Abstract
Invasive species exert disproportionate impacts in wetlands and pose particular challenges for rare species persisting at small spatial scales. In the urbanized San Francisco Estuary (SFE), which contains 90% of California’s remaining coastal wetlands, invasive and rare species often co-occur. One narrow endemic taxon, the federally listed Suisun thistle (Cirsium hydrophilum var. hydrophilum) is restricted to two or three locations where the invasive perennial pepperweed (Lepidium latifolium) has an increasing presence. Perennial pepperweed has invaded salt, brackish, and freshwater wetlands around the SFE, leading to high management concern. In this study, we investigated how perennial pepperweed may contribute to further rarity of the Suisun thistle, by conducting a removal experiment and surveying soil-plant relationships. Removing pepperweed led to a doubling of native species relative cover and an increase in native species richness by an average of one species per plot, positive effects on Suisun thistle cover, number, and reproductive output, and shifts in soil properties. Combined with survey data inside and outside of pepperweed stands, we conclude that pepperweed competes with Suisun thistle via competition for space, nutrients, and light, interferes with the Suisun thistle’s reproductive success, and alters brackish marsh soil physicochemical characteristics to further favor pepperweed. We recommend local control of pepperweed to prevent further loss of Suisun thistle. Further, the wide range of mechanisms by which this invasion may proceed if unchecked should be considered in other settings where rare or uncommon species are at risk from invaders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive plants are known to impact a variety of ecosystem functions (Vila et al. 2011), but these effects are not evenly distributed among ecosystems. Wetlands, which comprise 6% of the earth’s land mass, host 24% of the most invasive plant species (Zedler and Kercher 2004). These wetland invaders compete with native species and modify physical and biological environments through increased sediment trapping and altered nutrient dynamics, food webs, and water cycling (Zavaleta 2000; Blank and Young 2002; Able et al. 2003; Windham and Meyerson 2003; Zedler and Kercher 2004; Burkle et al. 2012; Wigginton et al. 2014; Robison et al. 2021).
In addition to their disproportionate presence in wetland systems, the effect of invasive plants on native plant species richness is more pronounced at smaller compared to larger spatial scales (Gaertner et al. 2009; Powell et al. 2011, 2013), perhaps due to the increased importance of competition and other ecological interactions at this level (Huston 1999). This presents a particular challenge for rare species confined to specific habitat types and/ or small geographic ranges, as they are already susceptible to extirpation from environmental stochasticity, genetic bottlenecks, and minimum density effects (Shaffer 1981; Frankham 2005).
Invasive plants vary widely in the mechanisms by which they impact rare species; past work has documented impacts from competition, decreased recruitment, facilitating increased predation, and reproductive interference. For example, direct competition from invasive Coronilla varia and Festuca arundinacea reduced seedling establishment and growth of the endangered Solidago shortii in a grassland system (Walck et al. 1999). Similarly, invasive annual grasses reduced seedling recruitment of the endangered Oenothera deltoides ssp. howellii, mainly due to a thick thatch layer and reduced soil disturbance associated with the grasses (Thomson 2005a). In coastal dunes, seed predation by Peromyscus maniculatus mice on the endangered Lupinus tidestromii increased to highly detrimental levels with proximity to invasive Ammophila arenaria, which provided habitat and cover for the mice (Dangremond et al. 2010). Invaders can also interfere with reproduction of rare species through competition for pollination; for example, the presence of pollen from the invasive Lythrum salicaria reduced seed set in its rare congener, Lythrum alatum (Brown and Mitchell 2001).
The highly urbanized nature of the San Francisco Estuary (SFE) has led to widespread invasion of non-native species, and some native species with inherently limited distributions have become increasingly rare. Because the SFE has retained at most only 18% of its historic coastal wetlands (Brophy et al. 2019), managers aim to restrict invasive species and enhance survival of rare species, towards the larger goals of restoring wetland function and biodiversity (U.S. Fish and Wildlife Service 2013; Goals Project 2015). One invader of particular interest, in part due to its rapid spread into habitats with sensitive species, is perennial pepperweed, Lepidium latifolium (hereafter, Lepidium; Schoenig et al. 2003; Fiedler et al. 2007; Hogle et al. 2007; Reynolds and Boyer 2010).
Native to Eurasia, Lepidium is the fifth most common weed in California. It has been a longtime nuisance in the Western United States, invading floodplains, rangelands, riparian areas, crop fields, and tidal marshes (Miller et al. 1986; Renz 2000; Schoenig et al. 2003). More recently, Lepidium has become highly invasive in several marshes throughout New England (Orth et al. 2006; Connolly and Hale 2016) and is invasive in Canada (Francis and Warwick 2007) and Mexico (Roldán et al. 2009). Lepidium plants live for several years, reproducing both by seeds and from root fragments. It tolerates a range of salinity and inundation regimes and in tidal marshes is found most dense at the transition zone and in the marsh plain (Grossinger et al. 1998; Whitcraft et al. 2011). It is insect-pollinated, with small but abundant and fragrant flowers. Dense monotypes establish quickly, with a thick litter layer inhibiting growth of other species (Young et al. 1995; Renz and Blank 2004). Lepidium may alter soil properties by increasing elemental cycling and reducing soil salinity (Blank and Young 2002; Renz and Blank 2004; Reynolds and Boyer 2010). Management has been particularly challenging in SFE wetlands, as mechanical control and herbicides are often impractical or prohibited (Spenst 2006; Hogle et al. 2007; Boyer and Burdick 2010).
In Suisun Marsh (northeast SFE), Lepidium co-occurs with a number of rare species, including the endemic federally listed endangered Suisun thistle (Cirsium hydrophilum var. hydrophilum, hereafter, Cirsium; Goals Project 2000; Whitcraft et al. 2011; USFWS 2013). A biennial plant reproducing by seed, Cirsium, grows in intact brackish tidal marsh plain, along borders of channels and creeks, where average inundation time is 12.5% in a 24-h period, with a minimum of 6% and maximum of 24% (USFWS 2013; Graham-Bruno et al. 2023). It is likely pollinated by bees (USFWS 2013). In 1892, the botanist Greene described Cirsium as “very common in the brackish marshes of Suisun Bay, California, where it grows within reach of tide water” (Greene 1892). Wetland diking and reclamation through the 1930s destroyed much of Cirsium’s original habitat, and it is now restricted to two or three populations in fragments of intact tidal marsh, the largest of which is at Rush Ranch, Suisun City (Whitcraft et al. 2011; USFWS 2013). Lepidium started invading Rush Ranch in the 1990s, with dense infestations developing in the mid-2000s (Whitcraft et al. 2011). A census of the Rush Ranch Cirsium population in 2003 found that Lepidium was Cirsium’s third most common plant associate, present among 85% of subpopulations (Fiedler et al. 2007). Due to this high degree of habitat overlap, there is a concern that Lepidium may be increasing rarity in Cirsium) through direct competition or other mechanisms (USFWS 2013). Additionally, there is a growing interest in understanding how Lepidium alters abiotic and biotic properties and interactions within ecosystems (Reynolds and Boyer 2010; Whitcraft et al. 2011; USFWS 2013; Wigginton et al. 2014; Wigginton et al. 2022).
