of Marine and Freshwater Research 34: 689–694. https://doi.org/10.1080/00288330.2000.9516970.
Hewitt, J.E., S.F. Thrush, P.K. Dayton, and E. Bonsdorff. 2007. The effect of spatial and temporal heterogeneity on the design and analysis of empirical studies of scale-dependent systems. The American Naturalist 169: 398–408. https://doi.org/10.1086/510925.
Huettel, M., P. Berg, and J.E. Kostka. 2014. Benthic exchange and biogeochemical cycling in permeable sediments. Annual Review of Marine Science 6: 23–51. https://doi.org/10.1146/annurev-marine-051413-012706.
Huettel, M., H. Røy, E. Precht, and S. Ehrenhauss. 2003. Hydrodynamical impact on biogeochemical processes in aquatic sediments In The interactions between sediments and water. Developments in Hydrobiology ed B Kronvang 169: 231–236. https://doi.org/10.1007/978-94-017-3366-3_31. Dordrecht: Springer.
Hunt, S. 2019. Summary of historic estuarine sedimentation measurements in the Waikato region and formulation of a historic baseline sedimentation rate. Technical Report 2019/08. Waikato Regional Council. https://www.waikatoregion.govt.nz/services/publications/tr201908/.
Keddy, P.A. 1982. Quantifying within-lake gradients of wave energy: Interrelationships of wave energy, substrate particle size and shoreline plants in Axe Lake, Ontario. Aquatic Botany 14: 41–58. https://doi.org/10.1016/0304-3770(82)90085-7.
Kilminster, K.L., D.I. Walker, P.A. Thompson, and J.A. Raven. 2008. Changes in growth, internode distance and nutrient concentrations of the seagrass Halophila ovalis with exposure to sediment sulphide. Marine Ecology Progress Series 361: 83–91. https://doi.org/10.3354/meps07479.
Kirk, J.T.O. 1985. Effects of suspensoids (turbidity) on penetration of solar radiation in aquatic ecosystems. Hydrobiologia 125: 195–208. https://doi.org/10.1007/BF00045935.
Kohler, K.E., and S.M. Gill. 2006. Coral point count with excel extensions (CPCe): A visual basic program for the determination of coral and substrate coverage using random point count methodology. Computers & Geosciences 32: 1259–1269. https://doi.org/10.1016/j.cageo.2005.11.009.
Kohlmeier, D., C.A. Pilditch, J.F. Bornman, and K. Bischof. 2014. Site specific differences in morphometry and photophysiology in intertidal Zostera muelleri meadows. Aquatic Botany 116: 104–109. https://doi.org/10.1016/j.aquabot.2014.02.011.
Krause-Jensen, D., J. Carstensen, S.L. Nielsen, T. Dalsgaard, P.B. Christensen, H. Fossing, and M.B. Rasmussen. 2011. Sea bottom characteristics affect depth limits of eelgrass Zostera marina. Marine Ecology Progress Series 425: 91–102. https://doi.org/10.3354/meps09026.
Krause-Jensen, D., S. Markager, and T. Dalsgaard. 2012. Benthic and pelagic primary production in different nutrient regimes. Estuaries and Coasts 35: 527–545. https://doi.org/10.1007/s12237-011-9443-1.
Kristensen, E., M.H. Jensen, and K.M. Jensen. 1997. Temporal variations in microbenthic metabolism and inorganic nitrogen fluxes in sandy and muddy sediments of a tidally dominated bay in the northern Wadden Sea. Helgoländer Meeresuntersuchungen 51: 295–320. https://doi.org/10.1007/BF02908717.
Lawton, R., and E. Conroy. 2019. Tauranga Moana State of the Environment report 2019. Environmental Publication 2019/04. Tauranga, New Zealand: Bay of Plenty Regional Council. https://atlas.boprc.govt.nz/api/v1/edms/document/A3324188/content
Lee, K.-S., S.R. Park, and Y.K. Kim. 2007. Effects of irradiance, temperature, and nutrients on growth dynamics of seagrasses: A review. Journal of Experimental Marine Biology and Ecology 350: 144–175. https://doi.org/10.1016/j.jembe.2007.06.016.
