Abstract
Coastal systems are immensely valuable to humans. They contain unique ecosystems that are biodiversity reservoirs and provide key ecosystem services as well as a wealth of cultural heritage. Despite their importance to humans, many coastal systems are experiencing degradation that threatens their integrity and provisioning of services. While much is known about the plant communities and associated wildlife in coastal areas, the importance of microorganisms represents a large knowledge gap. Here we review the ecology of plant-microbial symbioses in coastal systems, including mycorrhizae, nitrogen fixers, endophytes, rhizosphere microbes, and pathogens. We focus on four common coastal communities: sand dunes, marshes, mangroves, and forests/shrublands. We also assess recent research and the potential for using microbes in coastal restoration efforts to mitigate anthropogenic impacts. We find that microbial symbionts are largely responsible for the health of plants constituting the foundation of coastal communities by affecting plant establishment, growth, competitive ability, and stress tolerance, as well as modulating biogeochemical cycling in these stressful coastal systems. Current use of microbial symbionts to augment restoration of stressful and degraded coastal systems is still very much in its infancy; however, it holds great promise for increasing restoration success on the coast. Much research is still needed to test and develop microbial inocula for facilitating restoration of different coastal systems. This is an excellent opportunity for collaboration between restoration practitioners and microbial ecologists to work toward a common goal of enhancing resilience of our coastal ecosystems at a time when these systems are vulnerable to an increasing number of threats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coastal areas provide immense value to humans. They represent 22% of the land area worldwide while providing home to 38% of the human population and 50% of the world’s largest cities (Kummu et al. 2016). Coastal areas contain unique ecosystems that are biodiversity reservoirs, and they provide key ecosystem services including food production, storm surge protection, filtration of pollutants, carbon storage, recreation, and cultural heritage (UNEP 2006). Despite their importance to humans, many coastal systems are experiencing degradation from a number of stressors that threaten their integrity and provisioning of ecosystem services (Millennium Ecosystem Assessment 2005).
Previous reviews on coastal ecosystems have investigated the current status of coastal systems (Burke et al. 2001; UNEP 2006), climate change impacts (Field et al. 2001; Scavia et al. 2002), resilience to climate change (Bernhardt and Leslie 2013; Duarte et al. 2015), and management approaches (Powell et al. 2019; Spalding et al. 2014). These reviews have well documented that anthropogenic global change has a major impact on coastal systems. Climate warming is causing sea level rise and inundation and degradation of coastal ecosystems such as salt marshes (Jankowski et al. 2017; Kirwan and Megonigal 2013) and sand dunes (Feagin et al. 2005). Warming is also linked to increased intensity and frequency of storms making landfall in coastal areas (Knutson et al. 2010). Pollution from industry, agriculture, and oil spills has caused marsh and mangrove degradation and erosion (Kingsford et al. 2016; Silliman et al. 2012). Previous reviews have stressed the difficulties and idiosyncratic nature of restoration and recovery of coastal systems, with partial rather than full recovery prevailing and the existence of feedbacks that maintain coastal systems in degraded states (Duarte et al. 2015). Past work further highlights the importance of biodiversity and connectivity in sustaining the resilience of coastal systems (Bernhardt and Leslie 2013; Duarte et al. 2015).
The purpose of this review with a plant-microbial focus is twofold. First, despite much research on coastal systems, one identified knowledge gap is the ecology and importance of microorganisms (UNEP 2006). We aim to take a first step by reviewing literature on the ecology of plant-microbial symbioses in coastal ecosystems. A baseline understanding of these microscopic components of ecosystems is important for land managers and restoration ecologists, because microbes too are shifting with climate change and have important consequences for the functioning of coastal systems (Cavicchioli et al. 2019). Second, it is clear that there is an overwhelming sense of urgency to act expeditiously to reverse degradation from climate change in coastal areas because of our reliance on coastal ecosystems (UNEP 2006). While many general and specific solutions have been put forth to enhance restoration and management success (Bernhardt and Leslie 2013; Perrow and Davy 2002), the potential for plant-microbial interactions to improve restoration success of degraded coastal ecosystems has rarely been considered and not previously reviewed.
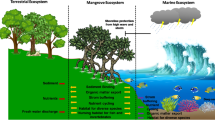
We focus on four main types of coastal ecosystems: sand dunes, marshes, mangroves, and forests/shrublands (Fig. 1). We chose these terrestrial systems, because they are dominated by plants (rather than algae) and have commonalities in how plant–microbe symbioses manifest. For a review of plant–microbe interactions in aquatic systems, see Srivastava et al. (2017). While sand dunes, marshes, mangroves, and forests/shrublands differ in notable ways (abiotic conditions, proximity to land–water interface, biotic communities), they are similar in that they are all stressful environments, as a result of low nutrients, high salt, or drought conditions. It is generally thought that stressful environments have more positive plant–microbe interactions (Bertness and Callaway 1994; Lekberg et al. 2018) and that stressful systems, in particular, would benefit from microbial mutualists to aide in successful establishment and persistence of plants during restoration (Valliere et al. 2020).
The Role of Plant–Microbe Symbioses in Ecosystems
Symbiosis (from Greek, “living together”) is a close and long-term biological interaction between two different species. Symbioses can be mutualistic (+/+), commensalistic (+/0), or parasitic/pathogenic (+/−). Microbes are important symbionts of plants that play key roles in ecosystems by affecting plant performance (as mutualists and pathogens) and by mediating nutrient cycling.
Fungi and prokaryotes (bacteria and archaea) play direct roles in plant establishment, growth, competitive ability, and stress tolerance. Essentially, all plants host microbes within and on every plant organ and tissue: leaves, stems, rhizomes, roots, flowers, and seeds (Partida-Martinez and Heil 2011). These microbiomes are so essential to the plant that a plant’s characteristics are a manifestation of highly coordinated and co-regulated plant and microbial genes. Microbial endophytes in plant seeds can increase germination rate (Berg and Raaijmakers 2018; Billingsley Tobias et al. 2017), and soil and root microbes such as arbuscular mycorrhizal fungi, ectomycorrhizal fungi, and actinorhizae can increase plant establishment (Koziol et al. 2018), especially in stressful habitats (Roy et al. 2007; Shemesh et al. 2020). Microbes also dictate plant competitive ability; for example, when fungicide is used to suppress fungal symbionts, dominant grasses no longer suppress subdominant plants to the same degree (Hartnett and Wilson 1999; O’Connor et al. 2002). Plant growth promoting rhizobacteria can enhance plant growth through mechanisms including nitrogen fixation, nutrient uptake, and production of plant growth hormones (Backer et al. 2018; van Loon 2007). Plant pathogens can have strong negative effects on plant growth and survival, as exemplified by the success of many invasive plant species who have escaped their pathogens in their native range (i.e., the enemy release hypothesis, Keane and Crawley 2002), as well as by exotic pathogen epidemics that have virtually wiped out native plant species (i.e., the chestnut blight, Anagnostakis 1987). Some endophytic fungi have been found to increase stress tolerance of plants; for example, inoculating plants with endophytes, especially endophytes isolated from saline areas, can substantially increase the growth of plants subjected to salt stress (Rodriguez et al. 2008; Soares et al. 2016). Because microbes benefit plants in stressful conditions, microbes will be key in helping plants tolerate stresses (e.g., heat, drought, salinity) due to climate change (Porter et al. 2019).
Microbial communities associated with plants play critical roles in biogeochemical cycling in ecosystems (Beinart 2019). Because symbionts are often protected by hosts and supported by host substrates, they can obtain large population sizes and high activity levels (Beinart 2019). Ectomycorrhizae and dark septate endophytes are plant symbionts that play important roles in decomposition, particularly of recalcitrant organic matter, through the production of extracellular degradative enzymes (Moreau et al. 2019). In the rhizosphere, plant roots produce exudates (labile carbon compounds) that stimulate symbiotic bacterial and fungal activity, called “priming the soil”. This rhizosphere priming typically enhances carbon (C) and importantly nitrogen (N) mineralization, which can then be used to meet microbial and plant N demands (Henneron et al. 2020; Moreau et al. 2019). Arbuscular mycorrhizal fungi (AMF), obligate plant symbionts whose hyphae extend in a complex network throughout the soil environment, play an important role in ecosystem carbon sequestration by promoting soil aggregate formation which physically protects soil organic matter (SOM) from degradation (Rillig 2004). Furthermore, ectomycorrhizal- and AMF-derived carbon is a significant component of SOM and contains recalcitrant compounds (e.g., glomalin, chitin) that resist decomposition (Parihar et al. 2020; Rillig 2004; Soudzilovskaia et al. 2015; Wilson et al. 2009). Plant-associated symbionts are also key players in the nitrogen cycle. Nitrogen fixation (conversion of N2 gas to biologically available ammonium) is often performed by internal root bacteria, such as Rhizobium (in legumes) and Frankia (in actinorhizal plants), or bacteria in the rhizosphere of plants (Moreau et al. 2019). Particularly in flooded, anaerobic, and wetland sediments, bacterial symbionts in the rhizosphere perform the vast amount of nitrification (oxidation of ammonium to nitrate that can be leached, taken up by plants, or denitrified), because it is an aerobic process that can be fueled by oxygen flux through plant roots (Penton et al. 2013; Reddy et al. 1989). Denitrification (nitrate and nitrite reduced to NO, N2O, and N2 gases and returned to the atmosphere) is often coupled to nitrification and can be stimulated by rhizodeposition under more anaerobic conditions (Penton et al. 2013; Reddy et al. 1989).
Plant symbionts also influence biogeochemical cycling in indirect ways via their effects on plant growth, plant species composition, and soil microbial composition (Beinart 2019; Rillig 2004). Both beneficial and disease-causing microbes influence plant growth and tissue quality, which in turn influence carbon and nutrient cycling via biomass, litter, and exudate production (Rillig 2004). Numerous studies have shown that symbiont community composition, for example, AMF composition, can alter plant community composition (Johnson et al. 2003; van der Heijden et al. 1998), which can thereby influence primary production and nutrient cycling via traits of the component plant species (Rillig 2004). Ectomycorrhizae and AMF can also affect soil fungal and bacterial composition (i.e., saprotrophs, nitrogen transformers, phosphate solubilizing bacteria), which in turn influence decomposition, carbon storage, and nutrient cycling (Nuccio et al. 2013; Rillig 2004; Soudzilovskaia et al. 2015).
Plant-Microbial Symbioses on Coasts
We focus on five major types of microbial symbionts: mycorrhizae, nitrogen fixers (also called diazotrophs), endophytes, rhizosphere microbial communities, and pathogens (see Box 1; Fig. 2). Some categories may overlap (for example, nitrogen fixers can be rhizosphere microbes), but these are common categories of symbionts discussed in microbiology and restoration.

