Abstract
Portunid crabs are an iconic, high value species in NSW, but catches are highly variable in space and time. Substantial variation in biomass is observed in both exploited and unfished populations, and environmental effects on distribution and abundance are an important factor contributing to this variability. Predicting and responding to this variability is a challenge for management and sustainability of exploited populations. We examine spatial and temporal variation in Blue Swimmer Crab (Portunus armatus) populations, over a 2.5 year trapping survey in two temperate estuaries that differ in tidal flow and riverine input. Specifically, monthly catch rates and distribution throughout the estuary are examined alongside variation in temperature and conductivity. In Wallis Lake, the shallower estuary with a restricted entrance, both water temperature and conductivity impacted abundance and distribution of crabs but there was no evidence that pulses of freshwater flow had a major impact. For a 10 °C increase in temperature the population on average shifted ~ 700 m closer to the ocean. Males were consistently located slightly further into the estuary compared to females, but there was no convincing evidence of estuarine egression in response to lower salinity. In Port Stephens, the deeper, tidal estuary, water temperature and conductivity also impacted abundance, but while males were more dispersed than females, the distribution of crabs within the estuary did not appear to be influenced by temperature, conductivity or flow. These results highlight the links between nuanced environmental relationships and estuarine geomorphology for Blue Swimmer Crab.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the abundance and biology of the species, and how they interact with the environment, is critical to effective fisheries management (Marks et al. 2021). The fisheries productivity for crustaceans can be influenced by environmental effects on a number of levels—abiotic factors can determine the abundance and distribution of larvae (Green et al. 2014), and physicochemical conditions can largely determine the success of recruitment (Gillson 2011; Taylor et al. 2017). In estuarine and coastal systems, environmental factors such as temperature, salinity and freshwater flow can also impact trophic interactions and the distribution of later life history stages within estuaries (Johnston et al. 2021). Defining these effects, and any synergistic impacts, is important for accounting for spatiotemporal fluctuations in exploited populations (Gillanders and Kingsford 2002).
Water temperature in south-eastern Australian estuaries is rapidly changing due to the increased poleward extension of the East Australian Current (EAC) (Scanes et al. 2020). The EAC has strengthened over the past 6 decades at a rate 2–3 times greater than the global average (Ridgway 2007). Increased occurrences of extreme temperatures have been recorded, and more severe extremes are predicted for the EAC in the future (Li et al. 2022). A recent study found that estuary temperatures in south-eastern Australia had increased by 2.16 °C on average over 12 years, at a rate of 0.2 °C per year (Scanes et al. 2020). Coupled with alterations in the magnitude and seasonality of freshwater inflow (and concomitant impacts on estuarine salinity), estuaries in south-eastern Australia will face substantial physicochemical variability in the future (Gillanders et al. 2011). This will have concomitant impacts on fisheries productivity (Gillson 2011), through influence on critical life history processes and the location and distribution of exploited life history stages. Therefore, temperature, conductivity and flow are most likely the important variables.
The Blue Swimmer Crab (BSC, Portunus armatus (originally Portunus pelagicus)) is found around most of the Australian coast, and east to New Caledonia while the congener Portunus pelagicus is widespread across south-eastern and eastern Asia, and is sympatric with P. armatus in the Northern Territory (Lai et al. 2010). BSC are a highly valued recreational and commercial species in Australia, but substantial variation in recreational and commercial portunid fisheries is observed throughout its range, which is thought to be driven by environmental variation (Loneragan 1999; de Lestang et al. 2003; Meynecke et al. 2012; Alberts-Hubatsch et al. 2014; Harris et al. 2017). Western Australia, South Australia and Queensland have larger commercial and recreational fisheries than New South Wales (NSW), where reported commercial catches are highly variable, typically ranging between 50 and 250 t. Approximately 85% of commercial landings in NSW are reported from four estuaries, with Wallis Lake accounting for around 60% of reported catch (Johnson 2020).
The main fishing season for BSC in NSW generally extends from November to July, peaking in late summer (Johnson et al. 2010). A recent study investigating the variability in BSC fishery in Wallis Lake found that high winter catches of crabs appears to negatively impact catch rates in the following year, likely due to the disproportionate high impact of this fishing on mated pre-spawned females, and that catches were also influenced by the Pacific Decadal Oscillation (PDO, which was likely to be subsuming a suite of climatic variables that impact physicochemical conditions in the estuary) (Schilling et al. 2023). Similar patterns have been found in Western Australia when mortality of mated pre-spawning females was shown to negatively effect catches (Johnston and Yeoh 2021). Factors impacting BSC abundance and productivity may include oceanography impacting larval dispersal (Hewitt et al. 2022b), spawning trigger (Johnston and Yeoh 2021) and changed migration patterns (male and female crabs (de Lestang et al. 2010), and the abundance and productivity of important habitats (Marks et al. 2020). As noted earlier, freshwater inflow, temperature and salinity may have substantial impacts on BSC in estuarine habitats; however, the mechanistic action of these variables has not yet studied in south-eastern Australian estuaries, but may well contribute to the observed variability in commercial catch and the influence of the PDO.