Livingston, R.J., S.E. McGlynn, and X. Niu. 1998. Factors controlling seagrass growth in a gulf coastal system: Water and sediment quality and light. Aquatic Botany 60: 135–159. https://doi.org/10.1016/S0304-3770(97)00079-X.
Lohrer, A.M., S.F. Thrush, and M.M. Gibbs. 2004. Bioturbators enhance ecosystem function through complex biogeochemical interactions. Nature 431: 1092–1095. https://doi.org/10.1038/nature03042.
Lohrer, A.M., J.E. Hewitt, and S.F. Thrush. 2006. Assessing far-field effects of terrigenous sediment loading in the coastal marine environment. Marine Ecology Progress Series 315: 13–18. https://doi.org/10.3354/meps315013.
Lohrer, A.M., M. Townsend, S.F. Hailes, I.F. Rodil, K. Cartner, D.R. Pratt, and J.E. Hewitt. 2016. Influence of New Zealand cockles (Austrovenus stutchburyi) on primary productivity in sandflat-seagrass (Zostera muelleri) ecotones. Estuarine, Coastal and Shelf Science 181: 238–248. https://doi.org/10.1016/j.ecss.2016.08.045.
MacIntyre, H.L., R.J. Geider, and D.C. Miller. 1996. Microphytobenthos: The ecological role of the “secret garden” of unvegetated, shallow-water marine habitats. I. Distribution, abundance and primary production. Estuaries 19: 186–201. https://doi.org/10.2307/1352224.
Mangan, S., K.R. Bryan, S.F. Thrush, R.V. Gladstone-Gallagher, A.M. Lohrer, and C.A. Pilditch. 2020a. Shady business: The darkening of estuaries constrains benthic ecosystem function. Marine Ecology Progress Series 647: 33–48. https://doi.org/10.3354/meps13410.
Mangan, S., A.M. Lohrer, S.F. Thrush, and C.A. Pilditch. 2020b. Water column turbidity not sediment nutrient enrichment moderates microphytobenthic primary production. Journal of Marine Science and Engineering 8: 732. https://doi.org/10.3390/jmse8100732.
McCartain, L.D., M. Townsend, S.F. Thrush, D.S. Wethey, S.A. Woodin, N. Volkenborn, and C.A. Pilditch. 2017. The effects of thin mud deposits on the behaviour of a deposit-feeding tellinid bivalve: Implications for ecosystem functioning. Marine and Freshwater Behaviour and Physiology 50: 239–255. https://doi.org/10.1080/10236244.2017.1364123.
Mcleod, E., G.L. Chmura, S. Bouillon, R. Salm, M. Björk, C.M. Duarte, C.E. Lovelock, W.H. Schlesinger, and B.R. Silliman. 2011. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Frontiers in Ecology and the Environment 9: 552–560. https://doi.org/10.1890/110004.
Migné, A., R.J. Trigui, D. Davoult, and N. Desroy. 2018. Benthic metabolism over the emersion - immersion alternation in sands colonized by the invasive Manila clam Ruditapes philippinarum. Estuarine, Coastal and Shelf Science 200: 371–379. https://doi.org/10.1016/j.ecss.2017.11.030.
Milliman, J. D., and R. Mei-e. 1995. Rivér flux to the sea: impact of human intervention on river systems and adjacent coastal areas. In Climate change Impact on coastal habitation. ed. D. Eisma, 57–83. Boca Raton, Florida: CRC Press. https://doi.org/10.1201/9781003069935-4
Mills, G. N., and H. Allen. 2021. Marine sediment contaminant state and trends in Tāmaki Makaurau / Auckland 2004–2019. State of the Environment Reporting. Technical Report 2021/10. Auckland, New Zealand: Auckland Council. https://knowledgeauckland.org.nz/publications/marine-sediment-contaminant-state-and-trends-in-tamaki-makaurau-auckland-2004-2019/
Moksnes, P.-O., L. Eriander, E. Infantes, and M. Holmer. 2018. Local regime shifts prevent natural recovery and restoration of lost eelgrass beds along the Swedish west coast. Estuaries and Coasts 41: 1712–1731. https://doi.org/10.1007/s12237-018-0382-y.
