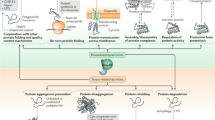
Abstract
Hsp70 proteins are key to maintaining intracellular protein homeostasis. To carry out this task, they employ a large number of cochaperones and adapter proteins. Here, we review what is known about the interaction between the chaperones and partners, with a strong slant toward structural biology. Hsp70s in general, and Hsc70 (HSPA8) in particular, display an amazing array of interfaces with their protein cofactors. We also review the known interactions between Hsp70s with lipids and with active compounds that may become leads toward Hsp70 modulation for treatment of a variety of diseases.











Similar content being viewed by others
References
Ahmad A, Bhattacharya A, McDonald RA, Cordes M, Ellington B, Bertelsen EB, Zuiderweg ER (2011) Heat shock protein 70 kDa chaperone/DnaJ cochaperone complex employs an unusual dynamic interface. Proc Natl Acad Sci U S A 108:18966–18971. doi:10.1073/pnas.1111220108
Arias E, Cuervo AM (2011) Chaperone-mediated autophagy in protein quality control. Curr Opin Cell Biol 23:184–189. doi:10.1016/j.ceb.2010.10.009
Arispe N, De Maio A (2000) ATP and ADP modulate a cation channel formed by Hsc70 in acidic phospholipid membranes. J Biol Chem 275:30839–30843. doi:10.1074/jbc.M005226200
Armijo G et al (2014) Interaction of heat shock protein 70 with membranes depends on the lipid environment. Cell Stress Chaperones 19:877–886. doi:10.1007/s12192-014-0511-x
Assimon VA, Southworth DR, Gestwicki JE (2015) Specific binding of tetratricopeptide repeat proteins to heat shock protein 70 (Hsp70) and heat shock protein 90 (Hsp90) is regulated by affinity and phosphorylation. Biochemistry 54:7120–7131. doi:10.1021/acs.biochem.5b00801
Atkin G, Paulson H (2014) Ubiquitin pathways in neurodegenerative disease. Front Mol Neurosci 7:63. doi:10.3389/fnmol.2014.00063
Bar-Lavan Y, Shemesh N, Ben-Zvi A (2016) Chaperone families and interactions in metazoa. Essays Biochem 60:237–253. doi:10.1042/ebc20160004
Bertelsen EB, Chang L, Gestwicki JE, Zuiderweg ER (2009) Solution conformation of wild-type E. coli Hsp70 (DnaK) chaperone complexed with ADP and substrate. Proc Natl Acad Sci U S A 106:8471–8476. doi:10.1073/pnas.0903503106
Bhattacharya A, Kurochkin AV, Yip GN, Zhang Y, Bertelsen EB, Zuiderweg ER (2009) Allostery in Hsp70 chaperones is transduced by subdomain rotations. J Mol Biol 388:475–490. doi:10.1016/j.jmb.2009.01.062
Bork P, Sander C, Valencia A, Bukau D (1992) A module of the DnaJ heat shock proteins found in malaria parasites. Trends Biochem Sci 17:129
Brinker A et al (2002) Ligand discrimination by TPR domains - relevance and selectivity of EEVD-recognition in Hsp70 center dot hop center dot Hsp90 complexes. J Biol Chem 277:19265–19275. doi:10.1074/jbc.M109002200
Britten CD et al (2000) A phase I and pharmacokinetic study of the mitochondrial-specific rhodacyanine dye analog MKT 077. Clinical cancer research : an official journal of the American Association for Cancer Research 6:42–49
Cesa LC, Patury S, Komiyama T, Ahmad A, Zuiderweg ER, Gestwicki JE (2013) Inhibitors of difficult protein-protein interactions identified by high-throughput screening of multiprotein complexes. ACS Chem Biol 8:1988–1997. doi:10.1021/cb400356m
Chang L et al (2011) Chemical screens against a reconstituted multiprotein complex: myricetin blocks DnaJ regulation of DnaK through an allosteric mechanism. Chemistry & biology 18:210–221. doi:10.1016/j.chembiol.2010.12.010
Cheeseman MD et al (2016) Exploiting protein conformational change to optimize adenosine-derived inhibitors of HSP70. J Med Chem 59:4625–4636. doi:10.1021/acs.jmedchem.5b02001
Connarn JN et al (2014) The molecular chaperone Hsp70 activates protein phosphatase 5 (PP5) by binding the tetratricopeptide repeat (TPR) domain. J Biol Chem 289:2908–2917. doi:10.1074/jbc.M113.519421