To address the question of how Lepidium affects Cirsium and its brackish marsh ecosystem, we conducted a Lepidium removal experiment at a small spatial scale and monitored the response of Cirsium, other plant species, and soil properties over two growing seasons. To further quantify habitat and plant-soil relationships for Lepidium and Cirsium, we surveyed soil properties and plant characteristics in additional unmanipulated survey sites over two growing seasons, also at a small spatial scale of our study marsh.
We hypothesized that the invasion of Lepidium into Cirsium habitat presents a further challenge for a narrow endemic species already experiencing limitations at several life history stages, e.g., seed set, seed predation, and seed dispersal (Schneider and Boyer 2021; Graham-Bruno et al. 2023). Specifically, we expected that Cirsium abundance and reproductive output would increase following experimental Lepidium removal, as it would no longer face competition for light and nutrients from the invader. Alternatively, given that Cirsium was rare before Lepidium invasion, competition with native dominant species recruiting following Lepidium removal could be similar to effects of Lepidium, and Cirsium might not benefit.
At unmanipulated survey sites, we expected soil salinity, moisture, and organic matter to be lower beneath Lepidium than Cirsium growing in the absence of Lepidium, as has been demonstrated for soils beneath the invader compared to soils beneath pickleweed (Salicornia pacifica) (Reynolds and Boyer 2010). A change in these and other soil properties in removal plots would suggest that Lepidium modifies them, perhaps in addition to colonizing areas that already have these characteristics.
Methods and Materials
Study Site
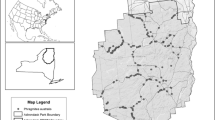
Rush Ranch in Suisun Marsh (38° 12′ 31.7″ N, 122° 1′ 31.9″ W) contains 425 ha of brackish tidal marsh within a 2000 ha preserve that is part of the National Estuarine Research Reserve System (Fig. 1). Like the surrounding Suisun Marsh, Rush Ranch is invaded by Lepidium; uniquely, however, it retains relatively large areas of undiked, intact tidal marsh and is home to several rare, threatened, and endangered species, including the California black rail (Laterallus jamaicensis coturniculus), California Ridgway’s rail (Rallus obsoletus obsoletus), salt marsh harvest mouse (Reithrodontomys raviventris), soft bird’s beak (Chloropyron molle ssp. molle), Suisun Marsh aster (Symphyotrichum lentum), and Cirsium (Goals Project 1999; Whitcraft et al. 2011). This study took place in the mid marsh zone within the First Mallard Branch area, which contains several subpopulations of Cirsium and is highly invaded by Lepidium.
Lepidium Removal Experiment
One pair of removal and control plots was established in March 2011 at each of six sites, with > 2 m between plots (Fig. 1). Sites were chosen where Cirsium rosettes were growing within dense Lepidium stands. In all plots, Lepidium was the dominant species present; percent cover of leaves (basal rosettes remaining green year-round) ranged from 19 to 52%, and percent cover of either dead stems or horizontal thatch exceeded 55% (Table S1, Online Resource 1). Cirsium rosettes (2–3) were present in each plot with an average cover of 4.8% (Table S1). Orthometric heights (elevation relative to a tidal datum) of the soil surfaces of all plots were within a range of 6 cm, and paired plots within a site had similar elevations (1–4 cm, Leica Real-Time Kinematic Global Positioning System, accurate to + / − 2 cm). Control and removal treatments were randomly assigned; treatment areas were 4 m2, centered on a 1 m2 study plot. In removal treatment areas, Lepidium was clipped to remove aboveground biomass; dead stems and thatch were also removed. Resprouts and seedlings of Lepidium (but not thatch) were removed monthly throughout the experiment. In September 2012, light levels (photosynthetically active radiation, PAR) at the soil surface were measured using a spherical quantum sensor (LI-COR LI-193, Lincoln, NE) and compared for each pair of removal and control plots.
Plant cover was assessed on a quarterly basis over two growing seasons using a 1-m2 quadrat grid with 25 sampling points. At each sampling point, a fiberglass rod was placed perpendicular to the ground, and plant species with green tissue touching the rod were recorded. Species present in the plot but not sampled by any of the points were recorded and used to determine species richness. Number of Cirsium plants was also recorded, and thatch depth was measured at four randomly chosen locations within each plot.
In August 2012, inflorescences per plant on all flowering Cirsium individuals were counted. In both August 2011 and 2012, three inflorescences in each plot that were past flowering but had not yet developed seeds were covered in a cloth bag with drawstrings (dimensions 6.5 cm × 8 cm) for later collection to determine seed set, seed predation by the beetle Lasioderma haemorrhoidale (family Anobiidae) observed during preliminary field visits, and seed germination levels in the lab (2012 only) (Surles and Kok 1978). Inflorescences were randomly chosen whenever possible, though some plots had three or fewer inflorescences that met our criteria, in which case inflorescences closest to the plot were used, including some outside of the treatment area. Maximum height of the tallest individual in each plot was also recorded.
Soil samples were collected quarterly for two growing seasons using a PVC corer (diameter = 4 cm, depth = 10 cm) just inside the 1 m2 plots. In the lab, soil moisture was determined gravimetrically. Soils were ground with a mortar, pestle, and mechanical grinder (Cuisinart Grind Central Coffee Grinder) to pass through a 2-mm sieve; salinity was determined using saturated soil pastes and a Leica refractometer (Reichert Analytical Instruments, Depew, NY) and percent organic matter determined by loss on ignition (500 °C for 3 h, Isotemp Programmable Muffle Furnace Model 650-126, Fisher Scientific, Pittsburgh, PA). A subset of samples 1 year apart representing spring conditions (March 2011 and March 2012) was analyzed for phosphorus and extractable cations (A and L Western Agricultural Labs, Modesto, CA; Bray and Kurtz 1945) and for carbon and nitrogen content (Costech ECS4010, Costech Analytical Technologies Inc., Valencia, CA).
Statistical Analyses
Statistical analyses were conducted in R Studio Version 1.1.453 (R Studio 2016, PBC, Boston, MA). Relative percent cover of native species in each plot was calculated by summing cover values for all native species and dividing by the sum of cover values for all species. This procedure was repeated for non-native species other than Lepidium. Species richness was compared over time using a repeated measures ANOVA with month, treatment, and month*treatment as factors. Because percent cover values could not be adequately transformed to meet the assumption of normality, separate t-tests or Wilcoxon rank sum tests for treatment effects were conducted within each month.