Morrison, M. A., M. L. Lowe, D. M. Parsons, N. R. Usmar, and I. M. McLeod. 2009. A review of land-based effects on coastal fisheries and supporting biodiversity in New Zealand. New Zealand Aquatic Environment and Biodiversity Report No. 37. Wellington, New Zealand: Ministry of Fisheries. https://fs.fish.govt.nz/Page.aspx?pk=113&dk=22003
Munkes, B. 2005. Eutrophication, phase shift, the delay and the potential return in the Greifswalder Bodden, Baltic Sea. Aquatic Sciences 67: 372–381. https://doi.org/10.1007/s00027-005-0761-x.
Nordlund, L.M., E.W. Koch, E.B. Barbier, and J.C. Creed. 2016. Seagrass ecosystem services and their variability across genera and geographical regions. PLoS ONE 11: e0163091. https://doi.org/10.1371/journal.pone.0163091.
Oksanen, J., G. L. Simpson, F. G. Blanchet, P. Legendre, P. R. Minchin, R. B. O'Hara, M. H. H. Stevens, E. Szoecs, H. Wagner, M. Barbour, M. Bedward, B. Bolker, D. Borcard, G. Carvalho, M. Chirico, M. De Caceres, S. Durand, H. B. A. Evangelista, R. FitzJohn, M. Friendly, B. Furneaux, G. Hannigan, M. O. Hill, L. Lahti, D. McGlinn, M.-H. Ouellette, E. R. Cunha, T. Smith, A. Stier, C. J. F. ter Braak, and J. Weedon. 2022. vegan: community ecology package. R package version 2.6–4. https://cran.r-project.org/web/packages/vegan/index.html
Ouisse, V., A. Migné, and D. Davoult. 2011. Community-level carbon flux variability over a tidal cycle in Zostera marina and Z. noltii beds. Marine Ecology Progress Series 437: 79–87. https://doi.org/10.3354/meps09274.
Park, S.R., S. Kim, Y.K. Kim, C.-K. Kang, and K.-S. Lee. 2016. Photoacclimatory responses of Zostera marina in the intertidal and subtidal zones. PLoS ONE 11: e0156214. https://doi.org/10.1371/journal.pone.0156214.
Park, S.G. 2016. Extent of seagrass in the Bay of Plenty in 2011. Environmental Publication 2016/03. Whakatāne, New Zealand: Bay of Plenty Regional Council. https://atlas.boprc.govt.nz/api/v1/edms/document/A2347475/content
Pratt, D.R., A.M. Lohrer, C.A. Pilditch, and S.F. Thrush. 2014a. Changes in ecosystem function across sedimentary gradients in estuaries. Ecosystems 17: 182–194. https://doi.org/10.1007/s10021-013-9716-6.
Pratt, D.R., C.A. Pilditch, A.M. Lohrer, and S.F. Thrush. 2014b. The effects of short-term increases in turbidity on sandflat microphytobenthic productivity and nutrient fluxes. Journal of Sea Research 92: 170–177. https://doi.org/10.1016/j.seares.2013.07.009.
R Core Team. 2023. R: A language and environment for statistical computing. Vienne, Austria: R Foundation for Statistical Computing. https://www.R-project.org/
Rahmstorf, S. 2007. A semi-empirical approach to projecting future sea-level rise. Science 315: 368–370. https://doi.org/10.1126/science.1135456.
Ralph, P.J., M.J. Durako, S. Enríquez, C.J. Collier, and M.A. Doblin. 2007. Impact of light limitation on seagrasses. Journal of Experimental Marine Biology and Ecology 350: 176–193. https://doi.org/10.1016/j.jembe.2007.06.017.