Based upon the previous evidence of environmental impacts on portunid populations, we hypothesise that the spatiotemporal variation in abundance and distribution of BSC will be related to changes in temperature and salinity. Specifically, we aimed to examine relative catch rates, and sex-specific patterns in distribution, during 2.5 years of sampling covering three summers in two geomorphologically distinct south-eastern Australian estuaries. We interpret the observed patterns in the context of differences between the sampled estuaries and how they respond to abiotic variability.
Methods
Sampling Design and Collection
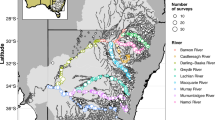
This study was conducted in two estuaries that support commercial and recreational BSC fisheries in south-eastern Australia (Fig. 1). Wallis Lake (32°18′S, 152°30′E) is a permanently open lake with a large waterway area and comparatively shallow depths with a restricted entrance to the ocean (Roy et al. 2001). It has two small rivers that discharge into the estuary, Wallingat and the Coolongolook (Griffith and Wilson 2008). Port Stephens (32°44′S, 152°3′E) is a drowned valley with a much larger and deeper entrance that enables a tidal signal to penetrate into the estuary, with freshwater flows from the Karuah and Myall Rivers (Creese et al. 2009). Monthly sampling was conducted using the small-mesh traps (Hanamseth et al. 2022) in Wallis Lake for a period from December 2018 to June 2021 and in Port Stephens from December 2018 to March 2021. The traps had a 25-mm diamond mesh of green–grey net (polyethylene, PE) tied with chaff rope to the top and bottom ring. In addition, the traps had four entry funnels instead of two (funnels were 300 × 200 mm on the outside and tapering to 200 × 50 mm (Hanamseth et al. 2022).
Map of a Wallis Lake and b East coast of Australia c Port Stephens. The black dots represent the sites and the colours representing the following: light green—seagrass, green—mangrove, orange—saltmarsh and red—oyster leases in the two estuaries (layers extracted from publicly available data- https://datasets.seed.nsw.gov.au/dataset/estuaries-including-macrophyte). Note that the sites are variously located with respect to distance from sea
Sampling sites (Fig. 1) were selected following input from commercial fishers and represented the range of areas in the estuary that were frequented by BSC. Following the first 8 months of sampling, data was examined, and the number of sampling sites was increased from 5 to 7 sites (in August 2019) to improve the spatial coverage of the independent survey within each estuary (4 small traps per site for first 6 months, then 6 small traps per site). For Wallis Lake, analysis of abundance used data collected from December 2018 to June 2021 and included all sites for which data was available, whereas the analysis of distribution included only data collected between August 2019 and June 2021 (i.e. only time points where data from 7 sites were available). For Port Stephens, analysis was structured similarly but only used data collected until March 2021.
Sampling occurred at depths of 2–4 m adjacent to shallow seagrass habitats, with traps deployed in the afternoon, and collected the following day (i.e. ~ 18 h soak time). Gear deployments were separated by > 50 m within each site to ensure independence. Traps were baited with two thawed Sea Mullet (Mugil cephalus) per trap (< 1 kg), chopped in half, with the two tails placed inside a 20 × 20 cm bait bag of 1 cm mesh, and the two heads skewered on a metal hook, with the baits secured to the base of the trap. GPS waypoints were used to mark the location of each gear deployment, made within each estuary. Traps were deployed and retrieved in the same order to ensure consistent soak times (between 18 and 22 h each). Upon retrieval of gear, crabs were immediately immersed in an ice slurry to pacify them so that they could be measured (Bellchambers et al. 2005). Individual crabs were sexed and measured for carapace length (the distance between the frontal notch and the posterior carapace margin) measured to nearest millimetre using vernier callipers and then released. Based on previous evidence of environmental impacts on portunid biology populations, continuous temperature and conductivity (as a proxy for salinity) measurements were recorded using Hobo water quality loggers (HOBO U24-002-C) that were installed in each estuary for the duration of the survey and periodically downloaded (Fig. 2). Conductivity was analysed instead of salinity because the calculation of salinity includes temperature and are therefore correlated (Williams 1986). For analysis, continuous temperature and conductivity data was aggregated to mean measurements across the 30 days prior to each sampling event. To also capture the impact of large flow pulses on crabs in the estuaries, daily inflow data was extracted from WaterNSW public database (https://realtimedata.waternsw.com.au/water.stm). The daily lagged flow data was categorised as pulse and no pulse, by categorising days when the daily flow was in the highest 10% of occurrences as a ‘pulse’ event (Schilling et al. 2023).