Box 1 Definitions and descriptions: common microbial symbionts of plants. A AMF structures (vesicles, hyphae, and arbuscules) in mangrove roots (photo credit: Mareli Sanchez Julia). B N fixers in root nodules of a legume (photo credit: Emily Farrer). C Dark septate endophytes in Spartina alterniflora roots (photo credit: Sunshine Van Bael). D Rhizosphere microbes (photo credit: Andrea Porras-Alfaro). E Leaf spot pathogen (could be from a number of different fungal taxa) in Phragmites australis (photo credit: Warwick Allen). Mycorrhizae: A symbiotic relationship between plants and fungi. The relationship is typically mutualistic, but can be parasitic. The fungi colonize the roots of the host plant providing nutrients and water to the plant in exchange for carbohydrates (photosynthate). Two major types are ectomycorrhizae and endomycorrhizae. Ectomycorrhizae ensheath the root but usually do not penetrate the root cells. Endomycorrhizae penetrate the root cells; the most widespread group of endomycorrhizae is the arbuscular mycorrhizal fungi (AMF). Less common are orchid and ericoid mycorrhizae. Nitrogen fixers (diazotrophs): Nitrogen-fixing bacteria and archaea capable of transforming nitrogen gas (N2) from the atmosphere into ammonia (NH4). Nitrogenase, the enzyme that catalyzes the reaction is degraded by oxygen; thus, nitrogen fixation often occurs in anaerobic conditions. Nitrogen fixers can be free-living, or they can live within a host plant’s roots like the Rhizobia that colonize legumes and the Frankia that colonize actinorhizal plants. Endophytes: Bacteria or fungi that live within a host plant without causing symptoms of disease. Endophytes can be mutualistic or have neutral impacts on hosts. Endophytes can increase host nitrogen acquisition, reduce abiotic stressors like drought or salinity, and can inhibit or facilitate plant pathogens (Busby et al. 2016; Porras-Alfaro and Bayman 2011). Rhizosphere microbes: The rhizosphere is the narrow zone surrounding and influenced by plant roots. Rhizosphere microorganisms include fungi, bacteria, archaea, and algae. Many of these organisms are so-called plant growth promoting microbes which improve plant nutrient acquisition, protect against pathogen attack, facilitate plant growth, and help plants tolerate abiotic stress. However, pathogens can also populate the rhizosphere. Pathogens: Microorganisms that cause disease. Pathogens include fungi, bacteria, oomycetes (“water molds”, a group of filamentous protists), viruses, and nematodes (roundworms). Interestingly, archaea have not been found to be pathogens (Cavicchioli et al. 2003); however, it may be that they have not yet been discovered.
Mycorrhizae
Mycorrhizal fungi form mutualistic associations with plant roots in most habitats, including coastal areas such as salt marshes (d’Entremont et al. 2018), estuaries (Carvalho et al. 2001), mangroves (Sengupta and Chaudhuri 2002), sand dunes (Koske et al. 2008), and heaths (Botnen et al. 2015). The symbiosis between mycorrhizae and plants is especially key for plants in stressful environments (e.g., primary succession in dunes), since mycorrhizae facilitate plants through uptake of nutrients as well as increase tolerance to drought and salt stress (Koske et al. 2008). Arbuscular mycorrhizal fungi are found in association with 74% of plant species (Brundrett 2009), including many trees, shrubs, and grasses that grow on coasts. However, some plant families/taxa that are abundant on coasts are non-mycorrhizal (e.g., the Chenopodiaceae which includes Salicornia, pickleweed) or have low or variable mycorrhizal colonization (e.g., the Cyperaceae, which includes Carex, sedges) (Muthukumar et al. 2004). There is some debate on whether high levels of salinity – like those found in coastal waters and soils – inhibit arbuscular mycorrhizae (Evelin et al. 2009). Moreover, whether arbuscular mycorrhizae act as facilitators or antagonists of plant growth in saline environments may be context and host-species dependent (Evelin et al. 2009; Johnson-Green et al. 2001). Arbuscular mycorrhizal fungi are well known to be important to primary succession on sand dunes as they facilitate grasses, forbs, and creepers in this phosphorus deficient and droughty environment (Beena et al. 2000, 2001; Koske et al. 2008). Ectomycorrhizal fungi are associated with approximately 9% of shrub and tree species (Brundrett 2009). Coastal forests are particularly reliant on associations with ectomycorrhizal fungi in temperate (Obase et al. 2009) and tropical regions (Séne et al. 2015), and primary succession in sand dune systems colonized by Salix, Pinus, and other woody species depends on a high diversity of ectomycorrhizal fungi (Ashkannejhad and Horton 2006; Roy-Bolduc et al. 2015; van der Heijden et al. 2000). There are many gaps in knowledge that exist with respect to mycorrhizal fungi – both for all terrestrial plants and for those along coasts. For example, via nutrient uptake and increased coastal plant growth, mycorrhizae could contribute to carbon sequestration along coasts. Mangroves have pan-tropical distribution, and a few studies have showed that their roots associate with arbuscular mycorrhizal fungi (e.g. Kumar and Ghose 2008), yet no studies have estimated the contribution of mycorrhizae to mangrove nutrient budgets and/or performance. Further study is warranted on the role of mycorrhizae in coastal plants, to better understand their benefits to plants and their contributions to soil formation and a resilient coastal ecosystem.
N-Fixers
Primary production and decomposition are N limited in mangroves (Bashan and Holguin 2002), marshes (Lovell et al. 2000), and sand dunes (Dalton et al. 2004; Wahab and Wareing 1980), and coastal systems are highly dependent on N inputs from plant-associated diazotrophs (Lovell 2005; Morris 1991). For example, in mangrove systems, it is estimated that N fixers supply 40% (Van Der Valk and Attiwill 1984) to 60% (Zuberer and Silver 1978) of the nitrogen requirement of plants. Because N fixation is carbon limited, N fixers are very active in the root, rhizosphere, and litter layers of the soil, and N fixation rates are tightly coupled with plant photosynthesis and decomposition (Whiting et al. 1986). N fixation is an anaerobic reaction; thus, saturated anoxic environments like wetlands and mangroves are prime habitats for N fixation; however, many adaptations exist for fixing N in variable or high oxygen environments, like the rhizosphere or dry soils (Mitsch and Gosselink 2007; Mus et al. 2016).
Both symbiotic and free-living diazotrophs are abundant in coastal ecosystems. In coastal sand dunes and heathlands, symbiotic rhizobial associations (Rhizobium or Bradyrhizobium) are common in woody and herbaceous legumes (Rodríguez-Echeverría 2010; Sridhar et al. 2005) and actinorhizal (Frankia) associations are widespread in a number of important shrub species, especially in higher latitudes (Dudley et al. 1996; Swensen 1996). Legumes such as Lathyrus japonicus are often the very first colonizers of dunes and beaches (Brightmore and White 1963). Dune grasses have also been shown to be colonized by other endophytic N fixing taxa (Burkholderia) (Dalton et al. 2004) and also rely on N fixers in the rhizosphere to cope with low soil nutrients (Abdel Wahab 1975; Wahab and Wareing 1980). In mangrove systems, high rates of N fixation are associated with roots, the pneumatophore (aerial root) surface, the rhizosphere, tree bark, decomposing leaves, and the soil and comprise a diversity of organisms including cyanobacteria and many other phyla (Alfaro-Espinoza and Ullrich 2015; Holguin et al. 2001). In coastal systems, N fixation by free-living microbes contributes significantly to ecosystem N cycling, especially in systems like salt marshes and coastal forests that do not contain many symbiotic N fixing plants. In forest communities developing on lava flows in Hawai‘i, N fixers on leaf litter are important in successional development (Crews et al. 2001). Diazotrophs are very active in the rhizoplane and rhizosphere of salt marsh plants and come from diverse and novel bacterial and archaeal lineages (Davis et al. 2018; Lovell and Davis 2012). Much applied work on N fixers in coastal sand dunes/beaches (Potgieter et al. 2014; Rodríguez-Echeverría et al. 2009) and forests (Vitousek and Walker 1989) has addressed the impacts of invasive N fixing species, as N fixation ability can allow species like Casuarinas (sheoak), Acacias, and Myrica faya to successfully colonize and invade low nutrient coastal habitats. Research is needed to link how the soil legacy effects of N fixers (e.g., elevated N, altered microbial communities) present barriers to restoration and how to overcome these barriers (Nsikani et al. 2018). In other coastal systems, such as marshes and mangroves, the use or promotion of N fixing microorganisms to enhance restoration of plant communities has long been suggested (Bashan and Holguin 2002; Holguin et al. 2001), and current research is examining the best practices for inoculation and soil amendments (Murphy et al. 2018).
Endophytes
Endophytic fungi and bacteria live inside of roots, stems, leaves, and inflorescences of coastal grasses, sedges, forbs, and trees. Although not well studied, coastal plants have been found to host high diversities of endophytes (David et al. 2016; Lumibao et al. 2018), including bacteria and fungi that have been previously described from marine, soil, and freshwater habitats (Ananda and Sridhar 2002). By definition, endophytes live asymptomatically within plant tissues, with functions that include antagonism or facilitation of plant disease (Busby et al. 2016) and increased stress resilience to host plants (Ali et al. 2014; Rodriguez et al. 2008). Much research on endophyte function in planta, however, has been restricted to agricultural plants (Busby et al. 2016), with less attention to coastal plants. Work in coastal sand dune systems suggests that the presence of an Epichloë sp. fungal leaf endophyte greatly increases Ammophila breviligulata (American beachgrass) survival (Emery and Rudgers 2013), belowground biomass (Bell-Dereske et al. 2017), vegetative growth, and sand accumulation (Emery et al. 2015), which has important implications for dune succession and stabilization. A similar fungal endophyte (Periglandula sp.) in Ipomoea pes-caprae (beach morning glory) was present in 100% of populations sampled on Florida coasts, USA, suggesting strong benefit (Beaulieu et al. 2021). Other studies on coastal plant endophytes have mostly focused on the compounds produced by endophytes in vitro, such as searching within mangrove endophytes for enzymes (Castro et al. 2014; Ravindran et al. 2012) and for medicinally active compounds (Gayathri et al. 2010). Since introducing endophytes from one host species to another is feasible, endophytes may be useful in biotechnology. For example, several recent studies have demonstrated the potential of using endophytes to attenuate pollutants in coastal wetlands (e.g., bioremediation, Rehman et al. 2018; Saleem et al. 2019; Zheng et al. 2018). Another compelling example is the use of endophytes that were isolated from a mangrove species to inoculate and improve the growth of a tree species used for restoration in Brazil (Castro et al. 2018). Thus, the stress of living in a coastal area has led to adaptations for both hosts and their internal symbionts, so endophyte studies in the coastal environment hold promise for understanding stress resilience and symbioses.
Rhizosphere Microbes
As in other terrestrial systems, the rhizosphere microbial communities in coastal systems are significantly different than bulk soil with elevated abundances of taxa known to be endophytes (Sanka Loganathachetti et al. 2017), mycorrhizae (Estrada et al. 2013; Johansen et al. 2015), and plant growth promoting bacteria (Park et al. 2005). In sand dunes, plant roots are important sources of carbon for microbial communities in a matrix that is otherwise characterized by resource limitation and high physical stress (Rajaniemi and Allison 2009). In inundated coastal systems, too, roots provide an important source of carbon for fueling microbial processes important to plants (N fixation) and decomposition (sulfate reduction, an anaerobic process that reduces sulfates to hydrogen sulfide). Sulfate reducing bacteria are responsible for more than half of the total decomposition of organic matter in salt marshes (Howarth and Hobbie 1982) and have been found to tolerate environments like the rhizosphere with rapidly changing redox conditions (Rooney-Varga et al. 1997). In fact, rates of N fixation and sulfate reduction, sometimes performed by the same organism, are considerably higher in the rhizosphere than in bulk soil (Nielsen et al. 2001). Many wetland plants also have structural adaptations (aerenchyma and pneumatophores) that oxidize the soil, at least in the immediate vicinity of the root or root tip (Andersen and Kristensen 1988; Koop-Jakobsen et al. 2017), which ameliorates the detrimental effects of hydrogen sulfide, stimulates microbial heterotrophic activity, and affects CO2 emission (Hester et al. 2018). Several rhizosphere bacteria in marshes (Gong et al. 2018; Halda-Alija 2003; Mavrodi et al. 2018), mangroves (Bashan and Holguin 2002), and sand dunes (Godinho 2015; Jayaprakashvel et al. 2014) have plant growth promoting capabilities, including IAA (indole acetic acid, i.e., auxin) production, siderophores, and phosphate solubilization. Much current and future work in this area is in the context of bioprospecting saline rhizosphere habitats for microorganisms that can facilitate agricultural plants under conditions of soil salinization (Godinho 2015; Gong et al. 2018). Another important future direction is understanding the impacts of current restoration techniques and timescales necessary for restoring microbial function (Mavrodi et al. 2018), as well as the application of rhizosphere microbial communities for aiding restoration (Bashan and Holguin 2002).