Revelle, W. 2024. psych: Procedures for psychological, psychometric, and personality research. R package version 2.4.3. https://CRAN.R-project.org/package=psych
Rodil, I.F., A.M. Lohrer, L.D. Chiaroni, J.E. Hewitt, and S.F. Thrush. 2011. Disturbance of sandflats by thin terrigenous sediment deposits: Consequences for primary production and nutrient cycling. Ecological Applications 21: 416–426. https://doi.org/10.1890/09-1845.1.
Rodriguez, A.B., B.A. McKee, C.B. Miller, M.C. Bost, and A.N. Atencio. 2020. Coastal sedimentation across North America doubled in the 20th century despite river dams. Nature Communications 11: 3249. https://doi.org/10.1038/s41467-020-16994-z.
Sánchez-Moyano, J.E., I. García-Asencio, and J.C. García-Gómez. 2010. Spatial and temporal variation of the benthic macrofauna in a grossly polluted estuary from southwestern Spain. Helgoland Marine Research 64: 155–168. https://doi.org/10.1007/s10152-009-0175-6.
Sand-Jensen, K., C. Prahl, and H. Stokholm. 1982. Oxygen release from roots of submerged aquatic macrophytes. Oikos 38: 349–354. https://doi.org/10.2307/3544675.
Sandwell, D.R., C.A. Pilditch, and A.M. Lohrer. 2009. Density dependent effects of an infaunal suspension-feeding bivalve (Austrovenus stutchburyi) on sandflat nutrient fluxes and microphytobenthic productivity. Journal of Experimental Marine Biology and Ecology 373: 16–25. https://doi.org/10.1016/j.jembe.2009.02.015.
Schmidt, A.L., M. Coll, and H.K. Lotze. 2017. Regional-scale differences in eutrophication effects on eelgrass-associated (Zostera marina) macrofauna. Estuaries and Coasts 40: 1096–1112. https://doi.org/10.1007/s12237-016-0204-z.
Seneviratne, S. I., N. Nicholls, D. Easterling, C. M. Goodess, S. Kanae, J. Kossin, Y. Luo, J. Marengo, K. McInnes, M. Rahimi, M. Reichstein, A. Sorteberg, C. Vera, and X. Zhang. 2012. Changes in climate extremes and their impacts on the natural physical environment. In Managing the risks and extreme events and disasters to advance climate change adaptation. eds. C. B. Field, V. Barros, T. F. Stocker, D. Qin, D. J. Dokken, K. L. Ebi, M. D. Mastrandrea, K. J. Mach, G.-K. Plattner, S. K. Allen, M. Tignor and P. M. Midgley, Chapter 3, 109–230. Cambridge, UK: Cambridge University Press.
Shao, Z., K.R. Bryan, M.K. Lehmann, G.J.L. Flowers, and C.A. Pilditch. 2024. Scaling up benthic primary productivity estimates in a large intertidal estuary using remote sensing. Science of the Total Environment 906: 167389. https://doi.org/10.1016/j.scitotenv.2023.167389.
Short, F.T. 1987. Effects of sediment nutrients on seagrasses: Literature review and mesocosm experiment. Aquatic Botany 27: 41–57. https://doi.org/10.1016/0304-3770(87)90085-4.
Stutes, A.L., J. Cebrian, and A.A. Corcoran. 2006. Effects of nutrient enrichment and shading on sediment primary production and metabolism in eutrophic estuaries. Marine Ecology Progress Series 312: 29–43. https://doi.org/10.3354/meps312029.
Swales, A., R.B. Williamson, L.F. Van Dam, M.J. Stroud, and M.S. McGlone. 2002. Reconstruction of urban stormwater contamination of an estuary using catchment history and sediment profile dating. Estuaries 25: 43–56. https://doi.org/10.1007/BF02696048.
Terrados, J., C.M. Duarte, M.D. Fortes, J. Borum, N.S.R. Agawin, S. Bach, U. Thampanya, L. Kamp-Nielsen, W.J. Kenworthy, O. Geertz-Hansen, and J. Vermaat. 1998. Changes in community structure and biomass of seagrass communities along gradients of siltation in SE Asia. Estuarine, Coastal and Shelf Science 46: 757–768. https://doi.org/10.1006/ecss.1997.0304.