A permutational multivariate analysis of variance (PERMANOVA) using distance matrices (adonis function, vegan package, Oksanen et al. 2018) was used to assess effects of Lepidium removal, stratified by site, on plant community composition during the summer of the second growing season. Mean thatch depth was compared between removal and control plots within each month using a t-test or Wilcoxon rank sum test.
Effect of Lepidium removal on Cirsium percent cover, number, mean seed set per inflorescence, and mean maximum height was examined by calculating effect size using Hedges’ g (analogous to Cohen’s d but for unequal or small sample sizes) at the end of the experiment (August and September 2012, depending on the variable). Effect sizes were also calculated to compare mean inflorescences per plant (per plot), mean seeds germinated per inflorescence (per plot), and mean number of larvae of the seed predator L. haemorrhoidale per inflorescence (per plot for August 2012 data).
Repeated measures ANOVAs were used to test for treatment and site effects on soil organic matter and salinity over time. Because soil moisture data could not be transformed to meet assumptions of normality, separate two sample t-tests or Wilcoxon rank sum tests were used to test for treatment effect within each sampling month.
A principal component analysis (PCA) was conducted on the nine measured variables (soil salinity, moisture, organic matter, carbon, nitrogen, phosphorus and extractable cations Na+, K+, Mg2+, and Ca2+) from cores from before the experimental treatment (March 2011) and 1 year later (March 2012). One observation was omitted due to lack of sufficient sample for phosphorus and cation analyses. Scores from principal components with Eigenvalues > 1 were then used in a two-way repeated measures ANOVA to test for year and treatment effects. Principal component 2 (PC2) scores could not be adequately transformed to meet normality assumptions; separate Welch two-sample t-tests were conducted for overall year effect and within-year treatment effects.
Unmanipulated Survey Sites
In April 2011, soil survey sites were established in unmanipulated areas that included dense Lepidium stands, mixed stands of Lepidium and other species, and Cirsium (n = 7 for each vegetation type, Fig. 1). For Lepidium, sites were chosen using a random compass direction and distance; the first Lepidium encountered after walking the specified distance was classified as either dense (monotypic in April, with other species present later in the season) or mixed (other species present in April). For Cirsium, upon reaching known locations, sites were chosen by walking in a random compass direction, and soil was sampled beneath the first individual encountered. Lepidium was not present in these Cirsium sites.
At each survey site, two 5-cm diameter soil cores were taken to a depth of 6 cm and divided into 0–2 cm and 2–6 cm fractions to examine potential differences between surface and deeper soils as documented in previous Lepidium research (Reynolds and Boyer 2010). Cores were taken quarterly through July 2012 and were analyzed for moisture, salinity, and organic matter, as described above. A subset of soils from the peak growing season (samples from July 2011, 2–6 cm) was also analyzed for carbon, nitrogen, and phosphorus, as described above.
In August 2011 and 2012, plant measurements were taken at survey sites. At Cirsium sites, a 0.25-m2 quadrat was positioned haphazardly, ensuring that at least one flowering Cirsium was contained therein. Maximum canopy height, total number of stems, and number of stems per individual were recorded, and stem length was measured for up to five randomly chosen stems. A leaf was collected from the non-flowering thistle closest to the sampling point, as leaves on reproducing thistles had already started to senesce.
At Lepidium sites (both dense and mixed stands), the 0.25-m2 quadrat was placed randomly, while ensuring that Lepidium plants originated within. Maximum canopy height, approximate area covered by flowers, total stem number, and number of stems per plant of three randomly chosen individuals were recorded. Stem length was measured for five randomly chosen stems, and a leaf sample was taken.
Leaf samples were rinsed in deionized water, dried at 50 °C overnight, and ground using a mortar and pestle. Carbon and nitrogen content were determined using an elemental analyzer (Costech ECS4010, Costech Analytical Technologies Inc., Valencia CA); subsamples were sent to the UC Davis Analytical Laboratory for phosphorus analysis (extractable or acid digestion method).
For soil properties that were measured quarterly (salinity, moisture, and organic matter), repeated measures ANOVAs were conducted with month, treatment, month*treatment, and depth as fixed effects. We also calculated and analyzed the difference in soil moisture between the surface (0–2 cm) and deeper (2–6 cm) samples. When data could not be adequately transformed to meet assumptions of normality and homogeneity of variances, separate one-way ANOVAs or Kruskal–Wallis tests were conducted within each month at each depth. Measurements taken only once (leaf and soil % P, % N, % C, C:N, and N:P) were tested using one-way ANOVAs by treatment.
To examine the relationship between soil properties and plant characteristics for each species, a PCA was conducted on soil measurements taken beneath each species in July 2011. Linear regression models were run for species-specific plant measurements taken in August 2011 versus principal components that encompassed over 80% of the variation (PC1 and PC2 for Cirsium soils, and PC1, PC2, and PC3 for Lepidium soils).
Results
Response of Plant Community to Lepidium Removal
Revegetation of removal plots was rapid, with only 1.3% (95% CI = ± 2.7%) bare ground observed at the first quarterly sampling. Monthly clipping of Lepidium was effective in controlling the invader (Fig. 2a); though Lepidium resprouted vigorously in removal plots during the first month following removal, in subsequent months during the first growing season little to no removal was required. During the spring of the second growing season (April 2012), an average of 13 vegetative resprouts was present per 4-m2 treatment area. In September 2012, light levels at the soil surface varied between 1.8 µmol s−1 m−2 in the most vegetated control plot to 61 µmol in the least vegetated removal plot and were on average 2.02 times greater in removal plots than in paired control plots (95% CI ± 0.46), i.e., light levels were between 1.56 and 2.48 times greater in removal plots.
a Percent cover of live Lepidium over time in removal compared to control (no removal) plots. Relative cover of b native species and c non-native species besides Lepidium, and species richness of d native and e non-native species in removal and control plots over time. Error bars indicate 95% confidence intervals
By the second growing season of the experiment, Lepidium removal significantly increased cover of native species (p < 0.01 for effect of treatment in September 2011, March 2012, June 2012, and September 2012, Fig. 2b). Total species richness was significantly higher in removal compared to control plots (F1,79 = 23.00, p < 0.0001; treatment by time interaction not significant). Initially, this effect was due to an increase in non-native species at the first quarterly re-sampling (June 2011). In subsequent sampling, both native and non-native species were encouraged by Lepidium removal, with non-natives (primarily annuals and senescing perennials) less consistently enhanced in fall (Fig. 2b–e).