Pathogens
Pathogens are important in shaping the natural community dynamics in coastal systems. In coastal sand dunes, soil pathogens (pathogenic fungi and parasitic nematodes) can promote plant species replacement and facilitate ecological succession (Van der Putten and Peters 1997). Fungal pathogens are also natural components of coastal forests causing winter seedbank mortality in coastal sage scrub communities (Mordecai 2012). Ergot (Claviceps purpurea) epidemics have been observed in Spartina anglica marshes in the UK (Raybould et al. 1998) and Spartina alterniflora marshes in the East and Gulf Coasts of North America (Eleuterius 1970; Eleuterius and Meyers 1974), where ergot infection during epidemics greatly reduces seed fecundity of these dominant grasses. In mangrove systems, a survey found abundant plant pathogens (Eutypella, Phaeophleospora, Phaeosphaeria, Phaeoramularia, Mycosphaerella), root pathogens (Gaeumannomyces, Cytospora, Magnaporthe, Pyricularia), and leaf pathogens (Diaporthe, Ramulispora) in above- and belowground mangrove tissues (Arfi et al. 2012). A number of fungal diseases in mangroves have been identified, and some mangrove diebacks in Gambia, Australia, and Hawai‘i have been attributed to fungal pathogens (Osorio et al. 2016). However, much more work on the importance of pathogens to mangrove growth and forest dynamics is needed.
Pathogens have been notable for their (potential) involvement in two high profile die-off events in coastal systems in recent history. Sudden vegetation dieback describes large die-offs of Spartina alterniflora that occurred in the late 1990s and 2000s in the USA, during which time over 1000 km2 in the Gulf Coast and 10% of Cape Cod’s marshes turned brown and died over the course of a few months (Elmer et al. 2013). The underlying cause of sudden vegetation dieback is still not known for certain, and some hypotheses do not invoke pathogens; however, two hypotheses posit that pathogens played a role: (1) Fusarium spp., which were isolated and abundant in many dieback areas, were believed to cause plant death particularly in combination with drought stress that marshes experienced at that time (Elmer et al. 2013), and (2) the periwinkle snail which promotes growth of a facultative plant pathogen (Phaeosphaeria spartinicola) was also thought to cause high Spartina mortality during periods of drought (Silliman et al. 2005). Sudden oak death is an ongoing epidemic caused by Phytophthora ramorum, a recently emerged generalist water mold pathogen which has decimated oak and tanoak populations in California and Oregon affecting over 2000 km2 of coastal forest (Grünwald et al. 2019; Rizzo and Garbelotto 2003). The pathogen also causes a similar disease with high mortality in Japanese larch trees in UK plantations. The wide host range of P. ramorum (over 100 plant species in 40 genera) is particularly problematic, as it can survive and sporulate on many forest understory species (Grünwald et al. 2012, 2019). Sudden oak death has also severely affected the horticulture industry because of quarantine regulations on the wide range of nursery plants that host the disease (Grünwald et al. 2019).
Pathogens play a key role in influencing plant invasions in coastal habitats. On the West coast of North America, ergot epidemics can facilitate invasive Spartina species and cause decline of native Spartina foliosa since outbreaks only occur in the native species and greatly reduce seed set (Fisher et al. 2007). Pathogens are also implicated in Spartina alterniflora invasion in China, as pathogen spillover of Fusarium palustre from Spartina has caused dieback in native Phragmites australis stands in coastal marshes (Li et al. 2014). In coastal sand dunes in California, invasive Ammophila arenaria promotes its own invasion by accumulating local pathogens which have larger negative effects on native plants (Eppinga et al. 2006). In South African coastal sand dunes, invasion intensity of Ammophila arenaria is a balance between enemy release from parasitic nematodes and biotic resistance imparted by native soil pathogens, which inhibit its invasion (Knevel et al. 2004). Similarly, native pathogens may somewhat limit Bromus diandrus invasion into the Californian coastal sage scrub (Hilbig and Allen 2015). Overall, pathogens play a major role in the ecology of coastal habitats, and future work to understand and control pathogens responsible for die-off events, manage the pathogens involved in species invasions, and understand how changing environmental conditions influence disease dynamics will be essential for maintaining the integrity of coastal communities.
Microbes in Coastal Restoration
Many coastal systems are experiencing severe degradation from global environmental change including sea level rise, warming, oil spills and other pollutants, drought, hurricane frequency, and invasive species. Because degradation threatens the provisioning of ecosystem services critical to humans, including food production, storm surge protection, filtration of pollutants, and carbon storage, the restoration of coastal systems has become a huge focus of conservation groups and governmental entities alike. Because sand dunes, marshes, mangroves, and forests/shrublands are all stressful systems, microbial mutualists that assist plants in tolerating low nutrients, drought, and salinity stress will likely be beneficial in restoration (Table 1). Below we review research on how microbial symbionts of plants may be specifically used to enhance coastal restoration and identify important considerations when implementing restorations using microbes.
Review of the Literature
There is growing recognition from inland systems that microbiota can be leveraged to enhance restoration success (Eviner and Hawkes 2008; Maltz and Treseder 2015). To do this, one typically applies inoculum, material containing spores, fungal hyphae, or bacterial cells, to seeds or plantings at the restoration site or to potted nursery plants that will be outplanted. Inocula can be sourced from whole soil or roots collected from a reference ecosystem, or specific microbial taxa can be cultured and multiplied in the laboratory (for fungal endophytes/bacteria) or greenhouse (for AMF). Generic commercial inocula are becoming readily available for use in restoration practice (Fisher 2012; Perkins and Hatfield 2016); however, locally sourced microbes have proven to be a superior source of mutualistic partners (Emam 2015; Maltz and Treseder 2015; Middleton and Bever 2012; Wubs et al. 2016). The use of microbial inocula in restoration can increase survival, growth, and establishment of target species (Richter and Stutz 2002; Thrall et al. 2005) and can increase plant diversity in restored sites (Koziol and Bever 2017), providing benefits for up to several years in the field (Maltz and Treseder 2015).
Sand dunes are one coastal system in which the use of AMF microbial inocula in restoration has received a lot of attention. Sand dunes are low nutrient, low organic matter systems, and most dune plants rely on mycorrhizae for nutrient acquisition as well as for drought and salt tolerance (Sigren et al. 2014). Studies have generally shown that inoculation with AMF prior to outplanting can increase survivorship (Emery and Rudgers 2011), growth (Al Agely and Sylvia 2008; de Souza et al. 2010; Emery and Rudgers 2011; Gemma and Koske 1997; Sylvia et al. 1993), flowering (Gemma and Koske 1997), and phosphorus content (Al Agely and Sylvia 2008) of dune grasses and trees. We quantified how AMF affected the growth of dune species across those restoration experiments above that reported sufficient information using Hedges’ D (Nakagawa and Cuthill 2007) and found there was a positive effect of AMF in 42% of the cases (5/12) and no significant effect in the rest (7/12; Fig. 3). Indeed, many studies find that AMF responses are site specific, i.e., an increase in performance from AMF inoculation does not occur at all sites (Al Agely and Sylvia 2008; Emery and Rudgers 2011; Sylvia et al. 1993), which may depend on the abundance of the mycorrhizal community prior to restoration. Interestingly, even in cases in which plant growth was not increased, root colonization and soil hyphae were elevated with the AMF inoculum treatment, which has benefits for dune stabilization (de Souza et al. 2010; Sylvia et al. 1993). Similarly, even if the effects of AMF inoculation are short lived (e.g., an increase in plant growth for only 1–2 years), there is still value to temporarily increasing growth if the restoration goal is dune stabilization (Miller and Jastrow 1992). Two studies also noted that different varieties of dune grasses responded differently to inocula, suggesting the choice of plant variety is key to successful use of inoculum in restoration (Al Agely and Sylvia 2008; Emery and Rudgers 2011). And, as found in other systems, local AMF inocula typically were more beneficial (Al Agely and Sylvia 2008; Sylvia et al. 1993).
The effect of AMF fungi or local field soil inoculum on growth (biomass, tiller production, height) of coastal sand dune and shrubland species outplanted in field restoration experiments. Data were extracted from the figures of available published papers using Plot Digitizer 2.6.9. Data shown below are the calculated Hedges’ D values with 95% confidence intervals (Nakagawa and Cuthill 2007); a positive number indicates that microbes positively affected growth. Labels indicate the study from which the data came: Al Agely and Sylvia (2008), Emery and Rudgers (2011), Caravaca et al. (2005), Bashan et al. (2012), and Aprahamian et al. (2016). The multiple points for each study indicate multiple sites, species, or genotypes tested. We only display results from low density treatments and first sampling dates. The species tested in each study were the following: Al Agely (Uniola paniculata), Emery (Ammophila breviligulata), Caravaca (Cistus albidus, Quercus coccifera), Bashan (Prosopis articulata, Parkinsonia microphylla, Parkinsonia florida), Aprahamian (Deinandra fasciculata, Mirabilis laevis, Salvia columbariae, Salvia mellifera). A number of studies mentioned in the text could not be analyzed here, because they did not report error bars
Coastal shrubland systems are another area in which restoration success may be limited by a lack of mycorrhizal fungi at restoration sites (Bowler 2000). A greenhouse study in coastal sage scrub suggested that field inoculum may improve restoration outcomes, because native species benefitted more from inoculum than invasive plants (Bozzolo and Lipson 2013). Another greenhouse experiment showed that both invasive and native inoculum increased native shrub seedling biomass, but native inoculum resulted in higher colonization and diversity of AMF and non-AMF fungi (Phillips et al. 2020). However, a field study found that applying live native soil and commercial AMF inoculum prior to seeding had no effect on plant root colonization, growth, or flower production, and commercial inoculum actually had negative effects on plant height (Aprahamian et al. 2016). In Baja California, inoculation of plant growth promoting bacteria and mycorrhizae at planting had short-term (but not long-term) positive effects on growth of two out of three leguminous trees (Bashan et al. 2012). In the Mediterranean, there was a large positive growth effect of mycorrhizal spore inoculation to pots prior to outplanting two native shrub species (Caravaca et al. 2005). When we summarized the effect of mycorrhizae on shrub growth across these field restoration experiments using Hedges’ D, we found that the effect of mycorrhizae was positive in four cases, neutral in eight cases, and negative in one case (Fig. 3). Overall, results of these meta-analyses suggest that there is potential that microbes can enhance restoration success in coastal shrublands, but more work in these systems is warranted.
The use of microbes in restoration of wetland systems, such as coastal marshes and mangroves, is much less studied. However, many of these wetland systems are low in nutrients, suggesting that adding growth-promoting microbes responsible for nutrient cycling could increase planting success (Bashan and Holguin 2002). Many taxa of growth promoting bacteria and fungi have been isolated from coastal marshes (Bledsoe and Boopathy 2016; Mavrodi et al. 2018; Smith and Farrer, unpublished data) and mangroves (Bashan and Holguin 2002; do Carmo et al. 2011; Vazquez et al. 2000), providing a starting point for research aimed at using microbes in restoration. For example, in one study, a high percentage (4–60%) of fungal taxa isolated from marsh plant roots had the capability of inhibiting pathogens, solubilizing phosphate, or producing plant growth hormones (Fig. 4; Smith and Farrer, unpublished data). A number of isolated rhizosphere wetland bacteria from mangroves (do Carmo et al. 2011; Piedad Díaz et al. 2000) and marshes (Zheng et al. 2018) also degrade oil and could be used to enhance remediation and restoration after oil spills. Initial greenhouse experiments in the saltmarsh grass Spartina alterniflora suggest that inoculation with growth promoting bacterial consortia can increase growth and nutrient uptake in plants over the short-term (2 months) (Bledsoe and Boopathy 2016). In mangroves, inoculation of seedlings with diazotrophic (N fixing) cyanobacteria increased nitrogen content in leaves (Bashan et al. 1998). Overall, these findings suggest that more research into using microbial communities in wetland restoration may be fruitful.