Craig EA, Huang P (2005) Cellular functions of Hsp70 chaperones. Protein folding handbook. Wiley-V C H Verlag Gmbh, Weinheim
Cuervo AM, Dice JF (1996) A receptor for the selective uptake and degradation of proteins by lysosomes. Science (New York, NY) 273:501–503
Demand J, Luders J, Hohfeld J (1998) The carboxy-terminal domain of Hsc70 provides binding sites for a distinct set of chaperone cofactors. Mol Cell Biol 18:2023–2028
Dice JF (1990) Peptide sequences that target cytosolic proteins for lysosomal proteolysis. Trends Biochem Sci 15:305–309
Flaherty KM, Deluca-Flaherty C, McKay DB (1990) 3-dimensional structure of the ATPase fragment of a 70 k heat-shock cognate protein. Nature 346:623–628
Fontaine SN et al (2015) Isoform-selective genetic inhibition of constitutive cytosolic Hsp70 activity promotes client tau degradation using an altered Co-chaperone complement. J Biol Chem 290:13115–13127. doi:10.1074/jbc.M115.637595
Frydman J (2000) Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu Rev Biochem 70:603–647
Frydman J, Höhfeld J (1997) Chaperones get in touch: the Hip-Hop connection. Trends Biochem Sci. 22(3):87-92
Gamerdinger M, Hajieva P, Kaya AM, Wolfrum U, Hartl FU, Behl C (2009) Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J 28:889–901. doi:10.1038/emboj.2009.29
Goloubinoff P, De Los Rios P (2007) The mechanism of Hsp70 chaperones: (entropic) pulling the models together. Trends Biochem Sci 32:372–380. doi:10.1016/j.tibs.2007.06.008
Guidon PT Jr, Hightower LE (1986) Purification and initial characterization of the 71-kilodalton rat heat-shock protein and its cognate as fatty acid binding proteins. Biochemistry 25:3231–3239
Harrison CJ, Hayer-Hartl M, Di Liberto M, Hartl F, Kuriyan J (1997) Crystal structure of the nucleotide exchange factor GrpE bound to the ATPase domain of the molecular chaperone DnaK. Science (New York, NY) 276:431–435
Hohfeld J, Hartl FU (1994) Post-translational protein import and folding. Curr Opin Cell Biol 6:499–509
Hohfeld J, Minami Y, Hartl FU (1995) Hip, a novel cochaperone involved in the eukaryotic Hsc70/Hsp40 reaction cycle. Cell 83(4):589–598
Hubbard J1, Erlichman C, Toft DO, Qin R, Stensgard BA, Felten S, Ten Eyck C, Batzel G, Ivy SP, Haluska P (2011) Phase I study of 17-allylamino-17 demethoxygeldanamycin, gemcitabine and/or cisplatin in patients with refractory solid tumors. Invest New Drugs 29(3):473-80. doi:10.1007/s10637-009-9381-y
Jiang Y, Woronicz JD, Liu W, Goeddel DV (1999) Prevention of constitutive TNF receptor 1 signaling by silencer of death domains. Science (New York, NY) 283:543–546
Jiang JH, Ballinger CA, Wu YX, Dai Q, Cyr DM, Hohfeld J, Patterson C (2001) CHIP is a U-box-dependent E3 ubiquitin ligase - identification of Hsc70 as a target for ubiquitylation. J Biol Chem 276:42938–42944. doi:10.1074/jbc.M101968200
Jiang J, Maes EG, Taylor AB, Wang L, Hinck AP, Lafer EM, Sousa R (2007) Structural basis of J cochaperone binding and regulation of Hsp70. Mol Cell 28:422–433
Jinwal UK, Akoury E, Abisambra JF, O'Leary JC 3rd, Thompson AD, Blair LJ, Jin Y, Bacon J, Nordhues BA, Cockman M, Zhang J, Li P, Zhang B, Borysov S, Uversky VN, Biernat J, Mandelkow E, Gestwicki JE, Zweckstetter M, Dickey CA (2013) Imbalance of Hsp70 family variants fosters tau accumulation. FASEB J 27(4):1450-9. doi:10.1096/fj.12-220889
Kabbage M, Dickman MB (2008) The BAG proteins: a ubiquitous family of chaperone regulators. Cellular and molecular life sciences : CMLS 65:1390–1402. doi:10.1007/s00018-008-7535-2
Kampinga HH, Craig EA (2010) The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol 11:579–592. doi:10.1038/nrm2941
Kampinga H et al (2009) Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chap 14:105–111
Kellner R, Hofmann H, Barducci A, Wunderlich B, Nettels D, Schuler B (2014) Single-molecule spectroscopy reveals chaperone-mediated expansion of substrate protein. Proc Natl Acad Sci U S A 111:13355–13360. doi:10.1073/pnas.1407086111