Water quality data showing conductivity (blue line) and temperature (red line) in a Wallis Lake and b Port Stephens. Missing data (6%) which were interpolated are shown in black. Note that Wallis Lake is shallower and with less tidal and riverine flow than Port Stephens, but it still incurs significant changes in conductivity which is not evident in Port Stephens
Statistical Analyses
Statistical analyses were conducted in R v. 4.0.2 (Team 2020), and several graphical approaches and statistical tests were used. The temperature and conductivity loggers were missing data for 6% of days (due to logger failure) and these days were estimated using linear interpolation from surrounding data points (Fig. 2). Prior to analysis, all candidate explanatory variables (such as temperature, conductivity, freshwater flow) were assessed for collinearity (Pearson r > 0.6) using scatter plots and correlation coefficient tests and none were found to be collinear.
Variations in the abundance of BSC in response to varying environmental factors was evaluated using generalised linear mixed models (GLMMs) with a Poisson error distribution (with log link) for each estuary separately using a backwards stepwise model selection approach starting with the following equation:
where \({{\text{Crab}}\_{\text{Abund}}}_{i,j,k}\) represents the abundance of BSC at Site i on Date j in replicate trap, k Beta (β) represents the coefficient values of each explanatory variable where Tempj is the mean temperature of the 30 days prior to date j, Condj is the mean conductivity of the 30 days prior to date j and Flow is a categorical variable of whether in the past 30 days prior to date j there were any ‘pulse’ flow events. Both linear and quadratic forms of Temp and Cond were included in the model as there may be non-linear responses to these variables. Site and Date both represent random intercept effects which account for dependency in sampling design. Soak time (Soak) of each trap was used as an offset to control minor differences in sampling effort (i.e. soak time of traps).
The spatial analysis in each estuary consisted of creating a distance to sea variable for each trap deployment, calculated using a raster proximity map indicating the distance from the centre of each pixel to the mouth of the estuary. Combining all samples within a month, weighted kernel density estimates were then calculated for each sex based upon from the distance to sea and abundance of male and female BSC in each trap. We then extracted the median distance to sea and inter quartile range (25% and 75%) for male and female distributions. Each month was then treated as a replicate with one median and interquartile range for each sex, representing the median position and spread of the population in the estuary. Linear models were used to determine the influence of environmental parameters on the spatial distribution of BSC using a similar model selection process as described above starting with the following full model:
where MDSm is the median distance to sea in month m for sex s, IQRm is the interquartile range in month m for sex s and other variables are as defined previously. \({{\text{Sex}}}_{s}*{{\text{Flow}}}_{m}\) represents an interaction term between the categorical factors of Sex and Flow, allowing each sex to respond differently to flow events. During the model selection process, we retained Sex in all models due to the dependency structures within the data.
All models were fit using the ‘glmmTMB’ package (Brooks et al. 2017), residuals and assumptions of the model were checked using the ‘DHARMa’ package (Hartig 2020). Using these models, a manual backwards selection process, whereby each variable was removed one at a time and the Akaike information criterion values (AIC) compared between competing models. Models with ΔAIC < 2 of the lowest AIC model were considered equivalent (Burnham and Anderson 2002) and selection only stopped if the reduced model was not equivalent or better than the previous model (ΔAIC > 2). Model results were visualised by manually creating partial effects prediction plots showing the influence of each variable when all other variables were held at mean levels.
Results
General Observations
In total 23,691 BSC were caught in both estuaries over the 2.5 years. In Wallis Lake, 17,388 BSC were caught in 2096 trap lifts and in Port Stephens 6303 BSC were captured in 1965 trap lifts. In general, Wallis Lake supported the highest catch rates of crabs, with peak summer catch rates sometimes exceeding 20 crabs trap−1; in comparison, Port Stephens had peak summer catch rates of 4 crabs trap−1 (Fig. 3). Temporal fluctuations of catch rates were relatively consistent at all sites across both estuaries, such that sites generally appeared to peak at the same time (Fig. 3).
Overall, in Wallis Lake, site 1 had the highest number of captured BSC (mean catch rate) followed by sites 4 and 2 (Figs. 1 and 3). The overall relative density of BSC for both sexes across months were relatively similar, apart from differences in August 2019 with increased capture of male BSC than females (Fig. S1). Catches were lower in Port Stephens compared to Wallis Lake, rarely exceeding 4 crabs trap−1 throughout the entire time series. The summer peak occurred later in the season in 2019/20 and 2020/21, than 2018/19 in Port Stephens, similar to Wallis Lake. However, during the 2020/21 season, catches peaked early in October and then in March. Overall, in Port Stephens, Site 3 had the highest density followed by Sites 2 and 4 (Figs. 1 and 3), in general alignment with distance to sea. The summer peak occurred later in the season in 2019/20 and 2020/21, than 2018/19 in both estuaries. However, in Port Stephens during the 2020/21 season, catches peaked both early in October, and then again in March. The overall relative density of sexes across months in Port Stephens were relatively similar across months, apart from differences in October 2019 and May 2020 with increased capture of female BSC than males (Fig. S2).