Percent of fungal taxa isolated from the roots of coastal marsh plants in Louisiana that have different plant growth promotion abilities: inhibiting pathogens, solubilizing phosphate, and producing plant growth hormones (auxin). Fungi were isolated from roots of common fresh, brackish, and saline marsh plants (Sagittaria lancifolia, Spartina patens, Phragmites australis, Spartina alterniflora, Juncus roemerianus) and tested for their ability to inhibit growth of a common pathogen (Fusarium palustre), solubilize phosphorus, and produce auxin (IAA) (Smith and Farrer, unpublished data). Percentages are out of 40, 57, and 51 taxa screened, respectively. This suggests a large proportion of culturable fungi may be useful in restoration
While the vast majority of microbially minded coastal restoration is focused on belowground microbial associates, leaf endophytes deserve a brief mention. First, some plant species harbor a special type of aboveground, systemic fungal endophyte that is vertically transmitted via seeds (Panaccione et al. 2014). The dune grass Ammophila breviligulata is colonized by such Epichloë endophytes, which can greatly increase plant growth and can enhance dune stabilization and restoration (Bell-Dereske et al. 2017; Emery et al. 2015; Emery and Rudgers 2013). Because they are vertically transmitted, endophyte manipulation may involve introducing endophytes to the seed/seedling with a needle (Emery et al. 2015) or purchase of endophyte positive nursery stock (Emery et al. 2010). However, care must be taken because not all natural populations of Ammophila host the endophyte (Emery et al. 2010), and it may be detrimental to introduce endophyte-infected plants into areas where they are not native as they may displace locally adapted genotypes (Slaymaker et al. 2015). Second, foliar spraying of (non-vertically transmitted) leaf endophytes is a technique that has proven useful in agricultural settings (Vimal and Singh 2020). In a greenhouse trial, Egan et al. (2021) found that foliar application of an endophytic yeast reduced disease incidence from invasive powdery mildew on a critically endangered mint species endemic to coastal forests of O‘ahu. Overall, despite relatively little research on leaf endophytes in coastal systems, these studies suggest that they warrant more consideration and research into what they can bring to restoration.
Considerations for Using Microbes in Coastal Restoration
The potential for using microbes to enhance restoration in coastal systems will depend on three things: (1) the reliance of plants on microbes (including site conditions and degradation), (2) the type of inoculum used, and (3) the type of restoration employed (outplanting live plants or seeding).
Reliance of Plants on Microbes
Both plant characteristics and site conditions influence the dependence of plants on microbial symbionts. While most plants benefit from rhizosphere symbionts (Vacheron et al. 2013) and mycorrhizae, it is well known that some plant families are non-mycorrhizal (Brassicaceae: mustards, Caryophyllaceae: pinks, Chenopodioideae: chenopods, Proteaceae: proteas) (Cosme et al. 2018) or less dependent on mycorrhizae (annuals are less dependent than perennials) (Collier et al. 2003) and thus would not benefit from mycorrhizal inoculation prior to restoration. Site conditions and the degree of land degradation have long been known to influence restoration success (Bakker and Berendse 1999), and we propose that they also impact the degree to which microbes will be useful in improving restoration. For example, in sand dunes, many plants are thought to be reliant on AMF due to the low nutrient content and low moisture in dune soils (Koske et al. 2008), and, as seen above, these systems often benefit from AMF inoculation during restoration (Al Agely and Sylvia 2008; de Souza et al. 2010; Emery and Rudgers 2011; Gemma and Koske 1997; Sylvia et al. 1993). In coastal shrublands, it has been found that a legacy of fungal pathogens limits restoration of former citrus fields (Hilbig and Allen 2019); thus, restoration success may be increased by inoculating with symbionts that promote pathogen resistance. Site degradation by invasive species has also been found to dramatically reduce mycorrhizal abundance and alter the composition of mycorrhizal communities (Grove et al. 2017); thus, previously invaded sites in coastal areas would likely benefit from mycorrhizal inoculation during restoration. Another example of an area that may lack microbial associates of plants are coastal marshes created using dredged sediment for restoration purposes; these created marshes may benefit from microbial inoculation during outplanting, even though inoculation is not common practice in marshes at this time. Lastly, despite the old microbial adage “everything is everywhere, but the environment selects” (Becking 1934), it is well known that microbes can be dispersal limited (Peay et al. 2010) particularly in restoration settings (Chen et al. 2020; Murphy and Foster 2014); therefore, sites such as impounded wetlands (with little water flow) or sites far from other natural areas (surrounded by agriculture or human habitation) may especially benefit from microbial inoculation.
Type of Inoculum
Many types of inocula are used in restoration projects including whole soil, single taxon, consortia of taxa, and commercial inoculum, with microbial taxa ranging from mycorrhizae to rhizosphere bacteria to endophytes to all of the above. Current research is in agreement that the type and source of inoculum used in restoration are of utmost importance to maximize success (Al Agely and Sylvia 2008; Aprahamian et al. 2016; Bashan et al. 2014; Sylvia et al. 1993) – the wrong inoculum can actually hinder plant growth and limit restoration (Aprahamian et al. 2016). It is important to understand the ecology of the focal species when selecting inoculum. For example, some plant species are purely ectomycorrhizal (pines, oaks, birches, eucalyptus) (Bruns et al. 2002). And while many plants associate with AMF, plants can be locally adapted to their particular AMF community, performing best with local AMF assemblages (Rúa et al. 2016). Similarly, plant growth promoting rhizobacteria are thought to be highly generalist, but the growth promotion effects of these microbes can depend on plant genotype and the particular bacterial strain (Drogue et al. 2012; Vacheron et al. 2013). This suggests that whole soil inoculum taken from underneath focal plants in similar reference habitats or microbial taxa isolated or propagated from focal species or genotypes may be best to use in restoration compared to externally sourced or commercial inoculum, a finding which has been confirmed by manipulative experiments (Emam 2015; Maltz and Treseder 2015; Middleton and Bever 2012; Wubs et al. 2016). There are costs and benefits to using whole soil inoculum vs. cultured microbes and this decision will depend on access to reference sites for soil collection, laboratory/greenhouse equipment and expertise in isolating and culturing microbial taxa, and financial costs of laboratory/greenhouse work. It is also important to note that the vast majority (> 99%) of microbes are non-culturable (Schloss and Handelsman 2005); thus, whole soil will contain a broader array of mutualists but may also contain parasites and pathogens. As an aside, AMF are not culturable in agar but can be propagated using host plants in soil (see Koziol et al. 2017 for more disucussion of practical aspects of using AMF in restoration). While most restoration research has focused on root-associated microbial symbionts, leaf endophytes can also impart drought tolerance and disease resistance, and cultured leaf endophytes have been applied in agriculture to increase crop performance (Canellas et al. 2015; Wu et al. 2013). Partnerships among restoration practitioners and microbial ecologists will be particularly fruitful in researching and selecting the best inoculum for a given restoration project or system.
Type of Restoration
In coastal systems, such as sand dunes, forests/shrublands, marshes, and mangroves, outplanting live plants or saplings is the typical method of active restoration. This is in contrast to more commonly studied grassland systems in which restorations rely heavily on seeding (although seeding is sometimes used in coastal shrubland communities (Allen et al. 2013)). Interestingly, outplanting makes it much easier to ensure microbial inoculation of the plant, because this can be done prior to restoration. It can be accomplished by using a fraction (often 10% volume) of whole soil or AMF infected soil in the potting media (Koziol et al. 2017), by adding inoculum pellets (Bashan et al. 2012) or liquid culture to the potting media (Tiepo et al. 2018), or by soaking plants in microbial suspensions prior to outplanting (Yuan et al. 2016). Some restorations, particularly in sand dune systems, inoculate the soil during outplanting by adding whole soil from reference sites or AMF infected soil to the holes just before planting (de Souza et al. 2010; Emery and Rudgers 2011; Gemma and Koske 1997). For leaf endophytes, plants could be sprayed with inoculant prior to outplanting or after planting. Foliar spraying of leaf endophytes also opens up the interesting possibility of applying endophytes at a large scale to enhance plant growth in mature, existing restoration sites, as large-scale application of belowground mutualists is typically not successful (Canellas et al. 2015). However, it is generally thought that smaller-scale nursery application is more effective at transmitting symbionts and more cost-effective (Bashan et al. 2014).
Restorations using seeding have a much harder time dispersing microbes and ensuring subsequent microbial survival and colonization of roots (Koziol et al. 2017). Dispersing microbes with seeds has, however, been well studied in agriculture for which there is much interest in using microbial symbionts to boost yield and stress tolerance of crops. Coating the seed with inoculum prior to seeding and drilling granular inoculants in seedbed furrows with the seed at sowing time are two main methods currently used in agriculture (Bashan et al. 2014), and these methods are just beginning to be adapted for restoration (Koziol et al. 2017). Overall, the type of restoration used, outplanting or seeding (which often depends on the type of plant), is an important consideration when assessing the ease of incorporating microbial symbionts in a restoration project.
Conclusions
Mycorrhizae, nitrogen fixers, endophytes, rhizosphere microbes, and pathogens play key roles in the functioning of coastal ecosystems. They are responsible, in large part, for the health (or lack thereof) of the plants that make up the foundation of coastal communities, on which a large web of wildlife, fisheries, and humans rely. These microscopic symbionts affect plant establishment, growth, competitive ability, and stress tolerance and regulate biogeochemical cycling in coastal systems. Current use of microbial symbionts to augment restoration of stressful and/or degraded coastal systems is still very much in its infancy; however, it holds great promise for increasing success of restoration on the coast. Much research is warranted to address the utility of microbes in different coastal systems and will be necessary for the development of local, effective inocula for use in restoration sites across the globe. This is an excellent opportunity for collaboration between restoration practitioners and microbial ecologists to work toward a common goal of increasing resilience of our coastal ecosystems. Such collaborations are especially relevant at a time when these systems are increasingly vulnerable to a great number of threats and as their ecosystem services become more valuable with global climate change.
References
Abdel Wahab, A.M. 1975. Nitrogen fixation by Bacillus strains isolated from the rhizosphere of Ammophila arenaria. Plant and Soil 42: 703–708.
Al Agely, A., and D.M. Sylvia. 2008. Compatible host/mycorrhizal fungus combinations for micropropagated sea oats: II Field evaluation. Mycorrhiza 18: 257–261.
Alfaro-Espinoza, G., and M.S. Ullrich. 2015. Bacterial N2-fixation in mangrove ecosystems: Insights from a diazotroph-mangrove interaction. Frontiers in Microbiology 6: 445.
Ali, S., T.C. Charles, and B.R. Glick. 2014. Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiology and Biochemistry 80: 160–167.
Allen, E.B., C. McDonald, and B.E. Hilbig. 2013. Long-term prospects for restoration of coastal sage scrub: invasive species nitrogen deposition and novel ecosystems. In Chaparral Restoration Workshop. Arcadia, CA.
Anagnostakis, S.L. 1987. Chestnut blight: The classical problem of an introduced pathogen. Mycologia 79: 23–37.
Ananda, K., and K.R. Sridhar. 2002. Diversity of endophytic fungi in the roots of mangrove species on the west coast of India. Canadian Journal of Microbiology 48 (10): 871–878.
