Abstract
Blue crabs (Callinectes sapidus) are highly mobile, ecologically-important mesopredators that support multimillion-dollar fisheries along the western Atlantic Ocean. Understanding how blue crabs respond to coastal landscape change is integral to conservation and management, but such insights have been limited to a narrow range of habitats and spatial scales. We examined how local-scale to landscape-scale habitat characteristics and bathymetric features (channels and oceanic inlets) affect the relative abundance (catch per unit effort, CPUE) of adult blue crabs across a > 33 km2 seagrass landscape in coastal Virginia, USA. We found that crab CPUE was 1.7 × higher in sparse (versus dense) seagrass, 2.4 × higher at sites farther from (versus nearer to) salt marshes, and unaffected by proximity to oyster reefs. The probability that a trapped crab was female was 5.1 × higher in sparse seagrass and 8 × higher near deep channels. The probability of a female crab being gravid was 2.8 × higher near seagrass meadow edges and 3.3 × higher near deep channels. Moreover, the likelihood of a gravid female having mature eggs was 16 × greater in sparse seagrass and 32 × greater near oceanic inlets. Overall, we discovered that adult blue crab CPUE is influenced by seagrass, salt marsh, and bathymetric features on scales from meters to kilometers, and that habitat associations depend on sex and reproductive stage. Hence, accelerating changes to coastal geomorphology and vegetation will likely alter the abundance and distribution of adult blue crabs, challenging marine spatial planning and ecosystem-based fisheries management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ecological patterns and processes are linked to attributes of habitat structure and environmental heterogeneity across several spatial scales (Turner 1989; Wiens 1989; Levin 1992). However, understanding the drivers of ecological patterns such as population density can be difficult because their structuring processes often operate on different scales and can covary across space (Levin 1992). In marine and estuarine systems, free-swimming animals (nekton) are known to use multiple habitats across several spatial scales (Irlandi and Crawford 1997; Micheli and Peterson 1999; Pittman and McAlpine 2003), but understanding these species-habitat relationships has been limited by studies that evaluate single habitats and look solely at within-habitat scales (Irlandi 1997; Moore and Hovel 2010; Smith et al. 2010; Carroll et al. 2015; but see Pittman et al. 2004; Gullström et al. 2008; and Olds et al. 2012). Knowledge of how habitat structure affects faunal abundance at several spatial scales is important to ecosystem-based fisheries management and spatial conservation planning (e.g., marine reserves and coastal restoration; Roberts et al. 2003; Leslie 2005; Parsons et al. 2014; Duarte et al. 2020), and is especially urgent in the face of accelerating global degradation of coastal habitats (Lotze et al. 2006; Waycott et al. 2009; Halpern et al. 2019).
Seagrass meadows are model systems for understanding species-habitat relationships, as they create easily-quantifiable habitat structure that is heterogeneous over several spatial scales (Robbins and Bell 1994; Boström et al. 2006; Wedding et al. 2011). At within-meadow scales (tens to thousands of m2), seagrass meadows differ in shoot density, patch size and shape, and degree of fragmentation. Such within-meadow heterogeneity affects faunal diversity and abundance by modifying food availability, predator–prey interactions, and larval settlement and recruitment (Irlandi et al. 1995; Bologna and Heck 2000; Hovel and Fonseca 2005; Boström et al. 2006; Carroll et al. 2012, 2015). For example, scallop (Argopecten irradians) survival increases with seagrass shoot density (Carroll et al. 2015) and is highest at the center of seagrass patches compared to edges, while scallop growth shows the opposite trend (Carroll and Peterson 2013). The interactions between aspects of habitat structure at different within-meadow scales can also be important in affecting faunal abundance. For instance, the degree to which seagrass shoot density enhances juvenile blue crab (Callinectes sapidus) survival depends on broader-scale meadow patchiness (Hovel and Fonseca 2005). Conversely, patterns at the patch scale do not always influence broader scales. For example, although patch-scale edge effects are related to landscape fragmentation, juvenile blue crab mortality may be affected by fragmentation but not by distance to habitat patch edges (Yarnall and Fodrie 2020).
Beyond the scale of an individual seagrass meadow (hundreds of m2 to tens of thousands of m2), the configuration of seascape features such as biogenic habitats and bathymetric features (e.g., deep channels) becomes important as fauna move across the landscape (Irlandi and Crawford 1997; With et al. 1997; Micheli and Peterson 1999; Beets et al. 2003; Luo et al. 2009). As a result of faunal movement among seascape features (e.g., patches of certain habitats), landscape-scale habitat connectivity influences community composition, faunal abundance, and species richness (Dorenbosch et al. 2007; Gullström et al. 2008; Unsworth et al. 2008; Olds et al. 2012; Baillie et al. 2015; Sievers et al. 2016). For example, nekton abundance is greater in areas with adjacent seagrass meadows and salt marshes relative to areas supporting only one of these habitats (Baillie et al. 2015). Certain habitat configurations may also facilitate landscape-scale movement by creating corridors or temporary shelter for animals in transit. Blue crabs use vegetation for shelter while foraging among multiple oyster reefs (Micheli and Peterson 1999), while fish inhabiting seagrass meadows feed and shelter in nearby mangrove forests (Unsworth et al. 2008). Marine species also often exhibit ontogenetic changes in movements and migrations across a seascape (Pittman and McAlpine 2003); as a result, the influence of habitat structure on faunal abundance can vary with life stage and reproductive stage (Dorenbosch et al. 2005, 2007; Gullström et al. 2008; Luo et al. 2009). For instance, juvenile fish densities increase with distance from coral reef habitat, while adults exhibit the opposite pattern (Dorenbosch et al. 2005). Likewise, non-reproducing gray snapper (Lutjanus griseus) moves solely between nearshore seagrass and mangrove habitats, while reproductive gray snapper also moves onto offshore coral reefs (Luo et al. 2009).
Despite advances in understanding species-habitat relationships in seagrass ecosystems, most investigations focus on the within-meadow scale without considering the broader seascape, or vice versa. The few existing cross-scale studies occur in the tropics (e.g., examining seagrass meadows in relation to coral reefs and mangrove forests; Boström et al. 2011) and are likely not representative of temperate coastal landscapes and their ecosystems (e.g., salt marshes; but see Whaley et al. 2007 and Baillie et al. 2015). Though it is challenging to examine species-habitat relationships across scales, it is especially pertinent to effective marine conservation and management under accelerating coastal change. Understanding habitat patch configurations and connectivity across a seascape can inform restoration strategies for multiple habitat types (Weinstein et al. 2005; Waltham et al. 2021) and ensure that the appropriate area is protected for species that utilize multiple habitats (Gillanders et al. 2003; Meynecke et al. 2008).
To help resolve this gap in our understanding of how habitat structure affects faunal abundance across spatial scales, we determined how seagrass, salt marsh, oyster reef, and bathymetric features (deep channels and oceanic inlets) in a large, temperate coastal lagoon influence the relative abundance of adult blue crabs—highly mobile mesopredators that support multimillion-dollar fisheries throughout the western Atlantic Ocean (Bunnell et al. 2010). Specifically, we investigated (1) how within-meadow seagrass attributes and landscape-scale habitat connectivity variables affect adult blue crab catch per unit effort (CPUE), and (2) how these relationships change with crab sex and reproductive stage. Our study demonstrates the value of considering multiple spatial perspectives in marine ecology by showing that seagrass meadows, salt marshes, and bathymetric features influence adult blue crab CPUE across scales. Our results suggest that the loss, migration, and restoration of coastal vegetation and barrier islands will change the relative abundance and distribution of blue crabs, but responses will depend on spatial context, crab sex, and reproductive stage.
Methods
Study System
Blue crabs are widely distributed in estuarine and marine habitats along the Atlantic and Gulf coasts of the USA (Rathbun 1896), where they are important consumers of infaunal invertebrates (Hines et al. 1990) and constitute prey for birds, fishes, humans, and other blue crabs (Guillory and Elliot 2001). When foraging and seeking refuge, adult blue crabs live and move among multiple biogenic habitats, such as seagrass meadows, salt marshes, and oyster reefs (Hines et al. 1987; Ryer 1987; Wolcott and Hines 1990; Fitz and Wiegert 1991; Micheli and Peterson 1999; Glancy et al. 2003). Molting blue crabs exhibit sex-specific differences in habitat use: males seek refuge in tidal marsh creeks, whereas mature females prefer deeper waters (Hines et al. 1987; Wolcott and Hines 1990; Hines 2007). Reproducing female crabs also undergo a large-scale summer migration, where they mate in low-salinity headwaters and spawn at estuary mouths (Millikin and Williams 1984; Tankersley et al. 1998; Eggleston et al. 2015).