Patterns in Abundance
The best fit model for Wallis Lake abundance was the full model without flow which was equivalent to the full model (ΔAIC < 2; Table S1) containing temperature (previous 30-day average, both linear and quadratic) and conductivity (previous 30-day average, both linear and quadratic). This model explained a large portion of the variance (conditional r2 = 0.619, marginal r2 = 0.457). There was strong evidence of nonlinear impacts of both temperature (χ22 = 121.07, P < 0.001; Fig. 4a) and conductivity (χ22 = 81.34, P < 0.001; Fig. 4c). Abundance responded positively to temperatures above 20 °C (Fig. 4a), while abundance declined when conductivity was higher than 40 ms cm−1 (Fig. 4c). Adding flow to the model did not significantly improve the model (Table S1).
Predicted crab (or BSC) abundance from the generalised linear mixed models based upon varying temperature (panels a and c) and conductivity (panels b and d). The top row is for Wallis Lake while the bottom row is for Port Stephens. All predictions were made on the fixed effects only holding all other variables at the median level. The grey ribbons show the standard error of the mean predictions
The final model for Port Stephens abundance included temperature (previous 30-day average, both linear and quadratic) and conductivity (previous 30-day average). This model was equivalent to both the full model and a number of other models, but this was the simplest equivalent model (Table S2). The Port Stephens model explains 30% less variance than the Wallis Lake model (conditional r2 = 0.251, marginal r2 = 0.110). There was evidence of a nonlinear impact of temperature (χ22 = 8.44, P = 0.015) and a strong negative log-linear effect of conductivity (χ22 = 24.68, P < 0.001). Abundance showed a relationship with temperature with an optimum at approximately 21 °C (Fig. 4b), while abundance declined with increasing conductivity (Fig. 4d).
Analysis of Distribution
The model with the most parsimonious fit for the median distance to sea for Wallis Lake was the model containing temperature (previous 30-day average), conductivity (previous 30-day average) and sex (Table S3; marginal r2 = 0.67, conditional r2 = 0.67). There was strong evidence of a negative temperature effect with the median position of the population moving closer to the estuary mouth (ocean) with increasing temperatures (χ21 = 10.674, P = 0.001). The model indicated that a 1 °C increase in temperature would shift the population ~ 61 m (18.7 m SE) closer to the ocean (Fig. 5a). There was also strong evidence that as conductivity increased, the median position of the population moved further into the estuary (χ21 = 10.857, P = 0.001). For a 1 ms cm−1 increase in conductivity, the median position of the population shifted ~ 28 m (8.5 m SE) further into the estuary (Fig. 5b). Males were also consistently located slightly further into the estuary compared to females (χ21 = 58.982, P < 0.001; Fig. 5c). The model selection process for the interquartile spread of the population selected the simplest possible model of only Sex (Table S4), suggesting no meaningful effects of temperature, conductivity or flow. Within this model, there was no evidence of any differences between sexes (χ21 = 0.053, P = 0.817) on the overall spread of the population within Wallis Lake.
Predicted median distance to sea for the blue swimmer crab population in Wallis Lake under varying a temperature, b conductivity, and c sex. Predictions were made by holding all other variables except the plotted variable constant at their mean values. The error bar (in panel c) and grey ribbons show the standard error of the predictions
For distribution in Port Stephens, no model showed any significant improvement over the simplest (Sex only) model (Table S5; ΔAIC < 2). This suggests there were no meaningful effects of temperature, conductivity or flow on the location of the population within the estuary. The model itself also showed no evidence of a difference between sexes (χ21 = 0.968, P = 0.325). Similarly, to Wallis Lake, the most parsimonious model investigating the spread of the population was the model containing only Sex (Table S6). There was however a significant effect of Sex (χ21 = 6.222, P = 0.013), with males having a larger interquartile range compared to the females (Fig. 6).
Discussion
Our data showed that in both estuaries, the highest catch rates of BSC were in late summer, corresponding with the peak catches from the commercial fishery. We found that water temperature and conductivity had a significant effect on the relative abundance of BSC but there was no evidence that increased flow resulted in lower catch. Although patterns in catch rates were generally similar among estuaries, catch rates from Wallis lake were ≈5 × greater than Port Stephens during observed summer peaks. This study highlights the importance of estuary specific assessments of spatiotemporal variability in abundance and contributing factors, and estuary-specific geomorphology may affect the fashion in which physicochemical variation impacts habitats across the estuarine systems, as discussed below.
Patterns in Abundance and Distribution
In both estuaries, the highest catch rates of BSC were in the summer months, but the magnitude and timing varied between estuaries. For example, in Port Stephens, BSC catch rates were much lower in the later summer months, being < 20% of those in Wallis Lake. This is likely related to Port Stephens being more tidal, deeper and with more freshwater flow than Wallis Lake. Water temperature and conductivity had a significant effect on catch rates, and we acknowledge that the catch rate is a reflection of the BSC catchability which occurs at the monthly scale. Geographical features of Port Stephens make it different to Wallis Lake as the entrance to the mouth of the estuary is much larger than Wallis Lake. In addition, the Port Stephens estuary is a more linear than Wallis Lake. This also suggests that Wallis Lake experiences much less mixing and lesser stratification to Port Stephens as Wallis is a much shallower estuary than Port Stephens.