Andersen, F.Ø., and E. Kristensen. 1988. Oxygen microgradients in the rhizosphere of the mangrove Avicennia marina. Marine Ecology 44: 201–204.
Aprahamian, A.M., M.E. Lulow, M.R. Major, K.R. Balazs, K.K. Treseder, and M.R. Maltz. 2016. Arbuscular mycorrhizal inoculation in coastal sage scrub restoration. Botany 94: 493–499.
Arfi, Y., M. Buée, C. Marchand, A. Levasseur, and E. Record. 2012. Multiple markers pyrosequencing reveals highly diverse and host-specific fungal communities on the mangrove trees Avicennia marina and Rhizophora stylosa. FEMS Microbiology Ecology 79: 433–444.
Ashkannejhad, S., and T.R. Horton. 2006. Ectomycorrhizal ecology under primary succession on coastal sand dunes: Interactions involving Pinus contorta suilloid fungi and deer. New Phytologist 169: 345–354.
Backer, R., J.S. Rokem, G. Ilangumaran, J. Lamont, D. Praslickova, E. Ricci, S. Subramanian, and D.L. Smith. 2018. Plant growth-promoting rhizobacteria: Context mechanisms of action and roadmap to commercialization of biostimulants for sustainable agriculture. Frontiers in Plant Science 9: 1473.
Bakker, J.P., and F. Berendse. 1999. Constraints in the restoration of ecological diversity in grassland and heathland communities. Trends in Ecology & Evolution 14: 63–68.
Bashan, Y., L.E. de Bashan, S.R. Prabhu, and J.-P. Hernandez. 2014. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant and Soil 378: 1–33.
Bashan, Y., and G. Holguin. 2002. Plant growth-promoting bacteria: A potential tool for arid mangrove reforestation. Trees 16: 159–166.
Bashan, Y., M.E. Puente, D.D. Myrold, and G. Toledo. 1998. In vitro transfer of fixed nitrogen from diazotrophic filamentous cyanobacteria to black mangrove seedlings. FEMS Microbiology Ecology 26: 165–170.
Bashan, Y., B.G. Salazar, M. Moreno, B.R. Lopez, and R.G. Linderman. 2012. Restoration of eroded soil in the Sonoran Desert with native leguminous trees using plant growth-promoting microorganisms and limited amounts of compost and water. Journal of Environmental Management 102: 26–36.
Beaulieu, W.T., D.G. Panaccione, Q.N. Quach, K.L. Smoot, and K. Clay. 2021. Diversification of ergot alkaloids and heritable fungal symbionts in morning glories. Communications Biology 4: 1362.
Becking, L.B. 1934. Geobiologie of inleiding tot de milieukunde. The Hague, the Netherlands: WP Van Stockum & Zoon.
Beena, K.R., A.B. Arun, N.S. Raviraja, and K.R. Sridhar. 2001. Association of arbuscular mycorrhizal fungi with plants of coastal sand dunes of west coast of India. Tropical Ecology 42: 213–222.
Beena, K.R., N.S. Raviraja, and K.R. Sridhar. 2000. Seasonal variations of arbuscular mycorrhizal fungal association with Ipomoea pes-caprae of coastal sand dunes Southern India. Journal of Environmental Biology 21: 341–347.
Beinart, R.A. 2019. The significance of microbial symbionts in ecosystem processes. mSystems 4: e00127–00119.
Bell-Dereske, L., C. Takacs-Vesbach, S.N. Kivlin, S.M. Emery, and J.A. Rudgers. 2017. Leaf endophytic fungus interacts with precipitation to alter belowground microbial communities in primary successional dunes. FEMS Microbiology Ecology 93.
Berg, G., and J.M. Raaijmakers. 2018. Saving seed microbiomes. The ISME Journal 12: 1167–1170.
Bernhardt, J.R., and H.M. Leslie. 2013. Resilience to climate change in coastal marine ecosystems. Annual Review of Marine Science 5: 371–392.
Bertness, M.D., and R. Callaway. 1994. Positive interactions in communities. Trends in Ecology and Evolution 9: 191–193.
Billingsley Tobias, T., E.C. Farrer, A. Rosales, R.L. Sinsabaugh, K.N. Suding, and A. Porras-Alfaro. 2017. Seed-associated fungi in the alpine tundra: Both mutualists and pathogens could impact plant recruitment. Fungal Ecology 30: 10–18.
Bledsoe, R., and R. Boopathy. 2016. Bioaugmentation of microbes to restore coastal wetland plants to protect land from coastal erosion. International Biodeterioration & Biodegradation 113: 155–160.
Botnen, S., H. Kauserud, T. Carlsen, R. Blaalid, and K. Høiland. 2015. Mycorrhizal fungal communities in coastal sand dunes and heaths investigated by pyrosequencing analyses. Mycorrhiza 25: 447–456.
Bowler, P.A. 2000. Ecological restoration of coastal sage scrub and its potential role in habitat conservation plans. Environmental Management 26: S85–S96.
Bozzolo, F.H., and D.A. Lipson. 2013. Differential responses of native and exotic coastal sage scrub plant species to N additions and the soil microbial community. Plant and Soil 371: 37–51.
Brightmore, D., and P.H.F. White. 1963. Lathyrus japonicus Willd. Journal of Ecology 51: 795–801.
Brundrett, M.C. 2009. Mycorrhizal associations and other means of nutrition of vascular plants: Understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant and Soil 320: 37–77.
Bruns, T.D., M.I. Bidartondo, and D.L. Taylor. 2002. Host specificity in ectomycorrhizal communities: What do the exceptions tell us? Integrative and Comparative Biology 42: 352–359.
Burke, L., Y. Kura, K. Kassem, C. Revenga, M. Spalding, and D. McAllister. 2001. Pilot analysis of global ecosystems: Coastal ecosystems. Washington DC: World Resources Institute.
Busby, P.E., M. Ridout, and G. Newcombe. 2016. Fungal endophytes: Modifiers of plant disease. Plant Molecular Biology 90: 645–655.
Canellas, L.P., S.F. da Silva, D.C. Olk, and F.L. Olivares. 2015. Foliar application of plant growth-promoting bacteria and humic acid increase maize yields. Journal of Food Agriculture & Environment 13: 131–138.
Caravaca, F., M.M. Alguacil, R. Azcón, J. Parladé, P. Torres, and A. Roldán. 2005. Establishment of two ectomycorrhizal shrub species in a semiarid site after in situ amendment with sugar beet rock phosphate and Aspergillus niger. Microbial Ecology 49: 73–82.
Carvalho, L.M., I. Caçador, and M. Martins-Loução. 2001. Temporal and spatial variation of arbuscular mycorrhizas in salt marsh plants of the Tagus estuary (Portugal). Mycorrhiza 11: 303–309.
Castro, R.A., M.N. Dourado, J.R. Almeida, P.T. Lacava, A. Nave, I.S. Melo, J.L. Azevedo, and M.C. Quecine. 2018. Mangrove endophyte promotes reforestation tree (Acacia polyphylla) growth. Brazilian Journal of Microbiology 49: 59–66.
Castro, R.A., M.C. Quecine, P.T. Lacava, B.D. Batista, D.M. Luvizotto, J. Marcon, A. Ferreira, I.S. Melo, and J.L. Azevedo. 2014. Isolation and enzyme bioprospection of endophytic bacteria associated with plants of Brazilian mangrove ecosystem. Springerplus 3: 382.
Cavicchioli, R., P.M. Curmi, N. Saunders, and T. Thomas. 2003. Pathogenic archaea: Do they exist? BioEssays 25: 1119–1128.
Cavicchioli, R., W.J. Ripple, K.N. Timmis, F. Azam, L.R. Bakken, M. Baylis, M.J. Behrenfeld, A. Boetius, P.W. Boyd, A.T. Classen, T.W. Crowther, R. Danovaro, C.M. Foreman, J. Huisman, D.A. Hutchins, J.K. Jansson, D.M. Karl, B. Koskella, D.B. Mark Welch, J.B.H. Martiny, M.A. Moran, V.J. Orphan, D.S. Reay, J.V. Remais, V.I. Rich, B.K. Singh, L.Y. Stein, F.J. Stewart, M.B. Sullivan, M.J.H. van Oppen, S.C. Weaver, E.A. Webb, and N.S. Webster. 2019. Scientists’ warning to humanity: Microorganisms and climate change. Nature Reviews Microbiology 17: 569–586.
Chen, W., S. Jiao, Q. Li, and N. Du. 2020. Dispersal limitation relative to environmental filtering governs the vertical small-scale assembly of soil microbiomes during restoration. Journal of Applied Ecology 57: 402–412.
Collier, S.C., C.T. Yarnes, and R. Peter Herman. 2003. Mycorrhizal dependency of Chihuahuan Desert plants is influenced by life history strategy and root morphology. Journal of Arid Environments 55: 223–229.
Cosme, M., I. Fernández, M.G.A. Van der Heijden, and C.M.J. Pieterse. 2018. Non-mycorrhizal plants: The exceptions that prove the rule. Trends in Plant Science 23: 577–587.
Crews, T.E., L.M. Kurina, and P.M. Vitousek. 2001. Organic matter and nitrogen accumulation and nitrogen fixation during early ecosystem development in Hawaii. Biogeochemistry 52: 259–279.
d’Entremont, T.W., J.C. López-Gutiérrez, and A.K. Walker. 2018. Examining arbuscular mycorrhizal fungi in saltmarsh hay (Spartina patens) and smooth cordgrass (Spartina alterniflora) in the Minas Basin Nova Scotia. Northeastern Naturalist 25 (72–86): 15.
Dalton, D.A., S. Kramer, N. Azios, S. Fusaro, E. Cahill, and C. Kennedy. 2004. Endophytic nitrogen fixation in dune grasses (Ammophila arenaria and Elymus mollis) from Oregon. FEMS Microbiology Ecology 49: 469–479.
David, A.S., E.W. Seabloom, and G. May. 2016. Plant host species and geographic distance affect the structure of aboveground fungal symbiont communities, and environmental filtering affects belowground communities in a coastal dune ecosystem. Microbial Ecology 71: 912–926.
Davis, D.A., S.L. Malone, and C.R. Lovell. 2018. Responses of salt marsh plant rhizosphere diazotroph assemblages to drought. Microorganisms 6: 27.
de Souza, R.G., B.T. Goto, D.K. Alves da Silva, F.S. Barbosa da Silva, E.V.S.B. Sampaio, and L.C. Maia. 2010. The role of arbuscular mycorrhizal fungi and cattle manure in the establishment of Tocoyena selloana Schum in mined dune areas. European Journal of Soil Biology 46: 237–242.
do Carmo, F.L., H.F. dos Santos, E.F. Martins, J.D. van Elsas, A.S. Rosado, and R.S. Peixoto. 2011. Bacterial structure and characterization of plant growth promoting and oil degrading bacteria from the rhizospheres of mangrove plants. The Journal of Microbiology 49: 535.
Drogue, B., H. Doré, S. Borland, F. Wisniewski-Dyé, and C. Prigent-Combaret. 2012. Which specificity in cooperation between phytostimulating rhizobacteria and plants? Research in Microbiology 163: 500–510.
Duarte, C.M., A. Borja, J. Carstensen, M. Elliott, D. Krause-Jensen, and N. Marbà. 2015. Paradigms in the recovery of estuarine and coastal ecosystems. Estuaries and Coasts 38: 1202–1212.
Dudley, J.L., B. Michener, and K. Lajtha. 1996. The contributions of nitrogen-fixing symbioses to coastal heathland succession. The American Midland Naturalist 135: 334–342.