We focused on blue crab populations across the coastal lagoon–barrier island landscape of the Virginia Coast Reserve Long Term Ecological Research project (VCR LTER) located on the Delmarva Peninsula in Virginia, USA. This region is ideal for examining the effects of habitat structure on blue crab abundance because it is largely sheltered from many common human impacts (e.g., development, nutrient pollution) and lacks pronounced estuarine gradients (e.g., temperature, salinity, dissolved oxygen, pH, turbidity; Orth et al. 2012) that often confound landscape-scale observational studies in coastal regions (Pelton and Goldsborough 2008). The region is sparsely populated by humans and is rural, with about one-third of lands conserved (Clower and Bellas 2017). Due to low nitrogen inputs and frequent tidal exchange with the Atlantic Ocean via inlets between barrier islands (Fig. 1), water quality is high relative to other coastal bays in the USA and worldwide (chlorophyll a ≈ 2–6 µg/L, dissolved oxygen ≈ 6–9 mg/L, pH ≈ 8.0, salinity ≈ 29–32 PSU, total dissolved nitrogen ≈ 14–25 µmol/L, total suspended solids ≈ 26–60 mg/L; McGlathery et al. 2007; Orth et al. 2012; McGlathery and Christian 2020).
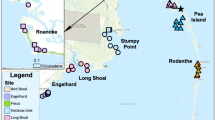
Map of study system. White circles indicate crab sampling sites within seagrass meadows (shown in green). Pale yellow and light blue areas show the distributions of salt marshes and intertidal oyster reefs, respectively. Dark blue areas show deep (> 3.4 m) channels. Bidirectional arrows show inlets connecting the coastal bays and the Atlantic Ocean (Imagery: Planet Team 2019)
Despite these relatively pristine conditions, a seagrass wasting disease epidemic in the 1930s (caused by the pathogen Labyrinthula zosterae) and a series of destructive hurricanes extirpated eelgrass (Zostera marina) populations in the Virginia coastal bays. To assist eelgrass recovery, practitioners restored the region in 2001 by seeding 2000–4000 m2 plots. Since then, eelgrass has expanded dramatically to cover a > 36 km2 landscape that spans several coastal bays, resulting in the largest successful seagrass restoration worldwide (Orth et al. 2006, 2020; Orth and McGlathery 2012). The landscape now hosts a complex mosaic of seagrass meadows, salt marshes (Spartina alterniflora), and oyster reefs (Crassostrea virginica) connected through open flats, channels, and tidal creeks (Fig. 1).
Blue Crab Surveys
We measured adult blue crab catch per unit effort (CPUE), or the number of individuals per trap per 24 h of soak time, from June through August 2019 at 24 fixed sites across > 33 km2 of seagrass meadows (Fig. 1; sites separated by 369–1137 m, mean = 672 m). We collected crabs with commercial crab traps (dimensions = 52 cm × 48 cm × 50 cm with four openings, each = 20 cm × 14 cm; mesh size = 5.5 cm × 3.5 cm) baited with Atlantic menhaden (Brevoortia tyrannus). We retrieved traps 24 h after deployment and repeated sampling five times at all sites (n = 120 total sampling events; sites sampled every 6–16 days). Although crab mobility may vary with diel and tidal cycles, we chose to retrieve traps after 24 h to maintain uniform trap soak times and replenish bait. Like all sampling methods, crab traps may present certain biases, such as potentially differing capture rates by sex (Bellchambers and de Lestang 2005); however, they are a standard and effective method for capturing adult blue crabs and excluding juveniles (Guillory 1998; Bellchambers and de Lestang 2005).
We counted the number of live crabs in each trap and recorded crab size (carapace width between lateral spines). We also recorded crab sex and female maturity based on apron shape (Fisher 1999). We noted the presence of eggs and their color (yellow, orange, brown, or black), which corresponds with embryonic development (Millikin and Williams 1984; Jivoff et al. 2007). Females with yellow or orange eggs were considered to have immature eggs, while females with brown or black eggs were considered to have mature eggs. Dead crabs were rare (1.2% of total). During each sampling event, we measured bottom dissolved oxygen (mean ± standard deviation; 7.7 ± 1.9 mg/L), water temperature (27 ± 1.4 °C), and salinity (30.5 ± 0.8 ppt) with a YSI Model 85 handheld meter (n = 120 total sampling events).
Seagrass and Landscape Measurements
To characterize local seagrass habitat structure for each site, we estimated shoot density on July 10–11, 2019 in ten 0.25 m2 quadrats spaced evenly along a 50 m transect (one transect per site). We were unable to collect data from one site, for which we instead interpolated seagrass shoot density by averaging measurements from the two nearest sites. We measured seagrass aboveground biomass (15 cm diameter core; 3 cores/site) and leaf area (3 shoots/core = 9 shoots/site); however, these variables were highly collinear with site-level estimates of shoot density (P < 0.001; R2 = 32–60%), so we omitted them from analyses.
To characterize landscape features, we analyzed aerial data products and satellite imagery in QGIS 3.14.0 (QGIS.org 2020). We used seagrass density maps based on high-resolution aerial photographs taken in 2019 (ground sample distance [GSD] = 24 cm; Orth et al. 2019) and salt marsh maps developed from 2011 field surveys and 2019 aerial photography (GSD = 30 cm; Berman et al. 2011; Planet Team 2019). We also used maps of intertidal oyster reefs created from 2002 and 2007 aerial imagery (GSD = 1 m; Ross and Luckenbach 2009) that were validated in 2015–2017 with field and LiDAR surveys (GSD < 1 m; Hogan and Reidenbach 2019; subtidal oyster reefs are not present in our study region). Specifically, we measured the Euclidean distance from each crab sampling site to the nearest seagrass meadow edge, as well as the minimum over-water distances between each site and the nearest salt marsh and oyster reef (Fig. 1). We estimated the minimum over-water distance between each site and the nearest oceanic inlet and deep channel (> 3.4 m depth below NAVD88; Fig. 1) using a 3-m-resolution bathymetry model (Richardson et al. 2014). We considered other landscape-scale predictors such as the area of seagrass surrounding each site at various radii, but these measures were highly collinear with distance to the edge of the meadow, so we omitted them from analyses (P < 0.001; R2 = 38–49%).
Statistical Analyses
We fit multiple generalized linear mixed models (GLMMs) to relate CPUE of adult blue crabs to seagrass meadow-scale and landscape-scale habitat variables. Specifically, we estimated the effects of mean seagrass shoot density as well as minimum distances to landscape habitat features (seagrass meadow edge, salt marsh, oyster reef, oceanic inlet, and deep channel) on total blue crab CPUE, probability of catching females, fecundity of females (i.e., presence of eggs), and egg maturity.
We modeled total crab CPUE using a negative binomial GLMM (log link; n = 120 trappings). We assessed sex-specific responses by modeling the probability that a trapped crab was female using a binomial GLMM (logit link; n = 845 crabs comprising 253 females and 592 males). Using binomial GLMMs, we quantified relationships with female reproductive stage by modeling the probability that a trapped mature female crab bore eggs (i.e., was gravid; n = 237 mature female crabs, with 100 gravid and 137 not gravid) and the probability that a gravid female crab bore mature eggs (n = 100 gravid females, with 13 bearing mature eggs and 87 with immature eggs).
We controlled for potential seasonal effects by including a term for day of the year (trap set date) and controlled for repeated measurements using a random intercept term for each site (Zuur et al. 2009; Diggle et al. 2013). We used site as a random effect in the model of total crab CPUE (negative binomial GLMM) to control for site-level variability. To control for potential trap-level effects, all models of individual crabs (binomial GLMMs) nested a random intercept of trap within site (Zuur et al. 2009).
Models were fit in R 4.0.3 (R Core Team 2020) using glmmTMB 1.0.2.1 (Magnusson et al. 2020). We applied Wald χ2 tests to GLMMs in a backwards model selection routine to identify the best-performing models (Bolker et al. 2009; Zuur et al. 2009). Terms that were not significant at the P < 0.05 level were dropped from candidate models (Pinheiro and Bates 2000; Zuur et al. 2009). We ensured the residuals of the best-performing models met the assumptions of linearity, normality, homoscedasticity, and zero-inflation via simulation using the DHARMa package (version 0.3.3.0; Hartig 2020). Spline correlograms showed no evidence of spatial autocorrelation and sample autocorrelation function analysis showed no evidence of temporal autocorrelation (Zuur et al. 2009). We assessed multicollinearity between model terms using the variance inflation factor (VIF), and in all cases, multicollinearity was low (VIF ≤ 1.3). We estimated marginal means and 95% confidence intervals (CIs) using ggeffects 1.0.1 (Lüdecke et al. 2020). Data and code to reproduce analyses are publicly available in Castorani and Cheng (2021).
Results
Total Blue Crab CPUE
Adult blue crab CPUE was negatively correlated with mean seagrass shoot density (Fig. 2a; Table 1, Table S1; χ2 = 4.9, P = 0.03), indicating that adult blue crabs were more abundant in sparser areas of seagrass than denser areas (predicted mean CPUE was 1.7 times higher at 100 shoots/m2 than at 720 shoots/m2). CPUE was positively correlated with distance from site to the nearest salt marsh (Fig. 2b; Table 1, Table S1; χ2 = 12.5, P < 0.001), indicating that adult crabs were more abundant farther from salt marshes (2.4 times higher CPUE at 1.6 km away than at 250 m away). All other landscape variables had no detectable effects (P > 0.05).