Aside from Cirsium, which is a biennial species, the most abundant species in experimental plots were all perennials. In order of decreasing total cover values over all months, these were Lepidium; Schoenoplectus americanus (chairmaker’s bulrush), a dominant, native brackish marsh species; Potentilla anserina ssp. pacifica (Pacific silverweed), a native herb; Cirsium; Epilobium ciliatum ssp. ciliatum (willow herb), a native herb; Grindelia stricta (marsh gumplant), a native shrub; and Juncus balticus, (Baltic rush), a widespread native rush at this site. Figure S1 (Online Resource 1) shows cover of some of these species over time in removal versus control plots. Notably, some non-native species, including the annual Atriplex prostrata (fat hen), annual or biennial Apium graveolens (wild celery), and annual or perennial Helminthotheca echioides (bristly ox-tongue), showed trends towards higher mean cover in removal plots during the first growing season, but not in the second. The first season was during a wet year, and the second season was much drier, with 75% of average rainfall in the region (National Weather Service 2022), and was the first of several years of drought (California Department of Water Resources 2021). The one removal plot with high cover of a non-native species (Helminthotheca echioides, bristly ox-tongue) in the first year also had the highest Cirsium cover and number of individuals by the end of the experiment.
The PERMANOVA showed a significant effect of Lepidium removal on plant species composition in June 2012 (F1,11 = 0.99, p = 0.03). Relative to control plots, species with the largest responses to Lepidium removal were the natives Schoenoplectus americanus, Epilobium ciliatum ssp. ciliatum, Juncus balticus, and Cirsium (Table S2, Online Resource 1).
Lepidium thatch did not build up in removal plots, and litter layer depth was significantly greater in control plots than removal plots (p < 0.05 in all months following initial Lepidium removal treatment; Table S3, Online Resource 1).
Response of Cirsium to Lepidium Removal
By September 2012, only one control plot contained Cirsium, compared to four removal plots (Fig. 3a). Removal showed a high effect size on percent cover of Cirsium (removal plot mean = 22.33, SD = 29.66, n = 6; control plot mean = 0.67, SD = 1.63, n = 6; Hedges’ g = 1.00, 95% CI = − 0.20, 2.20, Fig. 3b) and a high effect size on number of Cirsium (removal plot mean = 3.50, SD = 4.93, n = 6; control plot mean = 0.17, SD = 0.41, n = 6; Hedges’ g = 0.88, 95% CI = − 0.31, 2.06, Fig. 3c), though confidence intervals were large on the effect sizes (Cohen 1988). Recruitment of Cirsium was very high at one removal plot in particular, which contained 13 individuals at the end of the experiment.
Cirsium in removal and control plots over time. a Proportion of control plots containing Suisun thistle, b mean percent cover, c mean number of plants, d mean viable seeds per plant (Aug. 2012), e mean Lasioderma haemorrhoidale larvae per seedhead (Aug. 2012), f mean seed set per inflorescence (Aug. 2012), and g mean maximum height (Aug. 2012). Error bars indicate 95% confidence intervals
Annual Measurements of Reproductive Cirsium Plants
In 2012, removal showed a medium effect size on viable seed production per plant (removal plot mean = 124.78, SD = 113.68, n = 4; control plot mean = 51.79, SD = 28.07, n = 3; Hedges’ g = 0.68, 95% CI = − 0.80, 2.35, Fig. 3d) and a large effect size on number of L. haemorrhoidale larvae per seedhead (removal plot mean = 1.22, SD = 1.67, n = 6; control plot mean = 3.17, SD = 1.45, n = 4; Hedges’ g = 1.10, 95% CI = − 0.20, 2.59, Fig. 3e). Removal showed a medium effect size on mean seed set per inflorescence during 2012 (removal plot mean = 25.0, SD = 21.8, n = 6; control plot mean = 12.7, SD = 8.13, n = 4; Hedges’ g = 0.62, 95% CI = -0.64,1.98, Fig. 3f). Removal showed a low effect size on mean maximum height of Cirsium (removal plot mean = 115.10, SD = 20.61, n = 5; control plot mean = 120.92, SD = 7.32, n = 3; Hedges’ g = 0.29, 95% CI = − 1.73, 1.75, Fig. 3g.)
Effect of Lepidium Removal on Soil Properties
There was no treatment effect on soil salinity, moisture, or organic content. The PCA on nine soil variables (Fig. 4) resulted in three factors with Eigenvalues > 1. PC1 explained 37% of the variance in soil variables and was highly negatively correlated with Na+ cations (R2 = 0.73, p < 0.0001), moisture (R2 = 0.61, p < 0.0001), and salinity (R2 = 0.60, p < 0.0001). Strong interannual differences in these soil conditions are indicated by significantly higher PC1 scores in March 2011 than March 2012 (F1,15 = 4.80, p = 0.04). There was no Lepidium removal effect on PC1.
PC2 explained 32% of the variance in soil variables and was positively correlated with K+ cations (R2 = 0.76, p < 0.0001) and Mg2+ cations (R2 = 0.57, p < 0.0001) and negatively correlated with organic matter (R2 = 0.45, p = 0.0005) and moisture (R2 = 0.23, p = 0.02). Scores for PC2 were significantly higher in control plots than removal plots in 2012 (t = 2.3417, df = 8.701, p = 0.04); thus, removal of Lepidium was associated with lower K+ and Mg+ and higher organic matter and moisture. PC2 scores were higher in 2012 than in 2011 across all treatments (Wilcoxon rank sum test, W = 25, p = 0.01).
PC3 explained 15% of the variance in soil variables and was mainly correlated with phosphorus (R2 = 0.65, p < 0.0001). PC3 values were significantly higher in 2011 than 2012 (F1,18 = 5.75, p = 0.03) and showed no treatment effect.
Unmanipulated Survey Sites
In unmanipulated survey sites, soil salinity in the deeper section only (2–6 cm) was affected by month (F5,89 = 43.00; p < 0.0001), and there was a trend of a treatment effect, with salinity tending to be lowest beneath dense Lepidium, and higher beneath both Cirsium and mixed Lepidium (F2,17 = 2.69, p = 0.10, Fig. 5a). Soil organic content (0–2 cm depth only) differed significantly by treatment in July 2011 (mixed Lepidium > dense Lepidium, F2,18 = 4.15, p = 0.03) and July 2012 (mixed Lepidium > Cirsium, F2,18 = 4.23, p = 0.03) (Fig. 5b). Soil moisture was not significantly different by month or treatment. The difference between soil moisture at 0–2 cm and 2–6 cm tended to be lower beneath Cirsium and higher beneath Lepidium, most notably in July 2011 (F2,17 = 2.73, p = 0.09) and January 2012 (Kruskal–Wallis χ2 = 5.276, df = 2, p = 0.07) (Fig. 5c).