Egan, C.P., J.H. Koko, C.D. Muir, G. Zahn, S.O.I. Swift, A.S. Amend, and N.A. Hynson. 2021. Restoration of the mycobiome of the endangered Hawaiian mint Phyllostegia kaalaensis increases its resistance to a common powdery mildew. Fungal Ecology 52: 101070.
Eleuterius, L.N. 1970. Observations on Claviceps purpurea on Spartina alterniflora in the coastal marshes of Mississippi. Gulf and Caribbean Research 3: 105–109.
Eleuterius, L.N., and S.P. Meyers. 1974. Claviceps purpurea on Spartina in coastal marshes. Mycologia 66: 978–986.
Elmer, W.H., S. Useman, R.W. Schneider, R.E. Marra, J.A. LaMondia, I.A. Mendelssohn, M.M. Jiménez-Gasco, and F.L. Caruso. 2013. Sudden vegetation dieback in Atlantic and Gulf Coast salt marshes. Plant Disease 97: 436–445.
Emam, T. 2015. Local soil, but not commercial AMF inoculum, increases native and non-native grass growth at a mine restoration site. Restoration Ecology 24: 35–44.
Emery, S.M., L. Bell-Dereske, and J.A. Rudgers. 2015. Fungal symbiosis and precipitation alter traits and dune building by the ecosystem engineer, mmophila breviligulata. Ecology 96: 927–935.
Emery, S.M., and J.A. Rudgers. 2011. Beach restoration efforts influenced by plant variety, soil inoculum, and site effects. Journal of Coastal Research 27: 636–644.
Emery, S.M., and J.A. Rudgers. 2013. Impacts of simulated climate change and fungal symbionts on survival and growth of a foundation species in sand dunes. Oecologia 173: 1601–1612.
Emery, S.M., D. Thompson, and J.A. Rudgers. 2010. Variation in endophyte symbiosis, herbivory and drought tolerance of Ammophila breviligulata populations in the Great Lakes Region. The American Midland Naturalist 163 (186–196): 111.
Eppinga, M.B., M. Rietkerk, S.C. Dekker, P.C. De Ruiter, and W.H. Van der Putten. 2006. Accumulation of local pathogens: A new hypothesis to explain exotic plant invasions. Oikos 114: 168–176.
Estrada, B., M. Beltrán-Hermoso, J. Palenzuela, K. Iwase, J.M. Ruiz-Lozano, J.-M. Barea, and F. Oehl. 2013. Diversity of arbuscular mycorrhizal fungi in the rhizosphere of Asteriscus maritimus (L) Less, a representative plant species in arid and saline Mediterranean ecosystems. Journal of Arid Environments 97: 170–175.
Evelin, H., R. Kapoor, and B. Giri. 2009. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Annals of Botany 104: 1263–1280.
Eviner, V.T., and C.V. Hawkes. 2008. Embracing variability in the application of plant–soil interactions to the restoration of communities and ecosystems. Restoration Ecology 16: 713–729.
Feagin, R.A., D.J. Sherman, and W.E. Grant. 2005. Coastal erosion global sea-level rise and the loss of sand dune plant habitats. Frontiers in Ecology and the Environment 3: 359–364.
Field, J.C., D.F. Boesch, D. Scavia, R. Buddemeier, V.R. Burkett, D. Cayan, M. Fogarty, M. Harwell, R. Howarth, C. Mason, L.J. Pietrafesa, D. Reed, T. Royer, A. Sallenger, M. Spranger, and J.G. Titus. 2001. Chapter 16: Potential consequences of climate variability and change on coastal areas and marine resources. In Climate change impacts on the United States: The potential consequences of climate variability and change, ed. National Assessment Synthesis Team, 461–487. Cambridge, UK: Cambridge University Press Report for the US Global Change Research Program.
Fisher, A.J., J.M. DiTomaso, T.R. Gordon, B.J. Aegerter, and D.R. Ayres. 2007. Salt marsh Claviceps purpurea in native and invaded Spartina marshes in Northern California. Plant Disease 91: 380–386.
Fisher, N. 2012. Commercial microbial inocula – do they work? In Proceedings of the Seventh International Conference on Mine Closure, eds. A. Fourie and M. Tibbett, 449–457. Perth: Australian Centre for Geomechanics.
Gayathri, S., D. Saravanan, M. Radhakrishnan, R. Balagurunathan, and K. Kathiresan. 2010. Bioprospecting potential of fast growing endophytic bacteria from leaves of mangrove and salt-marsh plant species. Indian Journal of Biotechnology 9: 397–402.
Gemma, J.N., and R.E. Koske. 1997. Arbuscular Mycorrhizae in sand dune plants of the North Atlantic Coast of the U.S.: Field and greenhouse inoculation and presence of mycorrhizae in planting stock. Journal of Environmental Management 50: 251–264.
Godinho, A.L. 2015. Coastal sand dunes: A potential goldmine of bioresources. In Bioprospects of coastal eubacteria: Ecosystems of Goa, ed. S. Borkar, 1–24. Cham: Springer International Publishing.
Gong, Y., J.-L. Bai, H.-T. Yang, W.-D. Zhang, Y.-W. Xiong, P. Ding, and S. Qin. 2018. Phylogenetic diversity and investigation of plant growth-promoting traits of actinobacteria in coastal salt marsh plant rhizospheres from Jiangsu, China. Systematic and Applied Microbiology 41: 516–527.
Grove, S., K.A. Haubensak, C. Gehring, and I.M. Parker. 2017. Mycorrhizae invasions and the temporal dynamics of mutualism disruption. Journal of Ecology 105: 1496–1508.
Grünwald, N.J., M. Garbelotto, E.M. Goss, K. Heungens, and S. Prospero. 2012. Emergence of the sudden oak death pathogen Phytophthora ramorum. Trends in Microbiology 20: 131–138.
Grünwald, N.J., J.M. LeBoldus, and R.C. Hamelin. 2019. Ecology and evolution of the sudden oak death pathogen Phytophthora ramorum. Annual Review of Phytopathology 57: 301–321.
Halda-Alija, L. 2003. Identification of indole-3-acetic acid producing freshwater wetland rhizosphere bacteria associated with Juncus effusus L. Canadian Journal of Microbiology 49: 781–787.
Hartnett, D.C., and G.W.T. Wilson. 1999. Mycorrhizae influence plant community structure and diversity in tallgrass prairie. Ecology 80: 1187–1195.
Henneron, L., C. Cros, C. Picon-Cochard, V. Rahimian, and S. Fontaine. 2020. Plant economic strategies of grassland species control soil carbon dynamics through rhizodeposition. Journal of Ecology 108: 528–545.
Hester, E.R., S.F. Harpenslager, J.M.H. van Diggelen, L.L. Lamers, M.S.M. Jetten, C. Lüke, S. Lücker, and C.U. Welte. 2018. Linking nitrogen load to the structure and function of wetland soil and rhizosphere microbial communities. mSystems 3: e00214–00217.
Hilbig, B. E. and E. B. Allen (2015). Plant-soil feedbacks and competitive interactions between invasive Bromus diandrus and native forb species. Plant and Soil 392(1): 191-203.
Hilbig, B.E., and E.B. Allen. 2019. Fungal pathogens and arbuscular mycorrhizal fungi of abandoned agricultural fields: Potential limits to restoration. Invasive Plant Science and Management 12: 186–193.
Holguin, G., P. Vazquez, and Y. Bashan. 2001. The role of sediment microorganisms in the productivity conservation and rehabilitation of mangrove ecosystems: An overview. Biology and Fertility of Soils 33: 265–278.
Howarth, R.W., and J.E. Hobbie. 1982. The regulation of decomposition and heterotrophic microbial activity in salt marsh soils: a review. In Estuarine comparisons, ed. V.S. Kennedy. New York, N.Y.: Academic Press.
Jankowski, K.L., T.E. Törnqvist, and A.M. Fernandes. 2017. Vulnerability of Louisiana’s coastal wetlands to present-day rates of relative sea-level rise. Nature Communications 8: 14792.
Jayaprakashvel, M., V.K. Kumar, J. Abideen, M. Venkatramani. Swarnakala, and A.J. Hussain. 2014. Production of indole acetic acid and plant growth promotion by rhizobacteria from a less studied marine ecosystem. Biosciences Biotechnology Research Asia 11: 179–185.
Johansen, R.B., M. Vestberg, B.R. Burns, D. Park, J.E. Hooker, and P.R. Johnston. 2015. A coastal sand dune in New Zealand reveals high arbuscular mycorrhizal fungal diversity. Symbiosis 66: 111–121.
Johnson, N.C., J. Wolf, and G.W. Koch. 2003. Interactions among mycorrhizae, atmospheric CO2 and soil N impact plant community composition. Ecology Letters 6: 532–540.
Johnson-Green, P., N.C. Kenkel, and T. Booth. 2001. Soil salinity and arbuscular mycorrhizal colonization of Puccinellia nuttalliana. Mycological Research 105: 1094–1100.
Keane, R.M., and M.J. Crawley. 2002. Exotic plant invasions and the enemy release hypothesis. Trends in Ecology & Evolution 17: 164–170.
Kingsford, R.T., A. Basset, and L. Jackson. 2016. Wetlands: Conservation’s poor cousins. Aquatic Conservation: Marine and Freshwater Ecosystems 26: 892–916.
Kirwan, M.L., and J.P. Megonigal. 2013. Tidal wetland stability in the face of human impacts and sea-level rise. Nature 504: 53–60.
Knevel, I.C., T. Lans, F.B.J. Menting, U.M. Hertling, and W.H. van der Putten. 2004. Release from native root herbivores and biotic resistance by soil pathogens in a new habitat both affect the alien Ammophila arenaria in South Africa. Oecologia 141: 502–510.
Knutson, T.R., J.L. McBride, J. Chan, K. Emanuel, G. Holland, C. Landsea, I. Held, J.P. Kossin, A.K. Srivastava, and M. Sugi. 2010. Tropical cyclones and climate change. Nature Geoscience 3: 157–163.
Koop-Jakobsen, K., J. Fischer, and F. Wenzhöfer. 2017. Survey of sediment oxygenation in rhizospheres of the saltmarsh grass - Spartina anglica. Science of the Total Environment 589: 191–199.
Koske, R.E., J.N. Gemma, L. Corkidi, C. Sigüenza, and E. Rincón. 2008. Arbuscular mycorrhizas in coastal dunes. In Coastal dunes, eds. M.L. Martinez and N.P. Psuty, 173–187. Heidelberg: Springer Berlin.
Koziol, L., and J.D. Bever. 2017. The missing link in grassland restoration: Arbuscular mycorrhizal fungi inoculation increases plant diversity and accelerates succession. Journal of Applied Ecology 54: 1301–1309.
Koziol, L., P.A. Schultz, J.D. Bever, G. House, J. Bauer, and E. Middleton. 2017. A practical guide to inoculation with arbuscular mycorrhizal fungi in ecological restoration. In Department of Defense Strategic Environmental Research and Development Program (SERDP) Project RC-2330.
Koziol, L., P.A. Schultz, G.L. House, J.T. Bauer, E.L. Middleton, and J.D. Bever. 2018. The plant microbiome and native plant restoration: The example of native mycorrhizal fungi. BioScience 68: 996–1006.
Kumar, T., and M. Ghose. 2008. Status of arbuscular mycorrhizal fungi (AMF) in the Sundarbans of India in relation to tidal inundation and chemical properties of soil. Wetlands Ecology and Management 16: 471–483.
Kummu, M., H. de Moel, G. Salvucci, D. Viviroli, P.J. Ward, and O. Varis. 2016. Over the hills and further away from coast: global geospatial patterns of human and environment over the 20th–21st centuries. Environmental Research Letters 11: 034010.