Adult blue crab abundance (CPUE) was a negatively associated with seagrass shoot density and b positively associated with distance from the nearest salt marsh. Lines and shading show mean model predictions and 95% confidence intervals, respectively, after controlling for covariates. Points show actual data (Vector image attribution: Integration and Application Network [IAN; ian.umces.edu/media-library])
Sex-specific Differences in Blue Crab CPUE
The probability that a captured blue crab was female was negatively correlated with mean seagrass shoot density (χ2 = 6.5, P = 0.01), the distance from site to the nearest deep channel (χ2 = 6.7, P < 0.01), and the day of the year (Table 1, Table S1; χ2 = 11.0, P < 0.001). Specifically, the probability of a captured crab being female was 5.1 times greater in sparse seagrass (100 shoots/m2) compared to traps in dense seagrass (720 shoots/m2; Fig. 3a). Further, captured crabs were 8 times more likely to be female in traps closer to deep channels (1.6 km away) than in those farther away (4.6 km; Fig. 3b). Crabs were also 2.6 times more likely to be female in traps set at the beginning of the summer (June 20) than at the end of the summer (August 2; Fig. S1). Conversely, the probability of catching a male blue crab versus a female crab was higher in denser seagrass, farther from deep channels, and later in the summer. All other landscape variables had no detectable effects (P > 0.05).
The probability that a trapped crab was female was negatively associated with a seagrass shoot density and b distance to the nearest deep channel. Data points are transparent to show overlap and represent actual data. Lines, shading, and image attribution as in Fig. 2. Panels above and below model predictions show histograms of the number of females and males caught, respectively. Red vertical lines indicate median values for each histogram
Reproductive Stage of Female Crabs
The probability that a captured female blue crab was gravid was negatively correlated with the distance from the edge of the seagrass meadow (χ2 = 5.1, P = 0.02), the distance from the site to the nearest deep channel (χ2 = 4.4, P = 0.04), and the day of the year (Table 1, Table S1; χ2 = 7.3, P < 0.01). Specifically, the probability of a captured female crab being gravid was 2.8 times higher at seagrass meadow edges (50 m from edge) compared to interiors (580 m from edge; Fig. 4a), and 3.3 times higher close to deep channels compared to far from them (Fig. 4b). The probability of catching a gravid crab was also 2.5 times higher at the beginning of the summer than at the end (Fig. S2). Conversely, the probability of capturing non-gravid females was higher at seagrass meadow interiors, farther from deep channels, and later in the summer. All other landscape variables had no detectable effects (P > 0.05).
The probability that a trapped female crab was gravid (i.e., had eggs) was negatively related to the distances to a the seagrass meadow edge and b the nearest deep channel. Panels above and below model predictions show histograms of the number of gravid females and non-gravid females caught, respectively. Points, lines, shading, and image attribution as in Fig. 3
Egg Maturity
The probability of catching a gravid female crab with mature eggs was negatively correlated with mean seagrass shoot density (χ2 = 5.7, P = 0.02) and distance to the nearest oceanic inlet (Table 1, Table S1; χ2 = 7.7, P < 0.01). In particular, the probability of catching a gravid crab with mature eggs (brown to black coloration) was 16 times higher in sparse seagrass compared to dense seagrass (Fig. 5a), and 32 times higher close to oceanic inlets (1.4 km away) compared to very far from them (6 km away; Fig. 5b). The probability of catching a gravid crab with immature eggs (yellow to orange coloration) was higher in dense seagrass and far from oceanic inlets. All other landscape variables had no detectable effects (P > 0.05).
The probability that a gravid female crab bore mature eggs was negatively related to a seagrass shoot density and b distance to oceanic inlets. Panels above and below model predictions show histograms of the number of gravid females with mature and immature eggs caught, respectively. Points, lines, shading, and image attribution as in Fig. 3
Discussion
Our results demonstrate that coastal vegetation and bathymetric features influence the relative abundance and distribution of adult blue crabs across spatial scales ranging from a few meters to several kilometers. Nekton often respond to the composition and arrangement of multiple coastal habitats (Irlandi and Crawford 1997; Micheli and Peterson 1999; Pittman and McAlpine 2003; Whaley et al. 2007; Olds et al. 2012; Baillie et al. 2015), but understanding these relationships has been constrained by a historical emphasis on a single spatial scale, usually at the cost of a broader seascape perspective (Boström et al. 2006, 2011). Our findings look across spatial scales to demonstrate that adult blue crab CPUE is mediated by both local seagrass density and regional proximity to salt marshes. Moreover, specific relationships with seagrass structure (shoot density and within-meadow location) and bathymetric features (deep channels and oceanic inlets) were varied by sex and reproductive stage, reinforcing the idea that blue crabs exhibit stage-specific differences in their use of coastal habitats (Hines et al. 1987; Hines 2007; Ramach et al. 2009). These findings suggest that coastal geomorphic change and the loss and restoration of coastal vegetation in our study system will alter adult blue crab local abundances and distributions.
Relationships with Seagrass Habitat Structure at Local to Landscape Scales
Adult blue crabs were more abundant in sparse areas of seagrass compared to dense areas. Predation is often hindered by structured habitat such as seagrass (Savino and Stein 1982; Heck and Orth 2006), resulting in higher prey survival in densely vegetated habitats compared to sparse or bare sites (Rozas and Odum 1988; Hovel and Fonseca 2005; Carroll et al. 2015; but see Mattila et al. 2008). Hence, sparse seagrass may have been valuable for adult blue crabs by improving foraging efficiency (Hovel and Lipcius 2001; Hines 2007; Carroll et al. 2015), while still providing sufficient refuge from blue crab predators (relative to unvegetated areas; Micheli and Peterson 1999). Sparse seagrass, particularly at meadow edges, can be areas where blue crab prey are especially vulnerable to predation (Hovel et al. 2021). We did not observe an ensuing edge effect on adult blue crab CPUE, but this is not necessarily surprising, as edge effects vary greatly among habitats, faunal types, and seasons (Boström et al. 2006, 2011).
Female blue crabs appeared to drive the relationship between total blue crab CPUE and seagrass density. Crabs caught in sparse seagrass were more likely to be females or, specifically, females bearing mature eggs, whereas males were more common in denser seagrass, possibly due to tradeoffs between foraging ability and interference from other adult blue crabs (Goss-Custard et al. 1984; Micheli 1997). This result may also have been biased by our sampling method; crab traps may have provided a more structured habitat (i.e., refuge) relative to the surrounding sparse seagrass, attracting reproductive females (Guillory 1998; Sturdivant and Clark 2011). However, the impact of crab traps on catch sex ratios is equivocal (Bellchambers and de Lestang 2005), with variation in catch depending more on size and molt stage than sex (M. J. Williams and Hill 1982). Notably, blue crab habitat associations may differ in direction (Johnston and Lipcius 2012) or magnitude (Orth and van Montfrans 1987; Bromilow and Lipcius 2017) depending on crab size and life stage. For example, in contrast to our finding for adults, juvenile crab density tends to be higher in denser seagrass due to higher survival rates (Hovel and Lipcius 2001, 2002; Hovel and Fonseca 2005). Future work comparing adult and juvenile blue crabs may further elucidate ontogenetic shifts in habitat use and movement, and help identify critical habitats and home ranges (Grüss et al. 2011).
Gravid female blue crabs were more abundant closer to meadow edges, likely because moving between several habitats during their spawning migration increases their encounters with habitat edges (Epifanio 1995; Carr et al. 2005; Hovel et al. 2021). Gravid females may also have congregated near seagrass meadow edges because they support a higher diversity and quantity of food (Bologna and Heck 2002; Tanner 2005; Boström et al. 2006; Darnell et al. 2009; Macreadie et al. 2010). This pattern is consistent with prior work showing that seagrass edges pose higher predation risk for crab prey (Peterson et al. 2001; Gorman et al. 2009; Smith et al. 2011; Carroll et al. 2012; but see Hovel et al. 2021) and potentially lower predation risk for blue crabs, allowing them to forage more securely (Mahoney et al. 2018). Future landscape-scale studies that manipulate prey density and predation on adult crabs will help resolve how seagrass indirectly mediates blue crab abundance by changing food availability and foraging behavior.
Relationships with Biogenic Habitats at Landscape Scales
We found that adult blue crabs were more abundant farther from salt marshes, which could suggest that marshes are not ideal summer habitat for adult blue crabs in our system. This finding contrasts with literature showing that blue crabs commonly forage and molt at marsh edges, though several of these studies examined salt marshes in isolation from other vegetated habitats (Hines et al. 1987; Fitz and Wiegert 1991; Jivoff and Able 2003). In our system, where blue crabs have access to expansive intertidal salt marshes and subtidal seagrass meadows, crabs may favor seagrass habitat because it provides longer durations of inundation and generally supports a higher quantity and quality of prey (Ryer 1987; McDevitt-Irwin et al. 2016). Crabs may also have avoided marshes due to predation by wading birds (e.g., herons and egrets [Ardea spp.]; Erwin 1996; Maccarone and Brzorad 2005; Post 2008), which gather in high densities during summer (Austin 1995; B. Williams et al. 2007). Further study is needed to determine how seagrass and salt marsh differ in their foraging value and predation risk to blue crabs, and how these effects vary over space and time.