In comparison to Port Stephens, the higher catch rate in Wallis Lake had early summer peaks, when abundance strongly increased with temperatures above 20 °C, while catch rates declined when conductivity was higher than 40 ms cm−1. Higher catch rates in BSC have been reported in summer months in other estuaries (Sumpton et al. 2015; Johnston et al. 2021), and is often related to increased foraging behaviour, increased growth rates and metabolic activity. For example, the resting metabolic rate (RMR) of BSC increased by over 70% with temperature during the premoult stage (Junk et al. 2021). However, with increasing temperatures, the growth rates of decapod crustaceans tend to increase to a maxima, and then decline until the thermal tolerance boundaries are reached (Green et al. 2014). In addition, higher temperatures stimulate moulting with shorter inter-moult durations and increased rate of carapace hardening. However, the survival rate of P. pelagicus instars has been shown to decrease from 70% at 32 °C compared to 80% at 24 °C and 72% at 28 °C (Azra et al. 2018). Temperature was a significant positive environmental driver in catch rates of BSC in commercial fisheries across five estuaries in Western Australia, but there was a catch rate decline in some estuaries when elevated temperatures were encountered (Johnston et al. 2021). Early juvenile P. pelagicus have been shown to perform best in salinity ranges of 20–35 ppt, and outside this range their rate of survival, growth and development reduces (Romano and Zeng 2006). However, more recently adult BSC were found to be more resilient to increased salinity and temperature gradients as they are robust in adapting to environmental changes due to their ability to tolerate naturally variable habitats (Champion et al. 2020).
The most parsimonious model regarding the distribution of crabs within Wallis Lake was the full model which included temperature, conductivity and flow. There was strong evidence of a temperature effect with the median position of the population moving closer to the estuary mouth (ocean) with increasing temperatures. There was also strong evidence that as conductivity increased, the median position of the population moved further into the estuary. Despite the strong statistical evidence for changes in distribution, the magnitude of the movements was not substantial. However, there were clear differences in distribution of different sexes, with males consistently located slightly further into the estuary compared to females. Jivoff et al. (2017) found that salinity influenced the abundance of male blue crabs (Callinectes sapidus) and that areas of lower salinities had more abundant male crabs, which would align with our finding that males were further up the estuary where the salinity would naturally be slightly lower. The analysis of interquartile spread indicated that the range of temperature, conductivity and flow encountered in the study nor sex led to a detectable contraction or expansion in the area of estuary frequented by crabs. This suggests that other factors such as habitat quality or prey levels may influence these patterns, but also highlights the possibility our sites did not cover sufficient areas to allow such trends to be detected.
In Port Stephens, water temperature and conductivity also had a significant effect on the abundance of BSC, but environmental variables had no influence on distribution. Abundance declined with increasing conductivity, but showed a weak quadratic relationship with temperature with an optimum at ~ 21 °C (which implies that BSC abundances peaked at this temperature), which roughly corresponded with the peak temperature for reproductive output (~ 22–23 °C) for the species in Nolan et al. (2021). There was no evidence that catch of BSC decreased following high flow ‘pulse’ events in Port Stephens. This was surprising as environmental factors that govern habitat suitability for invertebrate fisheries are directly impacted by the natural variation in freshwater flows (Gillson 2011). Several species of invertebrate fisheries (not including BSC) have been reported to have a positive relationship between freshwater flow and their landings, often arising through flow-induced aggregation (Wilber 1994; Loneragan 1999; Lloret et al. 2001; Gillson 2011). While more rivers and creeks feed into Port Stephens than Wallis Lake (e.g. Tilligery and Karuah River are much larger than Wallis Lake tributaries), Port Stephens is a larger estuary with a more open entrance, and greater ocean connectivity likely dilutes and dampens the effects of inflow. The influence of flow needs to be further investigated across a greater number of flow events, to further clarify this finding.
Limitations and Future Work
Where catchability remains constant, abundance and catch rate provide useful indices of relative stock abundance (Maunder and Punt 2004). While sampling over a period covering three summers have provided an extensive dataset, outside of seasonal cycles of temperatures the amount of environmental variability encountered was somewhat low, with only a limited number of flow events occurring during the survey period. Ongoing sampling over a longer period would provide a more detailed picture of the interacting mechanisms that govern recruitment, sex ratios and size structure, and the influence of environmental variability on these. The study design established here is cost-effective and logistically feasible, and provides a useful template for ongoing independent surveys in estuaries important for BSC. This would support an increasing time series of data to further evaluate important processes at play. Many crab species including BSC have a minimum legal size that is set above the onset of sexual maturity (Johnson et al. 2010) to avoid recruitment overfishing (Broadhurst and Millar 2018; Herrmann et al. 2021; Yu et al. 2021, 2022). This aspect is highly essential for management and sustainability of crab stocks. Additionally, apart from sex, the effects of biotic and abiotic factors on the abundance and distribution of specific species may be length-dependent. Developing methods to establish the length-dependent patterns in distribution of BSC, like reported in many published papers (e.g. Cerbule et al. 2021; Yu et al. 2023) will be of significance in the future work.
