Abstract
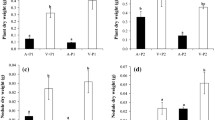
The ability to form symbiotic associations with soil microorganisms and the consequences for plant growth were studied for three woody legumes grown in five different soils of a Portuguese coastal dune system. Seedlings of the invasive Acacia longifolia and the natives Ulex europaeus and Cytisus grandiflorus were planted in the five soil types in which at least one of these species appear in the studied coastal dune system. We found significant differences between the three woody legumes in the number of nodules produced, final plant biomass and shoot 15N content. The number of nodules produced by A. longifolia was more than five times higher than the number of nodules produced by the native legumes. The obtained 15N values suggest that both A. longifolia and U. europaeus incorporated more biologically-fixed nitrogen than C. grandiflorus which is also the species with the smallest distribution. Finally, differences were also found between the three species in the allocation of biomass in the different studied soils. Acacia longifolia displayed a lower phenotypic plasticity than the two native legumes which resulted in a greater allocation to aboveground biomass in the soils with lower nutrient content. We conclude that the invasive success of A. longifolia in the studied coastal sand dune system is correlated to its capacity to nodulate profusely and to use the biologically-fixed nitrogen to enhance aboveground growth in soils with low N content.




Similar content being viewed by others
References
Azcón-Aguilar C, Barea JM (1996) Arbuscular mycorrhizas and biological control of soil-borne plant pathogens—an overview of the mechanisms involved. Mycorrhiza 6:457–464
Azcón R, Rubio R, Barea JM (1991) Selective interactions between different species of mycorrhizal fungi and Rhizobium meliloti strains, and their effects on growth, N2-fixation (15N) and nutrition of Medicago sativa L. New Phytol 117:399–404
Baker TJ, Gowen SR (1996) Staining nematodes and arbuscular mycorrhizae in the same root sample. Fundam Appl Nematol 19:607–608
Balbino LR (1968) O método de Egnér-Riehm na determinação do fósforo e do potássio “assimiláveis” em solos de Portugal. Rev Agron 51:46–56
Barea JM, Jeffries P (1995) Arbuscular mycorrhizas in sustainable soil–plant systems. In: Varma A, Hock B (eds) Mycorrhiza. Structure, function molecular biology and biotechnology. Springer, Berlin, pp 521–560
Bever JD (2003) Soil community feedback and the coexistence of competitors: conceptual framework and empirical tests. New Phytol 157:465–473
Bremner JM, Mulvaney CS (1982) Nitrogen-total. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2. Chemical and microbiological properties, 2nd edn. American Society of Agronomy Inc, Madison, Wisconsin, USA, pp 595–624
Castroviejo S et al (1999) Flora Ibérica: plantas vasculares de la Península Ibérica e Islas Baleares. CSIC, Madrid
Connell JH (1971) On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forests. In: den Boer PJ, Gradwell GR (eds) Dynamics in populations. Center for Agricultural Publishing and Documentation, Wageningen, The Netherlands, pp 298–312
Crisóstomo JA, Freitas H, Rodríguez-Echeverría S (2007) Comparison between relative growth rates of three woody legumes: implications in the process of ecological invasion. Web Ecol 7:22–26
Cronk QB, Fuller JL (1995) Plant invaders. Chapman and Hall, London
de la Peña E, Rodriguez-Echeverria S, van der Putten WH, Freitas H, Moens M (2006) Mycorrhizal fungi control migratory endoparasitic nematodes in Ammophila arenaria. New Phytol 169:829–840
Fogarty G, Facelli JM (1999) Growth and competition of Cytisus scoparius, an invasive shrub, and Australian native shrubs. Plant Ecol 144:27–35
Jakobsen I, Smith SE, Smith FA (2002) Function and diversity of arbuscular mycorrhizae in carbon and mineral nutrition. In: van der Heijden MGA, Sanders IR (eds) Mycorrhizal ecology. Springer-Verlag, GmbH, Berlin, Heidelberg, pp 75–92
Janzen DH (1970) Herbivores and the number of tree species in tropical forests. Am Nat 104:501–528
Jordan NR, Larson DL, Huerd SC (2007) Soil modification by invasive plants: effects on native and invasive species of mixed-grass prairies. Biol Invasions (online first)
Keeney DR, Nelson DW (1982) Nitrogen—inorganic forms. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2. Chemical and microbiological properties, 2nd edn. American Society of Agronomy, Inc, Madison, Wisconsin, USA, pp 643–698
Koske RE, Polson WR (1984) Are VA mycorrhizae required for sand dune stabilization? Bioscience 34:420–424
Lajtha K, Marshall JD (1994) Sources of variation in the stable isotopic composition of plants. In: Lajtha K, Michener RH (eds) Stables isotopes in ecology and environmental science. Blackwell Scientific, Oxford, pp 12–22
LQARS (1977) Ministerio da Agricultura. Lisboa, Portugal