Proximity to intertidal oyster reefs was unrelated to adult blue crab CPUE, despite their potential use as foraging ground (Wells 1961; Eggleston 1990; Micheli and Peterson 1999; Harding et al. 2010) and their importance to juvenile crab settlement and growth (Moksnes and Heck 2006; Shervette et al. 2011; Gain et al. 2017). However, blue crab abundance on oyster reefs is generally low compared to nearby seagrass and salt marsh (Coen et al. 1999; Glancy et al. 2003; Stunz et al. 2010), highlighting the need to consider multiple habitats across a seascape in studies of habitat use. Though oyster reefs are abundant with blue crab prey (Hines et al. 1990; Gain et al. 2017), the hard structure of oyster shells and narrow interstitial spaces (Hesterberg et al. 2017) provide ideal prey refuge (Shervette et al. 2011) and decrease blue crab foraging success (Hill and Weissburg 2013). Additionally, the oyster reefs in our study region are exclusively intertidal and are often small, fragmented, and isolated (Fig. 1; Hogan and Reidenbach 2019), requiring blue crabs to make potentially risky transits across exposed mudflats (Micheli and Peterson 1999).
Relationships with Bathymetric Features at Landscape Scales
Our findings that females were more common near deep channels and that crabs with mature eggs were more common near oceanic inlets can be understood through blue crab natural history. Mature females undergo their final molt in deep, open waters, while molting males inhabit shallow tidal marsh creeks (Hines et al. 1987; Wolcott and Hines 1990; Hines 2007). Gravid female blue crabs in particular often use deep channels to migrate seawards, where their eggs develop offshore (Epifanio 1995; Tankersley et al. 1998; Aguilar et al. 2005; Carr et al. 2005; Eggleston et al. 2015; Ogburn and Habegger 2015). Females with mature eggs may move closer to oceanic inlets in preparation for spawning compared to females with immature eggs, who are likely foraging and have not yet begun their directed seaward migration (Medici et al. 2006; Ramach et al. 2009). Bathymetric features such as inlets and deep channels may have a larger influence on blue crab distributions and migratory behavior in our polyhaline to euhaline coastal lagoons (Cargo 1958; Murphy and Secor 2006) compared to more brackish estuaries such as the Chesapeake Bay, where strong salinity gradients drive seasonal crab movements (Hines et al. 1987; Aguilar et al. 2005; Jivoff et al. 2017). More broadly, the importance of oceanic inlets and deep channels for blue crabs emphasizes the strong role that bathymetric features can play in structuring nekton distributions (Bell et al. 1988; Whaley et al. 2007; Cameron et al. 2014; Sievers et al. 2016).
Temporal Dimensions of Blue Crab Distributions
Consistent with previous observations, we found that female adult blue crabs and gravid crabs were more abundant earlier in the summer (Millikin and Williams 1984; Medici et al. 2006; Jivoff et al. 2017). Although we repeated sampling multiple times over a 6-week period, our study does not address seasonal patterns and interannual variation in crab abundances (Hines et al. 1987; Lipcius et al. 2003). Some of our findings were likely related to the female blue crab spawning migration that lasts from summer to autumn (Millikin and Williams 1984; Lipcius et al. 2003); as a result, we anticipate that blue crab habitat associations will vary seasonally with crab life history. Our study also did not account for fine-scale temporal variation (e.g., across a day or tidal cycle), though blue crabs exhibit changes in feeding and associated habitat use across these time periods (Ryer 1987, 1990; Fitz and Wiegert 1991). Future studies would benefit from examining spatial relationships across diel and tidal cycles, as well as across seasons and years to determine the degree to which these associations are temporally variable or connected to regional population trends.
Biogenic habitats, deep channels, and oceanic inlets in our study system are particularly dynamic over space and time due to active seagrass and oyster reef restorations (Orth and McGlathery 2012; Hogan and Reidenbach 2019), accelerating loss of salt marshes (Sun et al. 2018), extreme sea level rise (5.4 mm/y at the VCR; Sallenger et al. 2012; Aoki et al. 2020), and extraordinary rates of storm-driven geomorphic change (e.g., 15–40 m/y; Fenster and McBride 2015). Based on our findings, we anticipate that the spatial patterns of adult blue crabs will shift over the coming decades in response to projected changes in the distribution of barrier islands, coastal vegetation, and bathymetric features (Reeves et al. 2020; Wiberg et al. 2020; Oreska et al. 2021). Continued study will illuminate the importance of such geospatial changes relative to long-term trends and interannual variability.
Conclusions
Our study adds to a growing body of work demonstrating the importance of broad spatial perspectives to understanding species-habitat associations (Boström et al. 2011; Pittman et al. 2021). Because blue crab fisheries are highly valued, such knowledge may benefit marine spatial planning and ecosystem-based fisheries management (Crowder et al. 2008), especially with regard to the United Nations Sustainable Development Goal 14, “Life Below Water” (Duarte et al. 2020). Our finding that habitat associations depend on the sex and reproductive stage of adult blue crabs may inform fisheries management actions, such as setting sex-specific exploitation thresholds or designating sanctuary areas based on habitat distributions. For example, our results show that seagrass meadows near oceanic inlets in Virginia’s coastal bays may be a valuable habitat for gravid females during their spawning season, particularly because spawning females may remain offshore rather than return to estuaries (Gelpi et al. 2013). As such, there may be conservation value to including such habitats in blue crab spawning sanctuaries, which have been shown to provide effective protection (Lipcius et al. 2003; Lambert et al. 2006). Further clarifying blue crab-habitat relationships over the long-term will be essential for fisheries managers, coastal planners, and conservation practitioners in the context of rapid changes due to sea level rise, habitat loss, and restoration.
References
Aguilar, R., A.H. Hines, T.G. Wolcott, D.L. Wolcott, M.A. Kramer, and R.N. Lipcius. 2005. The timing and route of movement and migration of post-copulatory female blue crabs, Callinectes sapidus Rathbun, from the upper Chesapeake Bay. Journal of Experimental Marine Biology and Ecology 319: 117–128. https://doi.org/10.1016/j.jembe.2004.08.030.
Aoki, L.R., K.J. McGlathery, P.L. Wiberg, and A. Al-Haj. 2020. Depth affects seagrass restoration success and resilience to marine heat wave disturbance. Estuaries and Coasts 43: 316–328. https://doi.org/10.1007/s12237-019-00685-0.
Austin, H.M. 1995. Observations on daylight feeding periodicities of wading birds (Ardeidae) in Sarah’s Creek, York River, Virginia. Journal of the Elisha Mitchell Scientific Society 111: 137–145.
Baillie, C.J., J.M. Fear, and F.J. Fodrie. 2015. Ecotone effects on seagrass and saltmarsh habitat use by juvenile nekton in a temperate estuary. Estuaries and Coasts 38: 1414–1430. https://doi.org/10.1007/s12237-014-9898-y.
Beets, J., L. Muehlstein, K. Haught, and H. Schmitges. 2003. Habitat connectivity in coastal environments: patterns and movements of Caribbean coral reef fishes with emphasis on bluestriped grunt, Haemulon sciurus. Gulf and Caribbean Research 14: 29–42. https://doi.org/10.18785/gcr.1402.03.
Bell, J.D., A.S. Steffe, and M. Westoby. 1988. Location of seagrass beds in estuaries: Effects on associated fish and decapods. Journal of Experimental Marine Biology and Ecology 122: 127–146. https://doi.org/10.1016/0022-0981(88)90180-3.
Bellchambers, L.M., and S. de Lestang. 2005. Selectivity of different gear types for sampling the blue swimmer crab, Portunus pelagicus L. Fisheries Research 73: 21–27. https://doi.org/10.1016/j.fishres.2005.01.007.
Berman, M.R., H. Berquist, S. Killeen, C. Hershner, K. Nunez, K. Reay, T. Rudnicky, and D. E. Schatt. 2011. Northampton County – Shoreline Inventory Report, Comprehensive Coastal Inventory Program. Gloucester Point, Virginia, 23062: Virginia Institute of Marine Science, College of William and Mary.
Bolker, B.M., M.E. Brooks, C.J. Clark, S.W. Geange, J.R. Poulsen, M.H.H. Stevens, and J.-S.S. White. 2009. Generalized linear mixed models: A practical guide for ecology and evolution. Trends in Ecology & Evolution 24: 127–135. https://doi.org/10.1016/j.tree.2008.10.008.
Bologna, P.A.X., and K.L. Heck. 2000. Impacts of seagrass habitat architecture on bivalve settlement. Estuaries 23: 449–457. https://doi.org/10.2307/1353138.
Bologna, P.A.X., and K.L. Heck. 2002. Impact of habitat edges on density and secondary production of seagrass-associated fauna. Estuaries 25: 1033–1044. https://doi.org/10.1007/BF02691350.
Boström, C., E.L. Jackson, and C.A. Simenstad. 2006. Seagrass landscapes and their effects on associated fauna: A review. Estuarine, Coastal and Shelf Science 68: 383–403. https://doi.org/10.1016/j.ecss.2006.01.026.