Concluding Comments
While water temperature and conductivity were both important parameters that influenced the abundance of BSC, the overall magnitude of difference between the two study estuaries was substantial, with abundance substantially greater in Wallis Lake. This pattern concurs with commercial crab catches, which are much greater in Wallis Lake than all other estuaries in NSW, and suggests that there are geomorphic and ecological features of Wallis Lake that make this estuary highly suitable for BSC. It is likely that this is a combination of factors, including substantial seagrass cover which are important producers supporting BSC nutrition (Raoult et al. 2022), shallow bathymetry and slightly warmer temperatures, as well coastal oceanography that confers recruitment from a broad range of latitudes (Hewitt et al. 2022a). But further to this, the large waterway area in the central and southern sections of the estuary that are largely uninfluenced by high flows and low conductivities, may well provide some refuge from exposure to low salinity and provide a productive area for crabs to avoid adverse conditions without migrating to sea. This would have concomitant benefits for spawning activity and larval survival within the estuary.
References
Alberts-Hubatsch, H., S. Yip Lee, K. Diele, M. Wolff, and I. Nordhaus. 2014. Microhabitat use of early benthic stage mud crabs, Scylla serrata (Forskål, 1775), Eastern Australia. Journal of Crustacean Biology 34 (5): 604–610.
Azra, M.N., J.-C. Chen, M. Ikhwanuddin, and A.B. Abol-Munafi. 2018. Thermal tolerance and locomotor activity of blue swimmer crab Portunus pelagicus instar reared at different temperatures. Journal of Thermal Biology 74: 234–240.
Bellchambers, L., K. Smith, and S. Evans. 2005. Effect of exposure to ice slurries on nonovigerous and ovigerous blue swimmer crabs, Portunus pelagicus. Journal of Crustacean Biology 25 (2): 274–278.
Broadhurst, M.K., and R.B. Millar. 2018. Relative ghost fishing of portunid traps with and without escape gaps. Fisheries Research 208: 202–209 In English.
Brooks, M.E., K. Kristensen, K.J. Van Benthem, A. Magnusson, C.W. Berg, A. Nielsen, H.J. Skaug, M. Machler, and B.M. Bolker. 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9 (2): 378–400.
Burnham, K.P., and D.R. Anderson. 2002. 'A practical information-theoretic approach.' 2 edn.
Cerbule, K., B. Herrmann, E. Grimaldo, L. Grimsmo, and J. Vollstad. 2021. The effect of white and green LED-lights on the catch efficiency of the Barents Sea snow crab (Chionoecetes opilio) pot fishery. PLoS ONE 16 (10): e0258272.
Champion, C., M.K. Broadhurst, E.E. Ewere, K. Benkendorff, P. Butcherine, K. Wolfe, and M.A. Coleman. 2020. Resilience to the interactive effects of climate change and discard stress in the commercially important blue swimmer crab (Portunus armatus). Marine Environmental Research 159: 105009.
Creese, R., T. Glasby, G. West, and C. Gallen. 2009. Mapping the habitats of NSW estuaries. Nelson Bay, NSW.
de Lestang, S., N.G. Hall, and I.C. Potter. 2003. Reproductive biology of the blue swimmer crab (Portunus pelagicus, Decapoda: Portunidae) in five bodies of water on the west coast of Australia. Fishery Bulletin 101 (4): 745–757.
de Lestang, S., L.M. Bellchambers, N. Caputi, A.W. Thomson, M.B. Pember, D.J. Johnston, and D.C. Harris. (Eds). 2010. 'Stock recruitment environment relationship in a Portunus pelagicus fishery in Western Australia.' Biology and management of exploited crab populations under climate change.
Gillanders, B., and M. Kingsford. 2002. Impact of changes in flow of freshwater on estuarine and open coastal habitats and the associated organisms.
Gillanders, B.M., T.S. Elsdon, I.A. Halliday, G.P. Jenkins, J.B. Robins, and F.J. Valesini. 2011. Potential effects of climate change on Australian estuaries and fish utilising estuaries: A review. Marine and Freshwater Research 62 (9): 1115–1131.
Gillson, J. 2011. Freshwater flow and fisheries production in estuarine and coastal systems: Where a drop of rain is not lost. Reviews in Fisheries Science 19 (3): 168–186.
Green, B.S., C. Gardner, J.D. Hochmuth, and A. Linnane. 2014. Environmental effects on fished lobsters and crabs. Reviews in Fish Biology and Fisheries 24 (2): 613–638.