Leaf %P, %N, and %C were all significantly higher in Lepidium than in Cirsium and did not differ significantly between Lepidium stand types (Table 1). Plant C:N was significantly higher in Cirsium than in Lepidium, due to relatively lower %N in Cirsium. Plant N:P was not significantly different between Cirsium and Lepidium. Soil from beneath Cirsium and Lepidium did not contain significantly different %P, %N, %C, C:N, or N:P (Table 1).
PC1 explained 71% of the variance in soil properties beneath Cirsium and was most highly correlated with soil moisture (2–6 cm depth), %C, organic content (both depth segments), and %N (Table S4, Online Resource 1). Mean number of inflorescences per plant was significantly positively correlated with PC1 (R2 = 0.63, p = 0.04), as was mean number of stems per plant (R2 = 0.68, p = 0.03) (Fig. S2, Online Resource 1). PC2 explained 14% of the variance in soil properties and was not significantly correlated with any plant measurements. None of the Lepidium plant characteristics we measured in unmanipulated plots were significantly correlated with PC1 or PC2 scores for Lepidium soil properties. A summary of plant measurements is given in Table S5 (Online Resource 1).
Discussion
Both theoretical models and empirical research indicate that invasive species impacts on species richness are greater at small spatial scales (Gaertner et al. 2009; Powell et al. 2011, 2013) and that invasive species present critical additional stress to rare species (Walck et al. 1999; Brown and Mitchell 2001; Thomson 2005a, b; Dangremond et al. 2010; Combs et al. 2011). At the small spatial scale of our study, we also found evidence suggesting that Lepidium has a variety of effects on Cirsium, including competition for space, nutrients, and light, alteration of soil physicochemical characteristics, and interference with reproductive success, which taken together may be driving this endangered species along a path towards extinction (per Gilbert and Levine 2013; Downey and Richardson 2016). Though our results are specific to our study system, Lepidium impacts a variety of wetland habitats across different countries, and the interactions between invasive and rare species are of global relevance. We hope that this approach will inform research and management discussions in other places and systems.
Response of Plant Community to Lepidium Removal
Removal plots revegetated rapidly, with an increase in native species cover and overall species richness, indicating that the marsh contains a diversity of native propagules. The species that colonized removal plots generally reflected the vegetation composition adjacent to the plots, which varied across the study area. Although increased native cover and overall species richness was consistent with our hypothesis, native species also persisted in control plots, despite the presence of Lepidium. These areas of the marsh may be too frequently inundated for Lepidium to establish monotypic stands as it does in higher elevation zones (i.e., the marsh-terrestrial ecotone and levees).
Although some non-native species besides Lepidium were present in experimental plots, their abundance decreased during the drier second year; thus, removing Lepidium using this method did not facilitate establishment of other invasive plants. As mentioned above, the one removal plot with high cover of a non-native species (Helminthotheca echioides, bristly ox-tongue) in the first year also had the highest Cirsium cover and number of individuals by the end of the experiment.
Response of Cirsium
By the end of the Lepidium removal experiment, Cirsium was present in one control plot and four removal plots. Cirsium number and cover showed positive effects of Lepidium removal over time in removal plots, which was concordant with our hypothesis. Cirsium may have been released from direct competition with Lepidium for light and nutrients. Because native vegetation and associated litter allow more light to reach the soil surface than Lepidium and its thatch, Cirsium seeds may have had increased chances of germination and survival. As we found higher levels of %N and %P in Lepidium leaves than Cirsium leaves in survey sites, removing Lepidium likely left more nutrients for uptake by other species. As noted above, Cirsium inflorescences per plant and stems per plant were significantly positively correlated PC1 (which was highly correlated with %N) at unmanipulated survey sites. Interspecific competition combined with limited reproductive output in Cirsium (Schneider and Boyer 2021) probably explains why we did not see a larger increase in Cirsium with Lepidium removal.
Lepidium removal may have had a positive effect on Cirsium’s production of viable seeds per plant because they had more resources available to them. Additionally, while Lepidium blooms are vast, showy, and fragrant, dominant native species are either wind pollinated (Schoenoplectus americanus and Juncus balticus, for instance) or do not form large, dense blooms (e.g., Epilobium ciliatum ssp. ciliatum). Thus, Cirsium may have a greater chance of attracting pollinators when not growing directly with Lepidium. Alternatively, we may not have observed significant differences in seed set because all plots were surrounded by dense Lepidium; i.e., the matrix environment with respect to pollinators was fairly similar.
Notably, there were significant differences in Cirsium reproductive output between plants in experimental plots (removal and control plots combined) and those growing outside of dense Lepidium blooms measured in a separate study (Schneider and Boyer 2021). Seed set in experimental plots was 19.7 seeds per inflorescence (95% CI ± 4.5) compared to 33.7 seeds outside of the experiment (95% CI ± 4.7). There were 1.5 L. haemorrhoidale larvae per seedhead (95% CI ± 0.6) in experimental plots compared to 0.4 larvae per seedhead (95% CI ± 0.2) outside of the experiment. Finally, 17.4% of all seeds collected from the removal experiment germinated, compared to 34.2% of seeds collected from other areas. Vast Lepidium blooms may attract generalist pollinators, decreasing their visits to Cirsium plants, which could lead to reduced seed set in the latter. Lepidium may also provide suitable habitat for L. haemorrhoidale, or there may be other environmental variables affecting seed set, seed viability, and seed predation that correlate with Lepidium presence.
Effects of Lepidium on Soil Properties: Combined Evidence from Removal Experiment and Survey Sites
In addition to direct competition and interference with reproductive success, experimental and survey data point to a positive feedback loop in which Lepidium modifies soil properties towards conditions conducive to its growth (Reynolds and Boyer 2010). This feedback loop may increase rarity in Cirsium by magnifying the impacts of Lepidium discussed above and may impact the brackish marsh ecosystem as a whole through larger scale shifts in soil properties. Such ecosystem modifications have been demonstrated by other tidal marsh invaders such as non-native genotypes of Phragmites australis on the East Coast (Windham and Ehrenfeld 2003) and hybrid Spartina (Spartheina alterniflora × Spartina foliosa) (Levin et al. 2006).