Boström, C., S.J. Pittman, C. Simenstad, and R.T. Kneib. 2011. Seascape ecology of coastal biogenic habitats: Advances, gaps, and challenges. Marine Ecology Progress Series 427: 191–217. https://doi.org/10.3354/meps09051.
Bromilow, A.M., and R.N. Lipcius. 2017. Mechanisms governing ontogenetic habitat shifts: Role of trade-offs, predation, and cannibalism for the blue crab. Marine Ecology Progress Series 584: 145–159. https://doi.org/10.3354/meps12405.
Bunnell, D.B., D.W. Lipton, and T.J. Miller. 2010. The bioeconomic impact of different management regulations on the Chesapeake Bay blue crab fishery. North American Journal of Fisheries Management 30: 1505–1521. https://doi.org/10.1577/M09-182.1.
Cameron, M.J., V. Lucieer, N.S. Barrett, C.R. Johnson, and G.J. Edgar. 2014. Understanding community–habitat associations of temperate reef fishes using fine-resolution bathymetric measures of physical structure. Marine Ecology Progress Series 506: 213–229. https://doi.org/10.3354/meps10788.
Cargo, D.G. 1958. The migration of adult female blue crabs, Callinectes sapidus Rathbun, in Chincoteague Bay and adjacent waters. Journal of Marine Research 16: 180–191.
Carr, S.D., J.L. Hench, R.A. Jr. Luettich, R.B. Jr. Forward, and R.A. Tankersley. 2005. Spatial patterns in the ovigerous Callinectes sapidus spawning migration: results from a coupled behavioral-physical model. Marine Ecology Progress Series 294: 213–226. https://doi.org/10.3354/meps294213
Carroll, J.M., B.T. Furman, S.T. Tettelbach, and B.J. Peterson. 2012. Balancing the edge effects budget: Bay scallop settlement and loss along a seagrass edge. Ecology 93: 1637–1647. https://doi.org/10.1890/11-1904.1.
Carroll, J.M., L.J. Jackson, and B.J. Peterson. 2015. The effect of increasing habitat complexity on bay scallop survival in the presence of different decapod crustacean predators. Estuaries and Coasts 38: 1569–1579. https://doi.org/10.1007/s12237-014-9902-6.
Carroll, J.M., and B.J. Peterson. 2013. Ecological trade-offs in seascape ecology: Bay scallop survival and growth across a seagrass seascape. Landscape Ecology 28: 1401–1413. https://doi.org/10.1007/s10980-013-9893-x.
Castorani, M.C.N., and S.L. Cheng. 2021. Blue crab densities in a landscape context on the Virginia Coast, summer 2019 ver 10. Environmental data initiative. https://doi.org/10.6073/pasta/25042bc26f5eba3b4da79ed48eb0d5d4.
Clower, T.L., and D.D. Bellas. 2017. Socio-economic impacts of conserved land on Virginia’s Eastern Shore. George Mason University and Urban Analytics, Inc.
Coen, L.D., D.M. Knott, E.L. Wenner, N.H. Hadley, A.H. Ringwood, and M.Y. Bobo. 1999. Intertidal oyster reef studies in South Carolina: design, sampling and experimental focus for evaluating habitat value and function. In oyster reef habitat restoration: a synopsis and synthesis of approaches, ed. M. L. Luckenbach, R. Mann, and J. A. Wesson, 133–158. Williamsburg, VA: Virginia Institute of Marine Science. https://doi.org/10.21220/V5NK51.
Crowder, L.B., E.L. Hazen, N. Avissar, R. Bjorkland, C. Latanich, and M.B. Ogburn. 2008. The impacts of fisheries on marine ecosystems and the transition to ecosystem-based management. Annual Review of Ecology, Evolution, and Systematics 39: 259–278. https://doi.org/10.1146/annurev.ecolsys.39.110707.173406
Darnell, M.Z., D. Rittschof, K.M. Darnell, and R.E. McDowell. 2009. Lifetime reproductive potential of female blue crabs Callinectes sapidus in North Carolina, USA. Marine Ecology Progress Series 394: 153–163. https://doi.org/10.3354/meps08295.
Diggle, P.J., P.J. Heagerty, K.-Y. Liang, and S.L. Zeger. 2013. Analysis of longitudinal data, 2nd ed. New York, NY, USA: Oxford University Press.
Dorenbosch, M., M.G.G. Grol, I. Nagelkerken, and G. van der Velde. 2005. Distribution of coral reef fishes along a coral reef-seagrass gradient: Edge effects and habitat segregation. Marine Ecology Progress Series 299: 277–288. https://doi.org/10.3354/meps299277.
Dorenbosch, M., W.C.E.P. Verberk, I. Nagelkerken, and G. van der Velde. 2007. Influence of habitat configuration on connectivity between fish assemblages of Caribbean seagrass beds, mangroves and coral reefs. Marine Ecology Progress Series 334: 103–116. https://doi.org/10.3354/meps334103.
Duarte, C.M., S. Agusti, E. Barbier, G.L. Britten, J.C. Castilla, J. Gattuso, R.W. Fulweiler, et al. 2020. Rebuilding marine life. Nature 580: 39–51. https://doi.org/10.1038/s41586-020-2146-7.
Eggleston, D.B. 1990. Foraging behavior of the blue crab, Callinectes sapidus, on juvenile oysters, Crassostrea virginica - effects of prey density and size. Bulletin of Marine Science 46: 62–82.
Eggleston, D.B., E. Millstein, and G. Plaia. 2015. Timing and route of migration of mature female blue crabs in a tidal estuary. Biology Letters 11: 1–5. https://doi.org/10.1098/rsbl.2014.0936.
Epifanio, C.E. 1995. Transport of blue crab (Callinectes sapidus) larvae in the waters off Mid-Atlantic states. Bulletin of Marine Science 57: 713–725.
Erwin, R.M. 1996. Dependence of waterbirds and shorebirds on shallow-water habitats in the Mid-Atlantic coastal region: An ecological profile and management recommendations. Estuaries 19: 213–219. https://doi.org/10.2307/1352226.
Fenster, M.S., and R.A. McBride. 2015. Barrier-island geology and morphodynamics along the open-ocean coast of the Delmarva Peninsula: An overview. In Holocene Barrier-Island Geology and Morphodynamics of the Maryland and Virginia Open-Ocean Coasts: Fenwick, Assateague, Chincoteague, Wallops, Cedar, and Parramore Islands. Geological Society of America Field Guide 40: 310–334.
Fisher, M.R. 1999. Effect of temperature and salinity on size at maturity of female blue crabs. Transactions of the American Fisheries Society 128: 499–506. https://doi.org/10.1577/1548-8659(1999)128%3c0499:eotaso%3e2.0.co;2.
Fitz, H.C., and R.G. Wiegert. 1991. Utilization of the intertidal zone of a salt marsh by the blue crab Callinectes sapidus: Density, return frequency, and feeding habits. Marine Ecology Progress Series 76: 249–260. https://doi.org/10.3354/meps076249.
Gain, I.E., R.A. Brewton, M.M. Reese Robillard, K.D. Johnson, D.L. Smee, and G.W. Stunz. 2017. Macrofauna using intertidal oyster reef varies in relation to position within the estuarine habitat mosaic. Marine Biology 164: 1–16. https://doi.org/10.1007/s00227-016-3033-5.
Gelpi, C.G., Jr., B. Fry, R.E. Condrey, J.W. Fleeger, and S.F. Dubois. 2013. Using δ13C and δ15N to determine the migratory history of offshore Louisiana blue crab spawning stocks. Marine Ecology Progress Series 494: 205–218. https://doi.org/10.3354/meps10540.
Gillanders, B.M., K.W. Able, J.A. Brown, D.B. Eggleston, and P.F. Sheridan. 2003. Evidence of connectivity between juvenile and adult habitats for mobile marine fauna: An important component of nurseries. Marine Ecology Progress Series 247: 281–295. https://doi.org/10.3354/meps247281.
Glancy, T.P., T.K. Frazer, C.E. Cichra, and W.J. Lindberg. 2003. Comparative patterns of occupancy by decapod crustaceans in seagrass, oyster, and marsh-edge habitats in a northeast Gulf of Mexico estuary. Estuaries 26: 1291–1301. https://doi.org/10.1007/BF02803631.
Gorman, A.M., R.S. Gregory, and D.C. Schneider. 2009. Eelgrass patch size and proximity to the patch edge affect predation risk of recently settled age 0 cod (Gadus). Journal of Experimental Marine Biology and Ecology 371: 1–9. https://doi.org/10.1016/j.jembe.2008.12.008.
Goss-Custard, J.D., R.T. Clarke, and S.E.A.le V. dit Durell. 1984. Rates of food intake and aggression of oystercatchers Haematopus ostralegus on the most and least preferred mussel Mytilus edulis beds of the Exe Estuary. Journal of Animal Ecology 53: 233–245. https://doi.org/10.2307/4354.
Grüss, A., D.M. Kaplan, S. Guénette, C.M. Roberts, and L.W. Botsford. 2011. Consequences of adult and juvenile movement for marine protected areas. Biological Conservation 144: 692–702. https://doi.org/10.1016/j.biocon.2010.12.015.
Guillory, V. 1998. Blue crab, Callinectes sapidus, retention rates in different trap meshes. Marine Fisheries Review 60: 35–37.