Griffith, S.J., and R. Wilson. 2008. Wetland biodiversity in coastal New South Wales: The Wallis Lake catchment as a case study. Cunninghamia 10: 569–598.
Hanamseth, R., D.D. Johnson, H.T. Schilling, I.M. Suthers, and M.D. Taylor. 2022. Evaluation of a novel research trap for surveys of blue swimmer crab populations. Marine and Freshwater Research 73 (6): 812–822.
Harris, D., D. Johnston, J. Baker, and M. Foster. 2017 Fisheries Research Report No. 281, 2017.
Hartig, F. 2020. DHARMa: residual diagnostics for hierarchical regression models. In Comprehensive R Archive Network.
Herrmann, B., E. Grimaldo, J. Brčić, and K. Cerbule. 2021. Modelling the effect of mesh size and opening angle on size selection and capture pattern in a snow crab (Chionoecetes opilio) pot fishery. Ocean & Coastal Management 201: 105495.
Hewitt, D.E., Y. Niella, D.D. Johnson, I.M. Suthers, and M.D. Taylor. 2022a. Crabs go with the flow: Declining conductivity and cooler temperatures trigger spawning migrations for female Giant Mud Crabs (Scylla serrata) in subtropical estuaries. Estuaries and Coasts 45 (7): 2166–2180.
Hewitt, D.E., H.T. Schilling, R. Hanamseth, J.D. Everett, J. Li, M. Roughan, D.D. Johnson, I.M. Suthers, and M.D. Taylor. 2022b. Mesoscale oceanographic features drive divergent patterns in connectivity for co-occurring estuarine portunid crabs. Fisheries Oceanography 31 (6): 587–600.
Jivoff, P.R., J.M. Smith, V.L. Sodi, S.M. VanMorter, K.M. Faugno, A.L. Werda, and M.J. Shaw. 2017. Population structure of adult blue crabs, Callinectes sapidus, in relation to physical characteristics in Barnegat Bay. New Jersey. Estuaries and Coasts 40 (1): 235–250.
Johnson, D.D., C.A. Gray, and W.G. Macbeth. 2010. Reproductive biology of Portunus pelagicus in a south-east Australian estuary. Journal of Crustacean Biology 30 (2): 200–205.
Johnson, D.D. 2020. NSW Stock Status Summary 2018/19—Blue Swimmer Crab (Portunus armatus). . NSW Department of Primary Industries.
Johnston, D.J., and D.E. Yeoh. 2021. Temperature drives spatial and temporal variation in the reproductive biology of the blue swimmer crab Portunus armatus A. Milne-Edwards. 1861. Decapoda: Brachyura: Portunidae. Journal of Crustacean Biology 41 (3): 1–15.
Johnston, D.J., D.E. Yeoh, and D.C. Harris. 2021. Environmental drivers of commercial blue swimmer crab (Portunus armatus) catch rates in Western Australian fisheries. Fisheries Research 235: 105827.
Junk, E.J., J.A. Smith, I.M. Suthers, and M.D Taylor. 2021. Bioenergetics of blue swimmer crab (Portunus armatus) to inform estimation of release density for stock enhancement. Marine and Freshwater Research.
Lai, J.C., P.K. Ng, and P.J Davie. 2010 A revision of the Portunus pelagicus (Linnaeus, 1758) species complex (Crustacea: Brachyura: Portunidae), with the recognition of four species. Raffles Bulletin of Zoology 58(2).
Li, J., M. Roughan, and C. Kerry. 2022. Variability and drivers of ocean temperature extremes in a warming western boundary current. Journal of Climate 35 (3): 1097–1111.
Lloret, J., J. Lleonart, I. Solé, and J.M. Fromentin. 2001. Fluctuations of landings and environmental conditions in the north-western Mediterranean Sea. Fisheries Oceanography 10 (1): 33–50.
Loneragan, N.R. 1999. River flows and estuarine ecosystems: Implications for coastal fisheries from a review and a case study of the Logan River, southeast Queensland. J Australian Journal of Ecology 24 (4): 431–440.
Marks, R., S.A. Hesp, D. Johnston, A. Denham, and N. Loneragan. 2020. Temporal changes in the growth of a crustacean species, Portunus armatus, in a temperate marine embayment: Evidence of density dependence. ICES Journal of Marine Science 77 (2): 773–790.
Marks, R., S.A. Hesp, A. Denham, N.R. Loneragan, D. Johnston, and N. Hall. 2021. Factors influencing the dynamics of a collapsed blue swimmer crab (Portunus armatus) population and its lack of recovery. Fisheries Research 242: 106035.
Maunder, M.N., and A.E. Punt. 2004. Standardizing catch and effort data: A review of recent approaches. Fisheries Research 70 (2–3): 141–159.
Meynecke, J.-O., M. Grubert, and J. Gillson. 2012. Giant mud crab Scylla serrata catches and climate drivers in Australia – a large scale comparison. Marine and Freshwater Research 63 (1): 84–94.