The largest responses of soil properties to Lepidium removal were decreases in K+ and Mg2+. Notably, PC2 scores were lower in Lepidium removal plots than in controls after 1 year of treatment and higher in 2012 than in 2011, as were overall levels of salinity and Na+ in 2012. That K+ and Mg2+ were lower in removal plots, while at the same time salinity, Na+, K+, and Mg2+ were higher overall in 2012 than 2011, suggests that Lepidium differentially affects those cations. In another study, Lepidium was found to have higher levels of K+ and Mg2+ in its tissues than wheatgrass (Elytrigia elongata), so perhaps removing Lepidium led to a decrease of these cations in the soil (Blank and Young 2002). Lepidium may also be actively modifying its soil environment. Blank and Young (2002) followed Lepidium’s invasion of a grassland over time and documented that Lepidium increased Mg2+, K+, and Ca2+ in soils, possibly by preferentially binding these cations to the soil complex in place of Na+ to meet its higher requirements for these nutrients. The Na+ may then leach down in the soil when it becomes inundated (Renz and Blank 2004), possibly explaining why lower Na+ was observed beneath Lepidium in that study. Sodium cations, however, were not higher in removal than control plots, so perhaps anions or other factors contributing to salinity were at work in this study. Consistently higher mean salinity beneath mixed stands compared to dense Lepidium provides further evidence that Lepidium may be lowering soil salinity. Reynolds and Boyer (2010) also found lower salinity in dense Lepidium compared to Salicornia pacifica. Since 2012 was the first year of a prolonged drought (California Department of Water Resources 2021), it is possible that overall higher salinity levels in 2012 due to low rainfall may have made it difficult to detect changes in salinity resulting from Lepidium.
If Lepidium is indeed reducing soil salinity, this could put Cirsium and other native brackish marsh species at a disadvantage, as salinity tolerance can come with the tradeoff of reduced competitive ability (Bertness 1992; Pennings and Bertness 2001). Furthermore, brackish marsh plant community composition shifts during periods of higher and lower rainfall, with more saline periods favoring Cirsium (USFWS 2013). Additional implications could exist if certain native species have unusual requirements for specific cations.
Another notable soil response to Lepidium removal was an increase in organic matter. That Lepidium presence apparently leads to decreased soil organic matter is consistent with a study by Reynolds and Boyer (2010) who found lower organic matter beneath Lepidium than beneath S. pacifica. Indeed, organic matter tended to be higher in mixed than dense Lepidium stands. Lepidium’s persistent thatch layer may retard decomposition and increase conditions favorable for its growth.
Organic matter tended to be lower beneath Cirsium than at Lepidium sites. This is not surprising, as many congeners of Cirsium grow on poor soils, and its conspecific variety, Cirsium hydrophilum var. vaseyi (Mt. Tamalpais thistle) is restricted to serpentine soils, which have very low organic matter. Thus, in the absence of other impacts from Lepidium such as competition for nutrients and light, low organic matter does not appear to limit Cirsium. However, Lepidium’s reduction of soil organic matter has larger implications for the marsh, as organic matter accumulation is critical to marsh accretion in the face of sea level rise (Morris et al. 2002).
A few lines of evidence combine to suggest that Lepidium is decreasing soil moisture. Soil moisture in the Lepidium removal experiment was negatively correlated with PC2, which was higher in control plots than in removal plots. Additionally, the difference in soil moisture between 0–2 cm and 2–6 cm layers was greater in some months beneath Lepidium than Cirsium, suggesting that Lepidium is drying out deeper soil through evapotranspiration via roots. This may negatively affect Cirsium; as noted above, in the unmanipulated survey plots, both Cirsium inflorescences per plant and stems per plant were significantly positively correlated with PC1, which was most highly correlated with soil moisture (2–6 cm depth). Reynolds and Boyer (2010) found lower moisture beneath Lepidium stands compared to pickleweed stands at the same elevation and distance from channels. They suggested that evapotranspiration is greater from Lepidium than pickleweed due to its larger stature and non-succulent tissues. Many species present in the Lepidium removal experiment (such as Cirsium, Grindelia stricta, and Schoenoplectus americanus), by contrast, form tall canopies and have non-succulent tissues, which may explain why the soil moisture response was not greater in Lepidium removal plots.
Management Implications
By demonstrating how Lepidium affects Cirsium and its habitat, this study leads to a key management recommendation, to remove Lepidium in the vicinity of Cirsium, as the effects of Lepidium are reversible. Though labor intensive, the monthly reclipping method used in this study is effective and alleviates concerns about herbicides use and machinery in the vicinity of sensitive species. It could be carried out, potentially on a less frequent basis targeted to the spring and summer growing season, by staff or volunteers trained in discerning Lepidium leaves on early-season rosettes from those of other species. The timeframe for recovery would be expected to be at least two growing seasons, as some areas may initially experience an increase in other non-native species as seen in this experiment.
Treatment areas could be larger than in this study (4 m2) to minimize edge effects from Lepidium reseeding and could be combined with direct reseeding of Cirsium to increase chances of its persistence in these locations. Removal would ideally begin in early spring, before Lepidium resprouts vigorously. Areas in which Lepidium is less dense or just beginning to invade should be prioritized to prevent the development of dense infestations. Control efforts may also have increased effectiveness if timed to drought years; Lepidium has been shown to have lower stand density, seed production, and seed viability at high salinity sites (Leininger and Foin 2009), and recent work documented reduced vigor in Lepidium throughout the San Francisco Estuary due to salinity stress during the extreme drought of 2012–2016 (Kelso et al. 2020; Wigginton et al. 2020). In addition to increasing the presence of Cirsium, removal of Lepidium will also increase cover of other native species and prevent further shifts in soil properties demonstrated in this study such as decreases in organic matter, soil moisture, and soil salinity.
Conclusions
In this study, we examined whether invasive Lepidium is increasing rarity in the endangered Cirsium. Using a removal experiment and a survey of soil properties and plant characteristics in unmanipulated plots, we found evidence suggesting that Lepidium negatively affects Cirsium through several mechanisms, including competition for nutrients and light, changes to soil physicochemical characteristics, and interference with reproductive output and recruitment. As a narrow endemic species within an urbanized and altered estuary, additional impacts from Lepidium may be pushing Cirsium towards dangerously low population numbers. Other rare plants in Suisun Marsh and the larger San Francisco Estuary such as Chloropyron molle ssp. molle (soft bird’s beak) and Symphyotrichum lentum (Suisun Marsh aster) may be similarly disproportionately impacted by Lepidium due to their small population sizes, habitat requirements, and/or life history limitations. The approach and results from this study can inform research and management in other wetland systems experiencing invasion from Lepidium or other ecosystem engineer-type invasive plants.
Effects of Lepidium documented in this study are, of course, occurring within a larger ecosystem context. Not only Cirsium, but also other plant species along with their pollinators, consumers, and decomposers are facing new abiotic conditions and biotic interactions, particularly with respect to altered soil physiochemical processes created by the invader. As in this study, quantifying and elucidating the mechanisms at work will help identify appropriate management actions, as well as strengthen our empirical understanding of invasive species effects.