Lekberg, Y., J.D. Bever, R.A. Bunn, R.M. Callaway, M.M. Hart, S.N. Kivlin, J. Klironomos, B.G. Larkin, J.L. Maron, K.O. Reinhart, M. Remke, and W.H. van der Putten. 2018. Relative importance of competition and plant–soil feedback their synergy context dependency and implications for coexistence. Ecology Letters 21: 1268–1281.
Li, H., X. Zhang, R. Zheng, X. Li, W.H. Elmer, L.M. Wolfe, and B. Li. 2014. Indirect effects of non-native Spartina alterniflora and its fungal pathogen (Fusarium palustre) on native saltmarsh plants in China. Journal of Ecology 102: 1112–1119.
Lovell, C.R. 2005. Belowground interactions among salt marsh plants and microorganisms. In Interactions between macro- and microorganisms in marine sediments, ed. E. Kristensen, R.R. Haese, and J.E. Kostka, 61–83. Hoboken: John Wiley & Sons Inc.
Lovell, C.R., and D.A. Davis. 2012. Specificity of salt marsh diazotrophs for vegetation zones and plant hosts: Results from a North American marsh. Frontiers in Microbiology 3: 84–84.
Lovell, C.R., Y.M. Piceno, J.M. Quattro, and C.E. Bagwell. 2000. Molecular analysis of diazotroph diversity in the rhizosphere of the smooth cordgrass Spartina alterniflora. Applied and Environmental Microbiology 66: 3814–3822.
Lumibao, C.Y., S. Formel, V. Elango, J.H. Pardue, M. Blum, and S.A. Van Bael. 2018. Persisting responses of salt marsh fungal communities to the Deepwater Horizon oil spill. Science of the Total Environment 642: 904–913.
Maltz, M.R., and K.K. Treseder. 2015. Sources of inocula influence mycorrhizal colonization of plants in restoration projects: A meta-analysis. Restoration Ecology 23: 625–634.
Mavrodi, O.V., C.M. Jung, J.O. Eberly, S.V. Hendry, S. Namjilsuren, P.D. Biber, K.J. Indest, and D.V. Mavrodi. 2018. Rhizosphere microbial communities of Spartina alterniflora and Juncus roemerianus from restored and natural tidal marshes on Deer Island Mississippi. Frontiers in Microbiology 9: 3049.
Middleton, E.L., and J.D. Bever. 2012. Inoculation with a native soil community advances succession in a grassland restoration. Restoration Ecology 20: 218–226.
Millennium Ecosystem Assessment. 2005. Ecosystems and human well-being: Current state and trends. Washington, DC: Island Press.
Miller, R.M., and J.D. Jastrow. 1992. The application of VA mycorrhizae to ecosystem restoration and reclamation. In Mycorrhizal functioning an integrative plant-fungal process, ed. M.F. Allen, 438–467. New York: Chapman & Hall.
Mitsch, W.J., and J.G. Gosselink. 2007. Wetlands, 4th ed. Hoboken, New Jersey: John Wiley & Sons.
Mordecai, E.A. 2012. Soil moisture and fungi affect seed survival in California grassland annual plants. PLOS ONE 7: e39083.
Moreau, D., R.D. Bardgett, R.D. Finlay, D.L. Jones, and L. Philippot. 2019. A plant perspective on nitrogen cycling in the rhizosphere. Functional Ecology 33: 540–552.
Morris, J.T. 1991. Effects of nitrogen loading on wetland ecosystems with particular reference to atmospheric deposition. Annual Review of Ecology and Systematics 22: 257–279.
Murphy, C.A., and B.L. Foster. 2014. Soil properties and spatial processes influence bacterial metacommunities within a grassland restoration experiment. Restoration Ecology 22: 685–691.
Murphy, J.L., K.E. Boyer, and E.J. Carpenter. 2018. Restoration of cordgrass salt marshes: Limited effects of organic matter additions on nitrogen fixation. Wetlands 38: 361–371.
Mus, F., M.B. Crook, K. Garcia, A. Garcia Costas, B.A. Geddes, E.D. Kouri, P. Paramasivan, M.-H. Ryu, G.E.D. Oldroyd, P.S. Poole, M.K. Udvardi, C.A. Voigt, J.-M. Ané, and J.W. Peters. 2016. Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Applied and Environmental Microbiology 82: 3698–3710.
Muthukumar, T., K. Udaiyan, and P. Shanmughavel. 2004. Mycorrhiza in sedges–an overview. Mycorrhiza 14: 65–77.
Nakagawa, S., and I.C. Cuthill. 2007. Effect size confidence interval and statistical significance: A practical guide for biologists. Biological Reviews 82: 591–605.
Nielsen, L.B., K. Finster, D.T. Welsh, A. Donelly, R.A. Herbert, R. De Wit, and B.A. Lomstein. 2001. Sulphate reduction and nitrogen fixation rates associated with roots rhizomes and sediments from Zostera noltii and Spartina maritima meadows. Environmental Microbiology 3: 63–71.
Nsikani, M.M., B.W. van Wilgen, and M. Gaertner. 2018. Barriers to ecosystem restoration presented by soil legacy effects of invasive alien N2-fixing woody species: Implications for ecological restoration. Restoration Ecology 26: 235–244.
Nuccio, E.E., A. Hodge, J. Pett-Ridge, D.J. Herman, P.K. Weber, and M.K. Firestone. 2013. An arbuscular mycorrhizal fungus significantly modifies the soil bacterial community and nitrogen cycling during litter decomposition. Environmental Microbiology 15: 1870–1881.
O’Connor, P.J., S.E. Smith, and F.A. Smith. 2002. Arbuscular mycorrhizas influence plant diversity and community structure in a semiarid herbland. New Phytologist 154: 209–218.
Obase, K., J.Y. Cha, J.K. Lee, S.Y. Lee, J.H. Lee, and K.W. Chun. 2009. Ectomycorrhizal fungal communities associated with Pinus thunbergii in the eastern coastal pine forests of Korea. Mycorrhiza 20: 39–49.
Osorio, J.A., M.J. Wingfield, and J. Roux. 2016. A review of factors associated with decline and death of mangroves with particular reference to fungal pathogens. South African Journal of Botany 103: 295–301.
Panaccione, D.G., W.T. Beaulieu, and D. Cook. 2014. Bioactive alkaloids in vertically transmitted fungal endophytes. Functional Ecology 28: 299–314.
Parihar, M., A. Rakshit, V.S. Meena, V.K. Gupta, K. Rana, M. Choudhary, G. Tiwari, P.K. Mishra, A. Pattanayak, J.K. Bisht, S.S. Jatav, P. Khati, and H.S. Jatav. 2020. The potential of arbuscular mycorrhizal fungi in C cycling: A review. Archives of Microbiology 202: 1581–1596.
Park, M.S., S.R. Jung, M.S. Lee, K.O. Kim, J.O. Do, K.H. Lee, S.B. Kim, and K.S. Bae. 2005. Isolation and characterization of bacteria associated with two sand dune plant species Calystegia soldanella and Elymus mollis. Journal of Microbiology (Seoul) 43: 219-227.
Partida-Martinez, L.P., and M. Heil. 2011. The microbe-free plant: Fact or artifact? Frontiers in Plant Science 2: 100.
Peay, K.G., M. Garbelotto, and T.D. Bruns. 2010. Evidence of dispersal limitation in soil microorganisms: Isolation reduces species richness on mycorrhizal tree islands. Ecology 91: 3631–3640.
Penton, C.R., J.L. Deenik, B.N. Popp, G.L. Bruland, P. Engstrom, D. St. Louis, and J. Tiedje. 2013. Importance of sub-surface rhizosphere-mediated coupled nitrification–denitrification in a flooded agroecosystem in Hawaii. Soil Biology and Biochemistry 57: 362–373.
Perkins, L.B., and G. Hatfield. 2016. Can commercial soil microbial treatments remediate plant–soil feedbacks to improve restoration seedling performance? Restoration Ecology 24: 194–201.
Perrow, M.R., and A.J. Davy. 2002. Handbook of ecological restoration Volume 2: restoration in practice. Cambridge: Cambridge University Press.
Phillips, M.L., E.L. Aronson, M.R. Maltz, and E.B. Allen. 2020. Native and invasive inoculation sources modify fungal community assembly and biomass production of a chaparral shrub. Applied Soil Ecology 147: 103370.
Piedad Díaz, M., S.J.W. Grigson, C.J. Peppiatt, and J.G. Burgess. 2000. Isolation and characterization of novel hydrocarbon-degrading euryhaline consortia from crude oil and mangrove sediments. Marine Biotechnology 2: 522–532.
Porras-Alfaro, A., and P. Bayman. 2011. Hidden fungi emergent properties: Endophytes and microbiomes. Annual Review of Phytopathology 49: 291–315.
Porter, S.S., R. Bantay, C.A. Friel, A. Garoutte, K. Gdanetz, K. Ibarreta, B.M. Moore, P. Shetty, E. Siler, and M.L. Friesen. 2019. Beneficial microbes ameliorate abiotic and biotic sources of stress on plants. Functional Ecology 34: 2075–2086.
Potgieter, L.J., D.M. Richardson, and J.R.U. Wilson. 2014. Casuarina: Biogeography and ecology of an important tree genus in a changing world. Biological Invasions 16: 609–633.
Powell, E.J., M.C. Tyrrell, A. Milliken, J.M. Tirpak, and M.D. Staudinger. 2019. A review of coastal management approaches to support the integration of ecological and human community planning for climate change. Journal of Coastal Conservation 23: 1–18.
Rajaniemi, T.K., and V.J. Allison. 2009. Abiotic conditions and plant cover differentially affect microbial biomass and community composition on dune gradients. Soil Biology and Biochemistry 41: 102–109.
Ravindran, C., T. Naveenan, G.R. Varatharajan, R. Rajasabapathy, and R.M. Meena. 2012. Antioxidants in mangrove plants and endophytic fungal associations. Botanica Marina 55: 269–279.
Raybould, A.F., A.J. Gray, and R.T. Clarke. 1998. The long-term epidemic of Claviceps purpurea on Spartina anglica in Poole Harbour: Pattern of infection, effects on seed production and the role of Fusarium heterosporum. New Phytologist 138: 497–505.
Reddy, K.R., W.H. Patrick Jr., and C.W. Lindau. 1989. Nitrification-denitrification at the plant root-sediment interface in wetlands. Limnology and Oceanography 34: 1004–1013.
Rehman, K., A. Imran, I. Amin, and M. Afzal. 2018. Inoculation with bacteria in floating treatment wetlands positively modulates the phytoremediation of oil field wastewater. Journal of Hazardous Materials 349: 242–251.
Richter, B.S., and J.C. Stutz. 2002. Mycorrhizal inoculation of big sacaton: Implications for grassland restoration of abandoned agricultural fields. Restoration Ecology 10: 607–616.
Rillig, M.C. 2004. Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecology Letters 7: 740–754.
Rizzo, D.M., and M. Garbelotto. 2003. Sudden oak death: Endangering California and Oregon forest ecosystems. Frontiers in Ecology and the Environment 1: 197–204.
Rodriguez, R.J., J. Henson, E. Van Volkenburgh, M. Hoy, L. Wright, F. Beckwith, Y.-O. Kim, and R.S. Redman. 2008. Stress tolerance in plants via habitat-adapted symbiosis. The ISME Journal 2: 404–416.
Rodríguez-Echeverría, S. 2010. Rhizobial hitchhikers from Down Under: Invasional meltdown in a plant-bacteria mutualism? Journal of Biogeography 37: 1611–1622.
Rodríguez-Echeverría, S., J.A. Crisóstomo, C. Nabais, and H. Freitas. 2009. Belowground mutualists and the invasive ability of Acacia longifolia in coastal dunes of Portugal. Biological Invasions 11: 651–661.