Nolan, S.E., D.D. Johnson, R. Hanamseth, I.M. Suthers, and M.D. Taylor. 2021. Reproductive biology of female blue swimmer crabs in the temperate estuaries of south-eastern Australia. Marine and Freshwater Research 73 (3): 366–376.
Raoult, V., M. Taylor, R. Schmidt, I. Cresswell, C. Ware, and T. Gaston. 2022. Valuing the contribution of estuarine habitats to commercial fisheries in a seagrass-dominated estuary. Estuarine, Coastal and Shelf Science 274: 107927.
Ridgway, K.R. 2007. Long‐term trend and decadal variability of the southward penetration of the East Australian Current. Geophysical Research Letters 34(13).
Romano, N., and C. Zeng. 2006. The effects of salinity on the survival, growth and haemolymph osmolality of early juvenile blue swimmer crabs. Portunus Pelagicus. Aquaculture 260 (1): 151–162.
Roy, P.S., R.J. Williams, A.R. Jones, I. Yassini, P.J. Gibbs, B. Coates, R.J. West, P.R. Scanes, J.P. Hudson, and S. Nichol. 2001. Structure and function of south-east Australian estuaries. Estuarine, Coastal and Shelf Science 53 (3): 351–384.
Scanes, E., P.R. Scanes, and P.M. Ross. 2020. Climate change rapidly warms and acidifies Australian estuaries. Nature Communications 11 (1): 1803.
Schilling, H.T., D.D. Johnson, R. Hanamseth, I.M. Suthers, and M.D. Taylor. 2023. Long-term drivers of catch variability in south-eastern Australia’s largest portunid fishery. Fisheries Research 260: 106582.
Sumpton, W.D., M.J. Campbell, M.F. O'Neill, M.F. McLennan, A.B. Campbell, G.M. Leigh, Y.-G. Wang, and L. Lloyd-Jones. 2015. Assessment of the blue swimmer crab (Portunus armatus) fishery in Queensland.
Taylor, M.D., B. Fry, A. Becker, and N. Moltschaniwskyj. 2017. The role of connectivity and physicochemical conditions in effective habitat of two exploited penaeid species. Ecological Indicators 80: 1–11 In English.
Team, R.C. 2020. R: A language and environment for statistical computing. In R Foundation for Statistical Computing. (Vienna, Austria)
Wilber, D.H. 1994. The influence of Apalachicola River flows on blue crab, Callinectes sapidus, in north Florida. Fishery Bulletin 92 (1): 180–188.
Williams, W. 1986. Conductivity and salinity of Australian salt lakes. Marine and Freshwater Research 37 (2): 177–182.
Yu, M., L. Zhang, C. Liu, and Y. Tang. 2021. Improving size selectivity of round pot for Charybdis japonica by configuring escape vents in the Yellow Sea. China. Peerj 9: e12282.
Yu, M., C. Liu, Y. Tang, L. Zhang, and W. Zhao. 2022. Effects of escape vents on the size selection of whelk (Rapana venosa) and Asian paddle crab (Charybdis japonica) in the small-scale pot fishery of the Yellow Sea. China. Hydrobiologia 849 (14): 3101–3115.
Yu, M., B. Herrmann, K. Cerbule, C. Liu, L. Zhang, and Y. Tang. 2023. Effect of artificial lights on catch efficiency and capture patterns in Asian paddle crab (Charybdis japonica) gillnet fishery. Regional Studies in Marine Science 65: 103101.
Acknowledgements
We acknowledge the traditional owners and custodians of the Worimi nations where this research took place. The authors wish to thank B. Leach, M. Burns, L. Bidgood, J. McLeod and T. New for assistance in collecting samples. This project was supported by the Fisheries Research and Development Corporation on behalf of the Australian Government through a grant to MDT, DDJ and IMS (2017/006), and cofounded by the NSW Recreational Fishing Saltwater Trust. Sample collection was conducted under a Section 37 Scientific Collection Permit (permit P01/0059), and Animal Research Authority 13-08 issued by NSW Department of Primary Industries. RH was supported by an Australian Government RTP scholarship. HTS was supported by a NSW Office of the Chief Scientist and Engineer Research, Attraction and Acceleration Program (RAAP) Grant awarded to Sydney Institute of Marine Science. Funding bodies had no role in the design, data collection, analysis or interpretation of data. This work was produced at UNSW Sydney and therefore falls under the UNSW Open Access policy and the authors therefore retain all rights to the Author Accepted Manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. MDT serves as an Associate Editor for Estuaries and Coasts.
Additional information
Communicated by Weimin Quan
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hanamseth, R., Schilling, H.T., Johnson, D.D. et al. Abundance and Distribution of Blue Swimmer Crab in Response to Environmental Variation Across Two Contrasting Estuaries. Estuaries and Coasts 47, 1064–1074 (2024). https://doi.org/10.1007/s12237-024-01347-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-024-01347-6