Terrados, J., C.M. Duarte, L. Kamp-Nielsen, N.S.R. Agawin, E. Gacia, D. Lacap, M.D. Fortes, J. Borum, M. Lubanski, and T. Greve. 1999. Are seagrass growth and survival constrained by the reducing conditions of the sediment? Aquatic Botany 65: 175–197. https://doi.org/10.1016/S0304-3770(99)00039-X.
Thomas, S., C.A. Pilditch, S.F. Thrush, and C. Savage. 2022. Ecosystem function responses to nutrient enrichment mediated by mud content in soft sediment habitats. New Zealand Journal of Marine and Freshwater Research 56: 491–508. https://doi.org/10.1080/00288330.2022.2102043.
Thrush, S.F., J.E. Hewitt, R.D. Pridmore, and V.J. Cummings. 1996. Adult/juvenile interactions of infaunal bivalves: Contrasting outcomes in different habitats. Marine Ecology Progress Series 132: 83–92. https://doi.org/10.3354/meps132083.
Thrush, S.F., J.E. Hewitt, A. Norkko, P.E. Nicholls, G.A. Funnell, and J.I. Ellis. 2003. Habitat change in estuaries: Predicting broad-scale responses of intertidal macrofauna to sediment mud content. Marine Ecology Progress Series 263: 101–112. https://doi.org/10.3354/meps263101.
Thrush, S.F., J.E. Hewitt, V.J. Cummings, J.I. Ellis, C. Hatton, A. Lohrer, and A. Norkko. 2004. Muddy waters: Elevating sediment input to coastal and estuarine habitats. Frontiers in Ecology and the Environment 2: 299–306. https://doi.org/10.1890/1540-9295(2004)002[0299:MWESIT]2.0.CO;2.
Thrush, S.F., J.E. Hewitt, M. Gibbs, C. Lundquist, and A. Norkko. 2006. Functional role of large organisms in intertidal communities: Community effects and ecosystem function. Ecosystems 9: 1029–1040. https://doi.org/10.1007/s10021-005-0068-8.
Thrush, S.F., J.E. Hewitt, S. Parkes, A.M. Lohrer, C. Pilditch, S.A. Woodin, D.S. Wethey, M. Chiantore, V. Asnaghi, S. De Juan, C. Kraan, I. Rodil, C. Savage, and C. Van Colen. 2014. Experimenting with ecosystem interaction networks in search of threshold potentials in real-world marine ecosystems. Ecology 95: 1451–1457. https://doi.org/10.1890/13-1879.1.
Thrush, S.F., J.E. Hewitt, R.V. Gladstone-Gallagher, C. Savage, C. Lundquist, T. O’Meara, A. Vieillard, J.R. Hillman, S. Mangan, E.J. Douglas, D.E. Clark, A.M. Lohrer, and C. Pilditch. 2021. Cumulative stressors reduce the self-regulating capacity of coastal ecosystems. Ecological Applications 31: e02223. https://doi.org/10.1002/eap.2223.
Turner, S.J., J.E. Hewitt, M.R. Wilkinson, D.J. Morrisey, S.F. Thrush, V.J. Cummings, and G. Funnell. 1999. Seagrass patches and landscapes: The influence of wind-wave dynamics and hierarchical arrangements of spatial structure on macrofaunal seagrass communities. Estuaries 22: 1016–1032. https://doi.org/10.2307/1353080.
Turner, S., and A.-M. Schwarz. 2006. Management and conservation of seagrass in New Zealand: an introduction. Science for Conservation 264. Wellington, New Zealand: Science & Technical Publishing, Department of Conservation. https://www.doc.govt.nz/documents/science-and-technical/sfc264.pdf
Vitousek, P.M., J.D. Aber, R.W. Howarth, G.E. Likens, P.A. Matson, D.W. Schindler, W.H. Schlesinger, and D.G. Tilman. 1997. Human alteration of the global nitrogen cycle: Sources and consequences. Ecological Applications 7: 737–750. https://doi.org/10.1890/1051-0761(1997)007[0737:HAOTGN]2.0.CO;2.