Marchante H, Marchante E, Freitas H (2003) Invasion of the Portuguese dune ecosystems by the exotic species Acacia longifolia (Andrews) Willd.: effects at the community level. In: Child LE et al. (eds) Plant invasions: ecological threats and management solutions. Backhuys, Leiden, The Netherlands, pp 75–85
Marchante HS, Marchante EM, Buscardo E, Maia J, Freitas H (2004) Recovery potential of dune ecosystems invaded by an exotic Acacia species (Acacia longifolia). Weed Technol 18:1427–1433
McQueen JC, Tozer WC, Clarkson BD (2006) Consequences of alien N2-fixers on vegetation succession in New Zealand. In: Allen RB, Lee WG (eds) Biological invasions in New Zealand. Springer-Verlag, Berlin, Heidelberg
Mills KE, Bever JD (1998) Maintenance of diversity within plant communities: soil pathogens as agents of negative feedback. Ecology 79:1595–1601
Parker MA (2001) Mutualism as a constraint on invasion success for legumes and rhizobia. Divers Distrib 7:125–136
Parker MA, Malek W, Parker IM (2006) Growth of an invasive legume is symbiont limited in newly occupied habitats. Divers Distrib 12:563–571
Parker MA, Wurtz AK, Paynter Q (2007) Nodule symbiosis of invasive Mimosa pigra in Australia and in ancestral habitats: a comparative analysis. Biol Invasions 9:127–138
Parsons MJ, Douglas P, McMillain J (1998) Current names for wild plants in New Zealand. Manaaki Whenua Press, Lincoln, New Zealand
Peperkorn R, Werner C, Beyschlag W (2005) Phenotypic plasticity of an invasive acacia versus two native Mediterranean species. Funct Plant Biol 32:933–944
Pérez-Fernández MA, Lamont BB (2003) Nodulation and performance of exotic and native legumes in Australian soils. Aust J Bot 51:543–553
Poorter H, Nagel O (2000) The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantiative review. Aust J Plant Physiol 27:595–607
Reinhart KO, Packer A, Van der Putten WH, Clay K (2003) Plant–soil biota interactions and spatial distribution of black cherry in its native and invasive ranges. Ecol Lett 6:1046–1050
Richardson DM (2004) Plant invasion ecology—dispatches from the front line. Divers Distrib 10:315–319
Richardson DM, Allsopp N, D’Antonio CM, Milton SJ (2000) Plant invasions—the role of mutualisms. Biol Rev 75:65–93
Rodríguez-Echeverría S, Pérez-Fernández MA, Vlaar S, Finan TM (2003) Analysis of the legume-rhizobia symbiosis in shrubs from central western Spain. J Appl Microbiol 95:1367–1374
Rodríguez-Echeverría S, Crisóstomo JA, Freitas H (2007) Genetic diversity of rhizobia associated with Acacia longifolia in two stages of invasion of coastal sand dunes. Appl Environ Microbiol 73:5066–5070
Rossell RA, Gasparoni JC, Galantini JA (2001) Soil organic matter evaluation. In: Lal R, Kimble JM, Follett RF, Stewart BA (eds) Assessments methods for soil carbon. Lewis Publishers, USA, pp 676
Smith SE, Read DJ (1997) Mycorrhizal symbiosis, 2nd edn. Academic Press, London
Stephens BD, Neyra CA (1983) Nitrate and nitrite reduction in relation to nitrogenase activity in soybean nodules and Rhizobium japonicum bacteroids. Plant Physiol 71:731–735
Stepkowski T, Moulin L, Krzyzanska A, McInnes A, Law IJ, Howieson JG (2005) European origin of Bradyrhizobium populations infecting lupins and serradella in soils of Western Australia and South Africa. Appl Environ Microbiol 71:7041–7052
Ulrich A, Zaspel I (2000) Phylogenetic diversity of rhizobial strains nodulating Robinia pseudoacacia L. Microbiology 146:2997–3005
Vandenkoornhuyse P, Ridgway KP, Watson IJ, Fitter AH, Young JPW (2003) Co-existing grass species have distinctive arbuscular mycorrhizal communities. Mol Ecol 12:3085–3095
Watanabe FS, Olsen SR (1965) Test of an ascorbic acid method for determining phosphorus in water and Na HCO3 extracts from soil. Soil Sci Soc Am Proc 29:677–678
Weir BS, Turner SJ, Silvester WB, Park D-C, Young JM (2004) Unexpectedly diverse Mesorhizobium strains and Rhizobium leguminosarum nodulate native legume genera of New Zealand, while introduced legume weeds are nodulated by Bradyrhizobium species. Appl Environ Microbiol 70:5980–5987
Yelenik SG, Stock WD, Richardson DM (2007) Functional group identity does not predict invader impacts: differential effects of nitrogen-fixing exotic plants on ecosystem function. Biol Invasions 9:117–125
Acknowledgements
This work was supported by the project ROBIN (POCI/BIA-BDE/56941/2004) and by a post-doctoral research grant (SFRH/BPD/21066/2004) awarded to SRE, both from the Portuguese Foundation for Science and Technology (FCT) and the European Union (POCI 2010). We are grateful to Juan Antonio Galán for technical assistance during plant harvest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodríguez-Echeverría, S., Crisóstomo, J.A., Nabais, C. et al. Belowground mutualists and the invasive ability of Acacia longifolia in coastal dunes of Portugal. Biol Invasions 11, 651–661 (2009). https://doi.org/10.1007/s10530-008-9280-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-008-9280-8