References
Able, K.W., S.M. Hagan, and S.A. Brown. 2003. Mechanisms of marsh habitat alteration due to Phragmites: Response of young-of-the-year Mummichog (Fundulus heteroclitus) to treatment for Phragmites removal. Estuaries 26: 484–494.
Bertness, M.D. 1992. The ecology of New England salt marsh plant communities. American Scientist 80: 260–268.
Blank, R.R., and J.A. Young. 2002. Influence of the exotic invasive crucifer, Lepidium latifolium, on soil properties and elemental cycling. Soil Science 167: 821–829.
Boyer, K., and A. Burdick. 2010. Control of Lepidium latifolium (perennial pepperweed) and recovery of native plants in tidal marshes of the San Francisco Estuary. Wetlands Ecology and Management 18: 731–743.
Bray, R.H., and L. Kurtz. 1945. Determination of total, organic, and available forms of phosphorus in soils. Soil Science 59: 39–46.
Brophy, L.S., C.M. Greene, V.C. Hare, B. Holycross, A. Lanier, W.N. Heady, K. O’Connor, H. Imaki, T. Haddad, and R. Dana. 2019. Insights into estuary habitat loss in the western United States using a new method for mapping maximum extent of tidal wetlands. PLoS ONE 14 (8): e0218558.
Brown, B.J., and R.J. Mitchell. 2001. Competition for pollination: Effects of pollen of an invasive plant on seed set of a native congener. Oecologia 129: 43–49.
Burkle, L.A., J.R. Mihaljevic, and K.G. Smith. 2012. Effects of an invasive plant transcend ecosystem boundaries through a dragonfly-mediated trophic pathway. Oecologia 170: 1045–1052.
California Department of Water Resources. 2021. California’s drought of 2012–2016: An overview.
Cohen, J. 1988. Statistical power analysis for the behavioral sciences, 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates, Publishers.
Combs, J.K., S.H. Reichard, M.J. Groom, D.L. Wilderman, and P.A. Camp. 2011. Invasive competitor and native seed predators contribute to rarity of the narrow endemic Astragalus sinuatus Piper. Ecological Applications 21: 2498–2509.
Connolly, B.A., and I.L. Hale. 2016. Lepidium latifolium (Brassicaceae): Invasive perennial pepperweed observed in Rhode Island. Rhodora 118: 229–231.
Dangremond, E.M., E.A. Pardini, and T.M. Knight. 2010. Apparent competition with an invasive plant hastens the extinction of an endangered lupine. Ecology 91: 2261–2271.
Downey, P.O., and D.M. Richardson. 2016. Alien plant invasions and native plant extinctions: A six-threshold framework. AoB Plants. https://doi.org/10.1093/aobpla/plw047.
Fiedler, P.L., M.E. Keever, B.J. Grewell, and D.J. Partridge. 2007. Rare plants in the Golden Gate Estuary (California): The relationship between scale and understanding. Australian Journal of Botany 55: 206–220.
Francis, A., and S.I. Warwick. 2007. The biology of invasive alien plants in Canada. 8. Lepidium latifolium L. Canadian Journal of Plant Science 87: 639–658.
Frankham, R. 2005. Genetics and extinction. Biological Conservation 126: 131–140.
Gaertner, M., A. Den Breeyen, C. Hui, and D.M. Richardson. 2009. Impacts of alien plant invasions on species richness in Mediterranean-type ecosystems: A meta-analysis. Progress in Physical Geography 33: 319–338.
Gilbert, B., and J.M. Levine. 2013. Plant invasions and extinction debts. Proceedings of the National Academy of Sciences 110: 744–1749.
Goals Project. 1999. Baylands Ecosystem Habitat Goals. A report of habitat recommendations prepared by the San Francisco Bay Area Wetlands Ecosystem Goals Project. U.S. Environmental Protection Agency, San Francisco CA/ San Francisco Bay Regional Water Quality Control Board, Oakland, CA.
Goals Project. 2000. Baylands ecosystem species and community profiles: life histories and environmental requirements of key plants, fish and wildlife. San Francisco Bay Regional Water Quality Control Board, Oakland, CA.
Goals Project. 2015. The baylands and climate change: what we can do. Baylands Ecosystem Habitat Goals Science Update 2015. San Francisco Bay Area Wetlands Ecosystem Goals Project, California State Coastal Conservancy, Oakland, CA.
Graham-Bruno, R., M.A. Stickrod, and V.T. Parker. 2023. Constraints on Cirsium hydrophilum var. hydrophilum, the Suisun thistle, an endangered tidal wetland species. Wetlands Ecology and Management 31: 347–365.
Greene, E.L. 1892. New or noteworthy thistles. Proceedings of the National Academy of Sciences 44: 352–363.
Grossinger, R., J. Alexander, A.N. Cohen, J.N. and Collins. 1998. Introduced tidal marsh plants in the San Francisco Estuary. San Francisco Estuary Institute, Oakland, CA.
Hogle, I., R. Spenst, S. Leininger, and G. Block. 2007. San Pablo Bay national wildlife refuge Lepidium latifolium control plan. U.S. Fish and Wildlife Service, San Pablo Bay National Wildlife Refuge, Petaluma, CA.
Huston, M.A. 1999. Local processes and regional patterns: Appropriate scales for understanding variation in the diversity of plants and animals. Oikos 86: 393–401.
Kelso, M.A., R.D. Wigginton, and E.D. Grosholz. 2020. Nutrients mitigate the impacts of extreme drought on plant invasions. Ecology 101: e02980.
Leininger, S.P., and T.C. Foin. 2009. Lepidium latifolium reproductive potential and seed dispersal along salinity and moisture gradients. Biological Invasions 11: 2351–2365.
Levin, L.A., C. Neira, and E.D. Grosholz. 2006. Invasive cordgrass modifies wetland trophic function. Ecology 87: 419–432.
Miller, G.K., J.A. Young, and R.A. Evans. 1986. Germination of seeds of perennial pepperweed (Lepidium latifolium). Weed Science 34: 252–255.
Morris, J.T., P.V. Sundareshwar, C.T. Nietch, B. Kjerfve, and D.R. Cahoon. 2002. Responses of coastal wetlands to rising sea level. Ecology 83: 2869–2877.
National Weather Service. 2022. San Francisco / Monterey Bay area monthly climate summary. https://www.wrh.noaa.gov/mtr/climate/monthly_summary/summary.php
Oksanen, J., F. Guillaume Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P.R. Minchin, R.B. O'Hara, G.L. Simpson, P. Solymos, M.H. Stevens, E. Szoecs, and H. Wagner. 2018. Vegan: community ecology package. R package version 2.5–2. https://CRAN.R-project.org/package=vegan
Orth, J.F., M. Gammon, F. Abdul-Basir, R.D. Stevenson, D. Tsirelson, J. Ebersole, S. Speak, and R. Kesseli. 2006. Natural history, distribution, and management of Lepidium latifolium (Brassicaceae) in New England. Rhodora 108: 103–118.