Guillory, V., and M. Elliot. 2001. A review of blue crab predators. In Blue Crab Mortality Symposium 90: 69–83.
Gullström, M., M. Bodin, P.G. Nilsson, and M.C. Öhman. 2008. Seagrass structural complexity and landscape configuration as determinants of tropical fish assemblage composition. Marine Ecology Progress Series 363: 241–255. https://doi.org/10.3354/meps07427.
Halpern, B.S., M. Frazier, J. Afflerbach, J.S. Lowndes, F. Micheli, C. O’Hara, C. Scarborough, and K.A. Selkoe. 2019. Recent pace of change in human impact on the world’s ocean. Scientific Reports 9: 11609. https://doi.org/10.1038/s41598-019-47201-9.
Harding, J.M., M.J. Southworth, and R. Mann. 2010. Observations of blue crabs (Callinectes sapidus, Rathbun 1896) on Shell Bar oyster reef, Great Wicomico River, Virginia. Journal of Shellfish Research 29: 995–1004. https://doi.org/10.2983/035.029.0435.
Hartig, F. 2020. DHARMa: Residual diagnostics for hierarchical (multi-level/mixed) regression models (version 0.3.3.0).
Heck, K.J., and R.J. Orth. 2006. Predation in seagrass beds. In Seagrasses: Biology, Ecology and Conservation, 537–550. https://doi.org/10.1007/1-4020-2983-7_22
Hesterberg, S.G., C.C. Duckett, E.A. Salewski, and S.S. Bell. 2017. Three-dimensional interstitial space mediates predator foraging success in different spatial arrangements. Ecology 98: 1153–1162. https://doi.org/10.1002/ecy.1762.
Hill, J.M., and M.J. Weissburg. 2013. Habitat complexity and predator size mediate interactions between intraguild blue crab predators and mud crab prey in oyster reefs. Marine Ecology Progress Series 488: 209–219. https://doi.org/10.3354/meps10386.
Hines, A.H. 2007. Ecology of juvenile and adult blue crabs. In The Blue Crab: Callinectes Sapidus, ed. V. S. Kennedy and L. E. Cronin, 565–654. Maryland Sea Grant College.
Hines, A.H., A.M. Haddon, and L.A. Wiechert. 1990. Guild structure and foraging impact of blue crabs and epibenthic fish in a sub-estuary of Chesapeake Bay. Marine Ecology Progress Series 67: 105–126. https://doi.org/10.3354/meps067105.
Hines, A.H., R.N. Lipcius, and A.M. Haddon. 1987. Population dynamics and habitat partitioning by size, sex, and molt stage of blue crabs Callinectes sapidus in a subestuary of central Chesapeake Bay. Marine Ecology Progress Series 36: 55–64. https://doi.org/10.3354/meps036055.
Hogan, S., and M.A. Reidenbach. 2019. Quantifying and mapping intertidal oyster reefs utilizing LiDAR-based remote sensing. Marine Ecology Progress Series 630: 83–99. https://doi.org/10.3354/meps13118.
Hovel, K.A., J.E. Duffy, J.J. Stachowicz, P. Reynolds, C. Boström, K.E. Boyer, S. Cimon, et al. 2021. Joint effects of patch edges and habitat degradation on faunal predation risk in a widespread marine foundation species. Ecology 102. https://doi.org/10.1002/ecy.3316
Hovel, K.A., and M.S. Fonseca. 2005. Influence of seagrass landscape structure on the juvenile blue crab habitat-survival function. Marine Ecology Progress Series 300: 179–191. https://doi.org/10.3354/meps300179.
Hovel, K.A., and R.N. Lipcius. 2001. Habitat fragmentation in a seagrass landscape: Patch size and complexity control blue crab survival. Ecology 82: 1814–1829. https://doi.org/10.2307/2680049.
Hovel, K.A., and R.N. Lipcius. 2002. Effects of seagrass habitat fragmentation on juvenile blue crab survival and abundance. Journal of Experimental Marine Biology and Ecology 271: 75–98. https://doi.org/10.1016/S0022-0981(02)00043-6.
Irlandi, E.A. 1997. Seagrass patch size and survivorship of an infaunal bivalve. Oikos 78: 511–518. https://doi.org/10.2307/3545612.
Irlandi, E.A., W.G. Ambrose, and B.A. Orlando. 1995. Landscape ecology and the marine environment: How spatial configuration of seagrass habitat influences growth and survival of the bay scallop. Oikos 72: 307–313. https://doi.org/10.2307/3546115.
Irlandi, E.A., and M.K. Crawford. 1997. Habitat linkages: The effect of intertidal saltmarshes and adjacent subtidal habitats on abundance, movement, and growth of an estuarine fish. Oecologia 110: 222–230. https://doi.org/10.1007/s004420050154.
Jivoff, P.R., and K.W. Able. 2003. Evaluating salt marsh restoration in Delaware Bay: The response of blue crabs, Callinectes sapidus, at former salt hay farms. Estuaries 26: 709–719. https://doi.org/10.1007/bf02711982.
Jivoff, P.R., A.H. Hines, and L.S. Quackenbush. 2007. Reproduction biology and embryonic development. In The Blue Crab: Callinectes sapidus, ed. V. S. Kennedy and L. E. Cronin, 255–298. Maryland Sea Grant College.
Jivoff, P.R., J.M. Smith, V.L. Sodi, S.M. VanMorter, K.M. Faugno, A.L. Werda, and M.J. Shaw. 2017. Population structure of adult blue crabs, Callinectes sapidus, in relation to physical characteristics in Barnegat Bay, New Jersey. Estuaries and Coasts 40: 235–250. https://doi.org/10.1007/s12237-016-0127-8.
Johnston, C.A., and R.N. Lipcius. 2012. Exotic macroalga Gracilaria vermiculophylla provides superior nursery habitat for native blue crab in Chesapeake Bay. Marine Ecology Progress Series 467: 137–146. https://doi.org/10.3354/meps09935.
Lambert, D.M., R.N. Lipcius, and J.M. Hoenig. 2006. Assessing effectiveness of the blue crab spawning stock sanctuary in Chesapeake Bay using tag-return methodology. Marine Ecology Progress Series 321: 215–225. https://doi.org/10.3354/meps321215.
Leslie, H.M. 2005. A synthesis of marine conservation planning approaches. Conservation Biology 19: 1701–1713. https://doi.org/10.1111/j.1523-1739.2005.00268.x.
Levin, S.A. 1992. The problem of pattern and scale in ecology: The Robert H. MacArthur Award Lecture. Ecology 73: 1943–1967. https://doi.org/10.2307/1941447.
Lipcius, R.N., W.T. Stockhausen, R.D. Seitz, and P.J. Geer. 2003. Spatial dynamics and value of a marine protected area and corridor for the blue crab spawning stock in Chesapeake Bay. Bulletin of Marine Science 72: 453–469.
Lotze, H.K., H.S. Lenihan, B.J. Bourque, R.H. Bradbury, R.G. Cooke, M.C. Kay, S.M. Kidwell, M.X. Kirby, C.H. Peterson, and J.B.C. Jackson. 2006. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312: 1806–1809. https://doi.org/10.1126/science.1128035.
Lüdecke, D., F. Aust, S. Crawley, and M. Ben-Shachar. 2020. ggeffects: create tidy data frames of marginal effects for “ggplot” from model outputs (version 1.0.1).
Luo, J., J.E. Serafy, S. Sponaugle, P.B. Teare, and D. Kieckbusch. 2009. Movement of gray snapper Lutjanus griseus among subtropical seagrass, mangrove, and coral reef habitats. Marine Ecology Progress Series 380: 255–269. https://doi.org/10.3354/meps07911.
Maccarone, A.D., and J.N. Brzorad. 2005. Foraging microhabitat selection by wading birds in a tidal estuary, with implications for conservation. Waterbirds: The International Journal of Waterbird Biology 28: 383–391. https://doi.org/10.1675/1524-4695(2005)028[0383:fmsbwb]2.0.co;2.
Macreadie, P.I., J.S. Hindell, M.J. Keough, G.P. Jenkins, and R.D. Connolly. 2010. Resource distribution influences positive edge effects in a seagrass fish. Ecology 91: 2013–2021. https://doi.org/10.1890/08-1890.1.
Magnusson, A., H. Skaug, A. Nielsen, C. Berg, K. Kristensen, M. Maechler, K. van Bentham, et al. 2020. glmmTMB: generalized linear mixed models using template model builder (version 1.0.2.1).
Mahoney, R.D., M.D. Kenworthy, J.K. Geyer, K.A. Hovel, and F.J. Fodrie. 2018. Distribution and relative predation risk of nekton reveal complex edge effects within temperate seagrass habitat. Journal of Experimental Marine Biology and Ecology 503: 52–59. https://doi.org/10.1016/j.jembe.2018.02.004.
Mattila, J., K.L. Heck, E. Millstein, E. Miller, C. Gustafsson, S. Williams, and D. Byron. 2008. Increased habitat structure does not always provide increased refuge from predation. Marine Ecology Progress Series 361: 15–20. https://doi.org/10.3354/meps07392.