Rooney-Varga, J.N., R. Devereux, R.S. Evans, and M.E. Hines. 1997. Seasonal changes in the relative abundance of uncultivated sulfate-reducing bacteria in a salt marsh sediment and in the rhizosphere of Spartina alterniflora. Applied and Environmental Microbiology 63: 3895–3901.
Roy, S., D.P. Khasa, and C.W. Greer. 2007. Combining alders, frankiae, and mycorrhizae for the revegetation and remediation of contaminated ecosystems. Canadian Journal of Botany 85: 237–251.
Roy-Bolduc, A., E. Laliberté, and M. Hijri. 2015. High richness of ectomycorrhizal fungi and low host specificity in a coastal sand dune ecosystem revealed by network analysis. Ecology and Evolution 6: 349–362.
Rúa, M.A., A. Antoninka, P.M. Antunes, V.B. Chaudhary, C. Gehring, L.J. Lamit, B.J. Piculell, J.D. Bever, C. Zabinski, J.F. Meadow, M.J. Lajeunesse, B.G. Milligan, J. Karst, and J.D. Hoeksema. 2016. Home-field advantage? Evidence of local adaptation among plants soil and arbuscular mycorrhizal fungi through meta-analysis. BMC Evolutionary Biology 16: 122.
Saleem, H., M. Arslan, K. Rehman, R. Tahseen, and M. Afzal. 2019. Phragmites australis – a helophytic grass – can establish successful partnership with phenol-degrading bacteria in a floating treatment wetland. Saudi Journal of Biological Sciences 26: 1179–1186.
Sanka Loganathachetti, D., A. Poosakkannu, and S. Muthuraman. 2017. Fungal community assemblage of different soil compartments in mangrove ecosystem. Scientific Reports 7: 8560.
Scavia, D., J.C. Field, D.F. Boesch, R.W. Buddemeier, V. Burkett, D.R. Cayan, M. Fogarty, M.A. Harwell, R.W. Howarth, C. Mason, D.J. Reed, T.C. Royer, A.H. Sallenger, and J.G. Titus. 2002. Climate change impacts on U.S. coastal and marine ecosystems. Estuaries 25: 149–164.
Schloss, P.D., and J. Handelsman. 2005. Metagenomics for studying unculturable microorganisms: Cutting the Gordian knot. Genome Biology 6: 229.
Séne, S., R. Avril, C. Chaintreuil, A. Geoffroy, C. Ndiaye, A.G. Diédhiou, O. Sadio, R. Courtecuisse, S.N. Sylla, M.A. Selosse, and A. Bâ. 2015. Ectomycorrhizal fungal communities of Coccoloba uvifera (L) L mature trees and seedlings in the neotropical coastal forests of Guadeloupe (Lesser Antilles). Mycorrhiza 25: 547–559.
Sengupta, A., and S. Chaudhuri. 2002. Arbuscular mycorrhizal relations of mangrove plant community at the Ganges river estuary in India. Mycorrhiza 12: 169–174.
Shemesh, H., B.E. Boaz, C.I. Millar, and T.D. Bruns. 2020. Symbiotic interactions above treeline of long-lived pines: Mycorrhizal advantage of limber pine (Pinus flexilis) over Great Basin bristlecone pine (Pinus longaeva) at the seedling stage. Journal of Ecology 108: 908–916.
Sigren, J.M., J. Figlus, and A.R. Armitage. 2014. Coastal sand dunes and dune vegetation: Restoration erosion and storm protection. Shore & Beach 82: 5–12.
Silliman, B.R., J. van de Koppel, M.D. Bertness, L.E. Stanton, and I.A. Mendelssohn. 2005. Drought snails and large-scale die-off of southern U.S. salt marshes. Science 310: 1803–1806.
Silliman, B.R., J. van de Koppel, M.W. McCoy, J. Diller, G.N. Kasozi, K. Earl, P.N. Adams, and A.R. Zimmerman. 2012. Degradation and resilience in Louisiana salt marshes after the BP–Deepwater Horizon oil spill. Proceedings of the National Academy of Sciences 109: 11234.
Slaymaker, D.H., M.S. Peek, J. Wresilo, D.C. Zeltner, and Y.F. Saleh. 2015. Genetic structure of native and restored populations of American beachgrass (Ammophila breviligulata Fern) along the New Jersey Coast. Journal of Coastal Research 31: 1334–1343.
Soares, M.A., H.-Y. Li, K.P. Kowalski, M. Bergen, M.S. Torres, and J.F. White. 2016. Evaluation of the functional roles of fungal endophytes of Phragmites australis from high saline and low saline habitats. Biological Invasions: 1–14.
Soudzilovskaia, N.A., M.G.A. van der Heijden, J.H.C. Cornelissen, M.I. Makarov, V.G. Onipchenko, M.N. Maslov, A.A. Akhmetzhanova, and P.M. van Bodegom. 2015. Quantitative assessment of the differential impacts of arbuscular and ectomycorrhiza on soil carbon cycling. New Phytologist 208: 280–293.
Spalding, M.D., S. Ruffo, C. Lacambra, I. Meliane, L.Z. Hale, C.C. Shepard, and M.W. Beck. 2014. The role of ecosystems in coastal protection: Adapting to climate change and coastal hazards. Ocean & Coastal Management 90: 50–57.
Sridhar, K.R., A.B. Arun, N. Narula, A. Deubel, and W. Merbach. 2005. Patterns of sole-carbon-source utilization by fast-growing coastal sand dune rhizobia of the southwest coast of India. Engineering in Life Sciences 5: 425–430.
Srivastava, J.K., H. Chandra, S.J.S. Kalra, P. Mishra, H. Khan, and P. Yadav. 2017. Plant–microbe interaction in aquatic system and their role in the management of water quality: A review. Applied Water Science 7: 1079–1090.
Swensen, S.M. 1996. The evolution of actinorhizal symbioses: Evidence for multiple origins of the symbiotic association. American Journal of Botany 83: 1503–1512.
Sylvia, D.M., A.G. Jarstfer, and M. Vosátka. 1993. Comparisons of vesicular-arbuscular mycorrhizal species and inocula formulations in a commercial nursery and on diverse Florida beaches. Biology and Fertility of Soils 16: 139–144.
Thrall, P.H., D.A. Millsom, A.C. Jeavons, M. Waayers, G.R. Harvey, D.J. Bagnall, and J. Brockwell. 2005. Seed inoculation with effective root-nodule bacteria enhances revegetation success. Journal of Applied Ecology 42: 740–751.
Tiepo, A.N., M.F. Hertel, S.S. Rocha, A.K. Calzavara, A.L.M. De Oliveira, J.A. Pimenta, H.C. Oliveira, E. Bianchini, and R. Stolf-Moreira. 2018. Enhanced drought tolerance in seedlings of Neotropical tree species inoculated with plant growth-promoting bacteria. Plant Physiology and Biochemistry 130: 277–288.
UNEP. 2006. Marine and coastal ecosystems and human wellbeing: a synthesis report based on the findings of the Millennium Ecosystem Assessment: Nairobi: United Nations Environment Programme.
Vacheron, J., G. Desbrosses, M.-L. Bouffaud, B. Touraine, Y. Moënne-Loccoz, D. Muller, L. Legendre, F. Wisniewski-Dyé, and C. Prigent-Combaret. 2013. Plant growth-promoting rhizobacteria and root system functioning. Frontiers in Plant Science 4: 356–356.
Valliere, J.M., W.S. Wong, P.G. Nevill, H. Zhong, and K.W. Dixon. 2020. Preparing for the worst: utilizing stress-tolerant soil microbial communities to aid ecological restoration in the Anthropocene. Ecological Solutions and Evidence 1: e12027.
van der Heijden, E.W., F.W. de Vries, and T.W. Kuyper. 2000. Mycorrhizal associations of Salix repens L. communities in succession of dune ecosystems I Above-ground and below-ground views of ectomycorrhizal fungi in relation to soil chemistry. Canadian Journal of Botany 77: 1821–1832.
van der Heijden, M.G.A., T. Boller, A. Wiemken, and I.R. Sanders. 1998. Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecology 79: 2082–2091.
Van der Putten, W.H., and B.A.M. Peters. 1997. How soil-borne pathogens may affect plant communities. Ecology 78: 1785–1795.
Van Der Valk, A.G., and P.M. Attiwill. 1984. Acetylene reduction in an Avicennia marina community in Southern Australia. Australian Journal of Botany 32: 157–164.
van Loon, L.C. 2007. Plant responses to plant growth-promoting rhizobacteria. European Journal of Plant Pathology 119: 243–254.
Vazquez, P., G. Holguin, M.E. Puente, A. Lopez-Cortes, and Y. Bashan. 2000. Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biology and Fertility of Soils 30: 460–468.
Vimal, S.R., and J.S. Singh. 2020. Chapter 11 - Microbial services to nurture plant health under stressed soils. In Microbial services in restoration ecology, eds. J.S. Singh and S.R. Vimal, 157–179: Amsterdam: Elsevier.
Vitousek, P.M., and L.R. Walker. 1989. Biological invasion by Myrica faya in Hawai‘i: Plant demography nitrogen fixation ecosystem effects. Ecological Monographs 59: 247–265.
Wahab, A.M.A., and P.F. Wareing. 1980. Nitrogenase activity associated with the rhizosphere of Ammophila arenaria L. and effect of inoculation of seedlings with Azotobacter. New Phytologist 84: 711–721.
Whiting, G.J., E.L. Gandy, and D.C. Yoch. 1986. Tight coupling of root-associated nitrogen fixation and plant photosynthesis in the salt marsh grass Spartina alterniflora and carbon dioxide enhancement of nitrogenase activity. Applied and Environmental Microbiology 52: 108–113.
Wilson, G.W.T., C.W. Rice, M.C. Rillig, A. Springer, and D.C. Hartnett. 2009. Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: Results from long-term field experiments. Ecology Letters 12: 452–461.
Wu, J., Y. Wang, and X. Lin. 2013. Purple phototrophic bacterium enhances stevioside yield by stevia rebaudiana bertoni via foliar spray and rhizosphere irrigation. PLOS ONE 8: e67644.
Wubs, E.R.J., W.H. van der Putten, M. Bosch, and T.M. Bezemer. 2016. Soil inoculation steers restoration of terrestrial ecosystems. Nature Plants 2: 16107.
Yuan, Z., I.S. Druzhinina, J. Labbé, R. Redman, Y. Qin, R. Rodriguez, C. Zhang, G.A. Tuskan, and F. Lin. 2016. Specialized microbiome of a halophyte and its role in helping non-host plants to withstand salinity. Scientific Reports 6: 32467.
Zheng, M., W. Wang, M. Hayes, A. Nydell, M.A. Tarr, S.A. Van Bael, and K. Papadopoulos. 2018. Degradation of Macondo 252 oil by endophytic Pseudomonas putida. Journal of Environmental Chemical Engineering 6: 643–648.
Zuberer, D.A., and W.S. Silver. 1978. Biological dinitrogen fixation (acetylene reduction) associated with Florida mangroves. Applied and Environmental Microbiology 35: 567–575.
Funding
Funding was provided by the Louisiana State Board of Regents (LEQSF(2017–20)-RD-A-14), the Coypu Foundation, the National Science Foundation (DEB-2027920), and the US Department of Agriculture’s National Institute of Food and Agriculture (2020–67014-31922) to EF and the National Science Foundation (DEB-2116358) to SV.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Kenneth L. Heck
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farrer, E.C., Van Bael, S.A., Clay, K. et al. Plant-Microbial Symbioses in Coastal Systems: Their Ecological Importance and Role in Coastal Restoration. Estuaries and Coasts 45, 1805–1822 (2022). https://doi.org/10.1007/s12237-022-01052-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-022-01052-2