Waycott, M., C.M. Duarte, T.J.B. Carruthers, R.J. Orth, W.C. Dennison, S. Olyarnik, A. Calladine, J.W. Fourqurean, K.L. Heck, A.R. Hughes, G.A. Kendrick, W.J. Kenworthy, F.T. Short, and S.L. Williams. 2009. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences 106: 12377–12381. https://doi.org/10.1073/pnas.0905620106.
Wendländer, N.S., T. Lange, R.M. Connolly, E. Kristensen, R.M. Pearson, T. Valdemarsen, and M.R. Flindt. 2020. Assessing methods for restoring seagrass (Zostera muelleri) in Australia’s subtropical waters. Marine and Freshwater Research 71: 996–1005. https://doi.org/10.1071/MF19237.
Wickham, H., M. Averick, J. Bryan, W. Chang, L. D. McGowan, R. François, G. Grolemund, A. Hayes, L. Henry, J. Hester, M. Kuhn, T. L. Pedersen, E. Miller, S. M. Bache, K. Müller, J. Ooms, D. Robinson, D. P. Seidel, V. Spinu, K. Takahashi, D. Vaughan, C. Wilke, K. Woo, and H. Yutani. 2019. Welcome to the tidyverse. Journal of Open Source Software 4: 1686. https://doi.org/10.21105/joss.01686
Woodin, S.A., N. Volkenborn, C.A. Pilditch, A.M. Lohrer, D.S. Wethey, J.E. Hewitt, and S.F. Thrush. 2016. Same pattern, different mechanism: Locking onto the role of key species in seafloor ecosystem process. Scientific Reports 6: 26678. https://doi.org/10.1038/srep26678.
Zabarte-Maeztu, I., F.E. Matheson, M. Manley-Harris, R.J. Davies-Colley, M. Oliver, and I. Hawes. 2020. Effects of fine sediment on seagrass meadows: A case study of Zostera muelleri in Pāuatahanui Inlet, New Zealand. Journal of Marine Science and Engineering 8: 645. https://doi.org/10.3390/jmse8090645.
Zabarte-Maeztu, I., F.E. Matheson, M. Manley-Harris, R.J. Davies-Colley, and I. Hawes. 2021. Interaction of substrate muddiness and low irradiance on seagrass: A mesocosm study of Zostera muelleri. Aquatic Botany 175: 103435. https://doi.org/10.1016/j.aquabot.2021.103435.
Acknowledgements
We thank J Green for her assistance with the seagrass analyses and S Mangan for helping process the long-term light data. Thank you to R Gladstone-Gallagher, S Hailes and B Greenfield for their assistance with macrofauna identifications. We also thank the numerous people that assisted with field work and provided guidance with laboratory analysis from the University of Waikato, NIWA, and friends/family members. Funding was provided by the Bay of Plenty Regional Council, Sustainable Seas National Science Challenge (Project no. C01X1901) and the University of Waikato School of Science Trust. GF also thanks the Waikato Graduate Women Educational Trust Award and the Bay Trust Bruce Cronin Scholarship. We gratefully acknowledge the two anonymous reviewers for their constructive comments that improved the manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
CP conceived the study and secured funding. All authors contributed to the experimental design. All authors assisted with field and laboratory work led by GF. GF, CP, and HN conducted the statistical analysis. GF wrote the first draft of the manuscript, and all authors contributed to editing and finalising the paper.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Communicated by Masahiro Nakaoka
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Flowers, G.J.L., Needham, H.R., Bulmer, R.H. et al. The Effect of Sediment Mud Content on Primary Production in Seagrass and Unvegetated Intertidal Flats. Estuaries and Coasts 47, 1544–1560 (2024). https://doi.org/10.1007/s12237-024-01403-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-024-01403-1