Kityk R, Kopp J, Sinning I, Mayer MP (2012) Structure and dynamics of the ATP-bound open conformation of Hsp70 chaperones. Mol Cell 48:863–874. doi:10.1016/j.molcel.2012.09.023
Komarova EY, Meshalkina DA, Aksenov ND, Pchelin IM, Martynova E, Margulis BA, Guzhova IV (2015) The discovery of Hsp70 domain with cell-penetrating activity. Cell Stress Chaperones 20:343–354. doi:10.1007/s12192-014-0554-z
Leu JI, Pimkina J, Frank A, Murphy ME, George DL (2009) A small molecule inhibitor of inducible heat shock protein 70. Mol Cell 36:15–27. doi:10.1016/j.molcel.2009.09.023
Leu JI, Zhang P, Murphy ME, Marmorstein R, George DL (2014) Structural basis for the inhibition of HSP70 and DnaK chaperones by small-molecule targeting of a C-terminal allosteric pocket. ACS Chem Biol 9:2508–2516. doi:10.1021/cb500236y
Leung SM, Senisterra G, Ritchie KP, Sadis SE, Lepock JR, Hightower LE (1996) Thermal activation of the bovine Hsc70 molecular chaperone at physiological temperatures: physical evidence of a molecular thermometer. Cell Stress Chaperones 1:78–89
Leung SM, Hightower LE (1997) A 16-kDa protein functions as a new regulatory protein for Hsc70 molecular chaperone and is identified as a member of the Nm23/nucleoside diphosphate kinase family. J Biol Chem. 272(5):2607-14
Li Z, Hartl FU, Bracher A (2013) Structure and function of hip, an attenuator of the Hsp70 chaperone cycle. Nat Struct Mol Biol 20:929–935. doi:10.1038/nsmb.2608
Li X et al (2015) Validation of the Hsp70-Bag3 protein-protein interaction as a potential therapeutic target in cancer. Mol Cancer Ther. doi:10.1158/1535-7163.mct-14-0650
Lopez V, Cauvi DM, Arispe N, De Maio A (2016) Bacterial Hsp70 (DnaK) and mammalian Hsp70 interact differently with lipid membranes. Cell Stress Chaperones 21:609–616. doi:10.1007/s12192-016-0685-5
Ludlow RF, Verdonk ML, Saini HK, Tickle IJ, Jhoti H (2015) Detection of secondary binding sites in proteins using fragment screening. Proc Natl Acad Sci U S A 112:15910–15915. doi:10.1073/pnas.1518946112
Macazo FC, White RJ (2014) Monitoring charge flux to quantify unusual ligand-induced ion channel activity for use in biological nanopore-based sensors. Anal Chem 86:5519–5525. doi:10.1021/ac500832a
Majeski AE, Dice JF (2004) Mechanisms of chaperone-mediated autophagy. Int J Biochem Cell Biol 36:2435–2444. doi:10.1016/j.biocel.2004.02.013
Mayer MP, Bukau B (2005) Regulation of Hsp70 chaperones by co-chaperones. Protein folding handbook
Melero R et al (2015) Modulation of the chaperone DnaK allosterism by the nucleotide exchange factor GrpE. J Biol Chem 290:10083–10092. doi:10.1074/jbc.M114.623371
Morozova K et al (2016) Structural and biological interaction of hsc-70 protein with phosphatidylserine in endosomal microautophagy. J Biol Chem 291:18096–18106. doi:10.1074/jbc.M116.736744
Noguchi A, Ikeda A, Mezaki M, Fukumori Y, Kanemori M (2014) DnaJ-promoted binding of DnaK to multiple sites on sigma32 in the presence of ATP. J Bacteriol 196:1694–1703. doi:10.1128/jb.01197-13
Nylandsted J, Rohde M, Brand K, Bastholm L, Elling F, Jäättelä M (2000) Selective depletion of heat shock protein 70 (Hsp70) activates a tumor-specific death program that is independent of caspases and bypasses Bcl-2. Proc Natl Acad Sci U S A. 97(14):7871-6
Patury S, Miyata Y, Gestwicki JE (2009) Pharmacological targeting of the Hsp70 chaperone. Curr Top Med Chem 9:1337–1351
Pellecchia M, Montgomery DL, Stevens SY, Vander Kooi CW, Feng HP, Gierasch LM, Zuiderweg ER (2000) Structural insights into substrate binding by the molecular chaperone DnaK. Nat Struct Biol 7:298–303
Polier S, Dragovic Z, Hartl FU, Bracher A (2008) Structural basis for the cooperation of Hsp70 and Hsp110 chaperones in protein folding. Cell 133:1068–1079. doi:10.1016/j.cell.2008.05.022
Qi R et al (2013) Allosteric opening of the polypeptide-binding site when an Hsp70 binds ATP. Nat Struct Mol Biol 20:900–907. doi:10.1038/nsmb.2583