McDevitt-Irwin, J.M., J.C. Iacarella, and J.K. Baum. 2016. Reassessing the nursery role of seagrass habitats from temperate to tropical regions: A meta-analysis. Marine Ecology Progress Series 557: 133–143. https://doi.org/10.3354/meps11848.
McGlathery, K.J., and R.R. Christian. 2020. Water Quality Sampling - integrated measurements for the Virginia Coast, 1992–2019. Virginia Coast Reserve Long-Term Ecological Research Project Data Publication. https://doi.org/10.6073/pasta/db7f8fe720ddfcdfab1352adc9c22702.
McGlathery, K.J., K. Sundbäck, and I.C. Anderson. 2007. Eutrophication in shallow coastal bays and lagoons: The role of plants in the coastal filter. Marine Ecology Progress Series 348: 1–18. https://doi.org/10.3354/meps07132.
Medici, D.A., T.G. Wolcott, and D.L. Wolcott. 2006. Scale-dependent movements and protection of female blue crabs (Callinectes sapidus). Canadian Journal of Fisheries and Aquatic Sciences 63: 858–871. https://doi.org/10.1139/f05-263.
Meynecke, J.-O., S.Y. Lee, and N.C. Duke. 2008. Linking spatial metrics and fish catch reveals the importance of coastal wetland connectivity to inshore fisheries in Queensland, Australia. Biological Conservation 141: 981–996. https://doi.org/10.1016/j.biocon.2008.01.018.
Micheli, F. 1997. Effects of predator foraging behavior on patterns of prey mortality in marine soft bottoms. Ecological Monographs 67: 203–224. https://doi.org/10.1890/0012-9615(1997)067[0203:eopfbo]2.0.co;2.
Micheli, F., and C.H. Peterson. 1999. Estuarine vegetated habitats as corridors for predator movements. Conservation Biology 13: 869–881. https://doi.org/10.1046/j.1523-1739.1999.98233.x.
Millikin, M.R., and A.B. Williams. 1984. Synopsis of biological data on the blue crab. Callinectes sapidus Rathbun. NOAA Technical Reports NMFS: NOAA.
Moksnes, P.O., and K.L. Heck. 2006. Relative importance of habitat selection and predation for the distribution of blue crab megalopae and young juveniles. Marine Ecology Progress Series 308: 165–181. https://doi.org/10.3354/meps308165.
Moore, E.C., and K.A. Hovel. 2010. Relative influence of habitat complexity and proximity to patch edges on seagrass epifaunal communities. Oikos 119: 1299–1311. https://doi.org/10.1111/j.1600-0706.2009.17909.x.
Murphy, R.F., and D.H. Secor. 2006. Fish and blue crab assemblage structure in a U.S. mid-Atlantic coastal lagoon complex. Estuaries and Coasts 29: 1121–1131. https://doi.org/10.1007/BF02781814.
Ogburn, M.B., and L.C. Habegger. 2015. Reproductive status of Callinectes sapidus as an indicator of spawning habitat in the South Atlantic Bight, USA. Estuaries and Coasts 38. Springer: 2059–2069. https://doi.org/10.1007/s12237-015-9962-2.
Olds, A.D., R.M. Connolly, K.A. Pitt, and P.S. Maxwell. 2012. Primacy of seascape connectivity effects in structuring coral reef fish assemblages. Marine Ecology Progress Series 462: 191–203. https://doi.org/10.3354/meps09849.
Oreska, M.P.J., K.J. McGlathery, P.L. Wiberg, R.J. Orth, and D.J. Wilcox. 2021. Defining the Zostera marina (eelgrass) niche from long-term success of restored and naturally colonized meadows: Implications for seagrass restoration. Estuaries and Coasts 44: 396–411. https://doi.org/10.1007/s12237-020-00881-3.
Orth, R.J., W.C. Dennison, C. Gurbisz, M. Hannam, J. Keisman, J.B. Landry, J.S. Lefcheck, et al. 2019. Long-term annual aerial surveys of submersed aquatic vegetation (SAV) support science, management, and restoration. Estuaries and Coasts. https://doi.org/10.1007/s12237-019-00651-w.
Orth, R.J., J.S. Lefcheck, K.J. McGlathery, L. Aoki, M.W. Luckenbach, K.A. Moore, M.P.J. Oreska, R. Snyder, D. J. Wilcox, and B. Lusk. 2020. Restoration of seagrass habitat leads to rapid recovery of coastal ecosystem services. Science Advances 6: eabc6434. https://doi.org/10.1126/sciadv.abc6434.
Orth, R.J., M.L. Luckenbach, S.R. Marion, K.A. Moore, and D.J. Wilcox. 2006. Seagrass recovery in the Delmarva Coastal Bays, USA. Aquatic Botany 84: 26–36. https://doi.org/10.1016/j.aquabot.2005.07.007.
Orth, R.J., and K.J. McGlathery. 2012. Eelgrass recovery in the coastal bays of the Virginia Coast Reserve, USA. Marine Ecology Progress Series 448: 173–176. https://doi.org/10.3354/meps09596.
Orth, R.J., and J. van Montfrans. 1987. Utilization of a seagrass meadow and tidal marsh creek by blue crabs Callinectes sapidus. I. Seasonal and annual variations in abundance with emphasis on post-settlement juveniles. Marine Ecology Progress Series 41: 283–294. https://doi.org/10.3354/meps041283.
Orth, R.J., K.A. Moore, S.R. Marion, D.J. Wilcox, and D.B. Parrish. 2012. Seed addition facilitates eelgrass recovery in a coastal bay system. Marine Ecology Progress Series 448: 177–196. https://doi.org/10.3354/meps09522.
Parsons, E.C.M., B. Favaro, A.A. Aguirre, A.L. Bauer, L.K. Blight, J.A. Cigliano, M.A. Coleman, et al. 2014. Seventy-one important questions for the conservation of marine biodiversity. Conservation Biology 28: 1206–1214. https://doi.org/10.1111/cobi.12303.
Pelton, T., and B. Goldsborough. 2008. Bad water and the decline of blue crabs in the Chesapeake Bay. Chesapeake Bay Foundation.
Peterson, B.J., K.R. Thompson, J.H. Cowan, and K.L. Heck. 2001. Comparison of predation pressure in temperate and subtropical seagrass habitats based on chronographic tethering. Marine Ecology Progress Series 224: 77–85. https://doi.org/10.3354/meps224077.
Pinheiro, J.C., and D.M. Bates. 2000. Mixed-effects models in S and S-PLUS. New York, NY, USA: Springer.
Pittman, S.J., and C.A. McAlpine. 2003. Movements of marine fish and decapod crustaceans: process, theory and application. In Advances in Marine Biology, 44:205–294. Elsevier. https://doi.org/10.1016/S0065-2881(03)44004-2.
Pittman, S.J., C.A. McAlpine, and K.M. Pittman. 2004. Linking fish and prawns to their environment: A hierarchical landscape approach. Marine Ecology Progress Series 283: 233–254. https://doi.org/10.3354/meps283233.
Pittman, S.J., K.L. Yates, P.J. Bouchet, D. Alvarez-Berastegui, S. Andréfouët, S.S. Bell, C. Berkström, et al. 2021. Seascape ecology: Identifying research priorities for an emerging ocean sustainability science. Marine Ecology Progress Series 663: 1–29. https://doi.org/10.3354/meps13661.
Planet team. 2019. San Francisco, CA.: Planet Application Program Interface: In Space for Life on Earth.
Post, W. 2008. Food exploitation patterns in an assembly of estuarine herons. Waterbirds: The International Journal of Waterbird Biology 31: 179–192. https://doi.org/10.1675/1524-4695(2008)31[179:fepiaa]2.0.co;2.
QGIS.org. 2020. QGIS Geographic Information System. Open Source Geospatial Foundation Project.
R Core Team. 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria.
Ramach, S., M.Z. Darnell, N. Avissar, and D. Rittschof. 2009. Habitat use and population dynamics of blue crabs, Callinectes sapidus, in a high-salinity embayment. Journal of Shellfish Research 28: 635–640. https://doi.org/10.2983/035.028.0328.
Rathbun, M.J. 1896. The genus Callinectes. Proceedings of the United States National Museum 18: 349–375. https://doi.org/10.5479/si.00963801.18-1070.349.
Reeves, I.R.B., L.J. Moore, E.B. Goldstein, A.B. Murray, J.A. Carr, and M.L. Kirwan. 2020. Impacts of seagrass dynamics on the coupled long-term evolution of barrier-marsh-bay systems. Journal of Geophysical Research: Biogeosciences 125: e2019JG005416. https://doi.org/10.1029/2019JG005416.
Richardson, D.L., J. Porter, G. Oertel, R. Zimmerman, C. Carlson, and K. Overman. 2014. Integrated topography and bathymetry for the Eastern Shore of Virginia ver 10. Environmental Data Initiative. https://doi.org/10.6073/pasta/63a22558d6650fae8232b6a8814a90d5.
Robbins, B.D., and S.S. Bell. 1994. Seagrass landscapes: A terrestrial approach to the marine subtidal environment. Trends in Ecology & Evolution 9: 301–304. https://doi.org/10.1016/0169-5347(94)90041-8.