Pennings, S.C., and M.D. Bertness. 2001. Salt marsh communities. In: Marine Community Ecology. M. D. Bertness, S.D. Gaines, and M. Hay (Editors). Sinauer Associates, Sunderland, MA.
Powell, K.I., J.M. Chase, and T.M. Knight. 2011. A synthesis of plant invasion effects on biodiversity across spatial scales. American Journal of Botany 98: 539–548.
Powell, K.I., J.M. Chase, and T.M. Knight. 2013. Invasive plants have scale-dependent effects on diversity by altering species-area relationships. Science 339: 316–318.
R Studio Team. 2016. RStudio: integrated development for R. RStudio, Inc., Boston, MA. http://www.rstudio.com/
Renz, M.J., and R.R. Blank. 2004. Influence of perennial pepperweed (Lepidium latifolium) biology and plant-soil relationships on management and restoration. Weed Technology 18: 1359–1363.
Renz, M.J. 2000. Element Stewardship abstract for Lepidium latifolium L., perennial pepperweed, tall whitetop. The Nature Conservancy, Wildland Invasive Species Team. Arlington, VA.
Reynolds, L.K., and K.E. Boyer. 2010. Perennial pepperweed (Lepidium latifolium): Properties of invaded tidal marshes. Invasive Plant Science and Management 3: 130–138.
Robison, A.L., J.L. Berta, C.L. Mott, and K.J. Regester. 2021. Impacts of invasive Amur honeysuckle, Lonicera maackii, leaf litter on multiple trophic levels of detritus-based experimental wetlands. Freshwater Biology 66: 1464–1474.
Roldán, F.P., J.M. Pichardo, H. Vibrans, F.S. Flores, and I.G. Iniestra. 2009. Weeds of Mexico – Lepidium latifolium. http://www.conabio.gob.mx/malezasdemexico/brassicaceae/lepidium-latifolium/fichas/pagina1.htm. Accessed 14 July 2023.
Schneider, R.S., and K.E. Boyer. 2021. Limiting life history stages in the endangered Suisun thistle. Cirsium Hydrophilum Var. Hydrophilum. Madroño 68: 39–51.
Schoenig, S., S. Monheit, and M. Caldwell. 2003. California’s most wanted weeds. California Interagency Noxious Weed Coordinating Committee. Noxious times 4: 10–17.
Shaffer, M.L. 1981. Minimum population sizes for species conservation. BioScience 31: 131–134.
Spenst, R. 2006. The biology and ecology of Lepidium latifolium L. in the San Francisco Estuary and their implications for eradication of the invasive weed. PhD Dissertation, University of California, Davis, Davis, CA.
Surles, W.W., and L.T. Kok. 1978. Carduus thistle seed destruction by Rhinocyllus conicus. Weed Science 26: 264–269.
Thomson, D.M. 2005a. Measuring the effects of invasive species on the demography of a rare endemic plant. Biological Invasions 7: 615–624.
Thomson, D.M. 2005b. Matrix models as a tool for understanding invasive plant and native plant interactions. Conservation Biology 19: 917–928.
U.S. Fish and Wildlife Service. 2013. Recovery plan for tidal marsh ecosystems of Northern and Central California. Sacramento Fish and Wildlife Office, Sacramento, CA.
Vila, M., J.L. Espinar, M. Hejda, P.E. Hulme, V. Jarosik, J.L. Maron, J. Pergl, U. Schaffner, Y. Sun, and P. Pysek. 2011. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecology Letters 14: 702–708.
Walck, J.L., J.M. Baskin, and C.C. Baskin. 1999. Effects of competition from introduced plants on establishment, survival, growth and reproduction of the rare plant Solidago shortii (Asteraceae). Biological Conservation 88: 213–219.
Whitcraft, C.R., B.J. Grewell, and P.R. Baye. 2011. Estuarine vegetation at Rush Ranch Open Space Preserve, San Franciso Bay National Estuarine Research Reserve, California. San Francisco Estuary and Watershed Science 9 (3): 1–29.
Wigginton, R.D., J. Pearson, and C.R. Whitcraft. 2014. Invasive plant ecosystem engineer facilitates community and trophic level alteration for brackish marsh invertebrates. Ecosphere 5: 1–17.
Wigginton, R.D., M.A. Kelson, and E.D. Grosholz. 2020. Time-lagged impacts of extreme, multi-year rain on tidal salt marsh plant invasion. Ecosphere 11: e03155.
Wigginton, R.D., C. Van Grootheest, H. Spautz, J.L. Grenier, and C.R. Whitcraft. 2022. Stable isotope mixing models demonstrate the role of an invasive plant in wetland songbird food webs. Applications in Plant Sciences 10: e11486.
Windham, L., and J.G. Ehrenfeld. 2003. Net impact of a plant invasion on nitrogen-cycling processes within a brackish tidal marsh. Ecological Applications 13: 883–896.
Windham, L., and L.A. Meyerson. 2003. Effects of common reed (Phragmites australis) expansions on nitrogen dynamics of tidal marshes of the northeastern US. Estuaries 26: 452–464.
Young, J.A., C.E. Turner, and L.F. James. 1995. Perennial pepperweed. Rangelands 17: 121–123.
Zavaleta, E. 2000. The economic value of controlling an invasive shrub. Ambio 29: 462–467.
Zedler, J.B., and S. Kercher. 2004. Causes and consequences of invasive plants in wetlands: Opportunities, opportunists, and outcomes. Critical Reviews in Plant Sciences 23: 431–452.
Acknowledgements
We thank the many Boyer Lab students and interns who assisted in the field and lab. V. Thomas Parker, John Hafernik, and Matthew Ferner helped improve earlier versions of this manuscript. We appreciate study site access from the Solano Land Trust. This study was funded by the National Estuarine Research Reserve Graduate Research Fellowship, National Science Foundation Training in Research in Ecology and Evolution Fellowship, and awards from the California State University Council on Ocean Affairs, Science, and Technology, Northern California Botanists, and San Francisco State University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Holly Greening
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schneider, R.S., Buchbinder, M.V. & Boyer, K.E. Contributions of Perennial Pepperweed (Lepidium latifolium) Invasion to Rarity of the Endangered Suisun Thistle (Cirsium hydrophilum var. hydrophilum). Estuaries and Coasts 47, 622–636 (2024). https://doi.org/10.1007/s12237-024-01326-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-024-01326-x