Roberts, C.M., S. Andelman, G. Branch, R.H. Bustamante, J. Carlos Castilla, J. Dugan, B.S. Halpern, et al. 2003. Ecological criteria for evaluating candidate sites for marine reserves. Ecological Applications 13: 199–214. https://doi.org/10.1890/1051-0761(2003)013[0199:ECFECS]2.0.CO;2.
Ross, P., and M. Luckenbach. 2009. Population assessment of Eastern Oysters (Crassostrea virginica) in the seaside coastal bays. Virginia Institute of Marine Science, William & Mary.
Rozas, L.P., and W.E. Odum. 1988. Occupation of submerged aquatic vegetation by fishes: Testing the roles of food and refuge. Oecologia 77: 101–106. https://doi.org/10.1007/bf00380932.
Ryer, C.H. 1987. Temporal patterns of feeding by blue crabs (Callinectes sapidus) in a tidal-marsh creek and adjacent seagrass meadow in the lower Chesapeake Bay. Estuaries 10: 136–140. https://doi.org/10.2307/1352178.
Ryer, C.H. 1990. Utilization of a seagrass meadow and tidal marsh creek by blue crabs Callinectes-sapidus II. Spatial and temporal patterns of molting. Bulletin of Marine Science 46: 95–104.
Sallenger, A.H., K.S. Doran, and P.A. Howd. 2012. Hotspot of accelerated sea-level rise on the Atlantic coast of North America. Nature Climate Change 2: 884–888. https://doi.org/10.1038/nclimate1597.
Savino, J.F., and R.A. Stein. 1982. Predator-prey interaction between largemouth bass and bluegills as influenced by simulated, submersed vegetation. Transactions of the American Fisheries Society 111: 255–266. https://doi.org/10.1577/1548-8659(1982)111%3c255:piblba%3e2.0.co;2.
Shervette, V.R., F. Gelwick, and N. Hadley. 2011. Decapod utilization of adjacent oyster, vegetated marsh, and non-vegetated bottom habitats in a Gulf of Mexico estuary. Journal of Crustacean Biology 31: 660–667. https://doi.org/10.1651/10-3360.1.
Sievers, K.T., R.J. Barr, J.M. Maloney, N.W. Driscoll, and T.W. Anderson. 2016. Impact of habitat structure on fish populations in kelp forests at a seascape scale. Marine Ecology Progress Series 557: 51–63. https://doi.org/10.3354/meps11885.
Smith, T.M., J.S. Hindell, G.P. Jenkins, and R.M. Connolly. 2010. Seagrass patch size affects fish responses to edges. Journal of Animal Ecology 79: 275–281. https://doi.org/10.1111/j.1365-2656.2009.01605.x.
Smith, T.M., J.S. Hindell, G.P. Jenkins, R.M. Connolly, and M.J. Keough. 2011. Edge effects in patchy seagrass landscapes: The role of predation in determining fish distribution. Journal of Experimental Marine Biology and Ecology 399: 8–16. https://doi.org/10.1016/j.jembe.2011.01.010.
Stunz, G.W., T.J. Minello, and L.P. Rozas. 2010. Relative value of oyster reef as habitat for estuarine nekton in Galveston Bay, Texas. Marine Ecology Progress Series 406: 147–159. https://doi.org/10.3354/meps08556.
Sturdivant, S.K., and K.L. Clark. 2011. An evaluation of the effects of blue crab (Callinectes sapidus) behavior on the efficacy of crab pots as a tool for estimating population abundance. Fishery Bulletin 109: 48–55.
Sun, C., S. Fagherazzi, and Y. Liu. 2018. Classification mapping of salt marsh vegetation by flexible monthly NDVI time-series using Landsat imagery. Estuarine, Coastal and Shelf Science 213: 61–80. https://doi.org/10.1016/j.ecss.2018.08.007.
Tankersley, R.A., M.G. Wieber, M.A. Sigala, and K.A. Kachurak. 1998. Migratory behavior of ovigerous blue crabs Callinectes sapidus: Evidence for selective tidal-stream transport. The Biological Bulletin 195: 168–173. https://doi.org/10.2307/1542824.
Tanner, J.E. 2005. Edge effects on fauna in fragmented seagrass meadows. Austral Ecology 30: 210–218. https://doi.org/10.1111/j.1442-9993.2005.01438.x.
Turner, M.G. 1989. Landscape ecology: The effect of pattern on process. Annual Review of Ecology and Systematics 20: 171–197. https://doi.org/10.1146/annurev.es.20.110189.001131.
Unsworth, R.K.F., P.S. De León, S.L. Garrard, J. Jompa, D.J. Smith, and J.J. Bell. 2008. High connectivity of Indo-Pacific seagrass fish assemblages with mangrove and coral reef habitats. Marine Ecology Progress Series 353: 213–224. https://doi.org/10.3354/meps07199.
Waltham, N.J., C. Alcott, M.A. Barbeau, J. Cebrian, R.M. Connolly, L.A. Deegan, K. Dodds, et al. 2021. Tidal marsh restoration optimism in a changing climate and urbanizing seascape. Estuaries and Coasts 44: 1681–1690. https://doi.org/10.1007/s12237-020-00875-1.
Waycott, M., C.M. Duarte, T.J.B. Carruthers, R.J. Orth, W.C. Dennison, S. Olyarnik, A. Calladine, et al. 2009. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences of the United States of America 106: 12377–12381. https://doi.org/10.1073/pnas.0905620106.
Wedding, L.M., C.A. Lepczyk, S.J. Pittman, A.M. Friedlander, and S. Jorgensen. 2011. Quantifying seascape structure: Extending terrestrial spatial pattern metrics to the marine realm. Marine Ecology Progress Series 427: 219–232. https://doi.org/10.3354/meps09119.
Weinstein, M.P., S.Y. Litvin, and V.G. Guida. 2005. Considerations of habitat linkages, estuarine landscapes, and the trophic spectrum in wetland restoration design. Journal of Coastal Research 51–63.
Wells, H.W. 1961. The fauna of oyster beds, with special reference to the salinity factor. Ecological Monographs 31: 239–266. https://doi.org/10.2307/1948554.
Whaley, S.D., J.J. Burd, and B.A. Robertson. 2007. Using estuarine landscape structure to model distribution patterns in nekton communities and in juveniles of fishery species. Marine Ecology Progress Series 330: 83–99.
Wiberg, P.L., S. Fagherazzi, and M.L. Kirwan. 2020. Improving predictions of salt marsh evolution through better integration of data and models. Annual Review of Marine Science 12: 389–413. https://doi.org/10.1146/annurev-marine-010419-010610.
Wiens, J.A. 1989. Spatial scaling in ecology. Functional Ecology 3: 385–397. https://doi.org/10.2307/2389612.
Williams, B., D.F. Brinker, and B.D. Watts. 2007. The status of colonial nesting wading bird populations within the Chesapeake Bay and Atlantic barrier island-lagoon system. Waterbirds: The International Journal of Waterbird Biology 30: 82–92. https://doi.org/10.1675/1524-4695(2007)030[0082:tsocnw]2.0.co;2.
Williams, M.J., and B.J. Hill. 1982. Factors influencing pot catches and population estimates of the portunid crab Scylla serrata. Marine Biology 71: 187–192. https://doi.org/10.1007/BF00394628.
With, K.A., R.H. Gardner, and M.G. Turner. 1997. Landscape connectivity and population distributions in heterogeneous environments. Oikos 78: 151. https://doi.org/10.2307/3545811.
Wolcott, T.G., and A.H. Hines. 1990. Ultrasonic telemetry of small-scale movements and microhabitat selection by molting blue crabs (Callinectes sapidus). Bulletin of Marine Science 46: 83–94.
Yarnall, A.H., and F.J. Fodrie. 2020. Predation patterns across states of landscape fragmentation can shift with seasonal transitions. Oecologia 193: 403–413. https://doi.org/10.1007/s00442-020-04675-z.
Zuur, A.F., E.N. Ieno, N.J. Walker, A.A. Saveliev, and G.M. Smith. 2009. Mixed effects models and extensions in ecology with R. New York, NY, USA: Springer.
Acknowledgements
We are thankful to L. N. Johnson and S. M. Rosenberg for their fieldwork assistance. We thank R. J. Orth and D. J. Wilcox for providing aerial seagrass data through the Virginia Institute of Marine Science Submerged Aquatic Vegetation Program. We are also grateful to T. Barnes and P. L. Wiberg for providing bathymetric data. We thank the staff, scientists, and students of the VCR LTER, especially C. A. Baird, D. Lee, and J. Morreale, for their help with data collection and logistical support.
Funding
Funding for this project was provided by the National Science Foundation through sustained support of the Virginia Coast Reserve Long Term Ecological Research project (VCR LTER; award no. DEB-1832221).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Melisa C. Wong
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheng, S.L., Tedford, K.N., Smith, R.S. et al. Coastal Vegetation and Bathymetry Influence Blue Crab Abundance Across Spatial Scales. Estuaries and Coasts 45, 1701–1715 (2022). https://doi.org/10.1007/s12237-021-01039-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-021-01039-5