Radons J (2016) The human HSP70 family of chaperones: where do we stand? Cell Stress Chaperones 21:379–404. doi:10.1007/s12192-016-0676-6
Rauch JN, Zuiderweg ER, Gestwicki JE (2016) Non-canonical interactions between heat shock cognate protein 70 (Hsc70) and Bcl2-associated Anthanogene (BAG) Co-chaperones are important for client release. J Biol Chem 291:19848–19857. doi:10.1074/jbc.M116.742502
Rousaki A, Miyata Y, Jinwal UK, Dickey CA, Gestwicki JE, Zuiderweg ER (2011) Allosteric drugs: the interaction of antitumor compound MKT-077 with human Hsp70 chaperones. J Mol Biol 411:614–632. doi:10.1016/j.jmb.2011.06.003
Rout AK, Strub MP, Piszczek G, Tjandra N (2014) Structure of transmembrane domain of lysosome-associated membrane protein type 2a (LAMP-2A) reveals key features for substrate specificity in chaperone-mediated autophagy. J Biol Chem 289:35111–35123. doi:10.1074/jbc.M114.609446
Sahu R et al (2011) Microautophagy of cytosolic proteins by late endosomes. Dev Cell 20:131–139. doi:10.1016/j.devcel.2010.12.003
Schlecht R et al (2013) Functional analysis of Hsp70 inhibitors. PLoS One 8:e78443. doi:10.1371/journal.pone.0078443
Schroder H, Langer T, Hartl FU, Bukau B (1993) Dnak, Dnaj and Grpe form a cellular chaperone machinery capable of repairing heat-induced protein damage. EMBO J 12:4137–4144
Schuermann JP et al (2008) Structure of the Hsp110:Hsc70 nucleotide exchange machine. Mol Cell 31:232–243. doi:10.1016/j.molcel.2008.05.006
Shomura Y et al (2005) Regulation of Hsp70 function by HspBP1: structural analysis reveals an alternate mechanism for Hsp70 nucleotide exchange. Mol Cell 17:367–379. doi:10.1016/j.molcel.2004.12.023
Smith MC et al (2013) The E3 ubiquitin ligase CHIP and the molecular chaperone Hsc70 form a dynamic, tethered complex. Biochemistry 52:5354–5364. doi:10.1021/bi4009209
Sondermann H, Scheufler C, Schneider C, Hohfeld J, Hartl FU, Moarefi I (2001) Structure of a bag/Hsc70 complex: convergent functional evolution of Hsp70 nucleotide exchange factors. Science (New York, NY) 291:1553–1557
Suh WC, Burkholder WF, Lu CZ, Zhao X, Gottesman ME, Gross CA (1998) Interaction of the Hsp70 molecular chaperone, DnaK, with its cochaperone DnaJ. Proc Natl Acad Sci U S A 95:15223–15228
Suh WC, Lu CZ, Gross CA (1999) Structural features required for the interaction of the Hsp70 molecular chaperone DnaK with its cochaperone DnaJ. J Biol Chem 274:30534–30539
Suzuki H, Noguchi S, Arakawa H, Tokida T, Hashimoto M, Satow Y (2010) Peptide-binding sites as revealed by the crystal structures of the human Hsp40 Hdj1 C-terminal domain in complex with the octapeptide from human Hsp70. Biochemistry 49:8577–8584. doi:10.1021/bi100876n
Swain JF, Dinler G, Sivendran R, Montgomery DL, Stotz M, Gierasch LM (2007) Hsp70 chaperone ligands control domain association via an allosteric mechanism mediated by the interdomain linker. Mol Cell 26:27–39
Szyperski T, Pellecchia M, Wall D, Georgopoulos C, Wüthrich K (1994) NMR structure determination of the Escherichia coli DnaJ molecular chaperone: secondary structure and backbone fold of the N-terminal region (residues 2-108) containing the highly conserved J domain. Proc Natl Acad Sci USA 91:11343–11347
Takayama S, Xie Z, Reed JC (1999) An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem 274:781–786
Takenaka IM, Leung SM, McAndrew SJ, Brown JP, Hightower LE (1995) Hsc70-binding peptides selected from a phage display peptide library that resemble organellar targeting sequences. J Biol Chem 270:19839–19844
Terlecky SR, Chiang HL, Olson TS, Dice JF (1992) Protein and peptide binding and stimulation of in vitro lysosomal proteolysis by the 73-kDa heat shock cognate protein. J Biol Chem 267:9202–9209
Vega VL et al (2008) Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. Journal of immunology (Baltimore, Md : 1950) 180:4299–4307
Wang H, Pang Y, Kurochkin AV, Hu W, Flynn GC, Zuiderweg ERP (1998) The solution structure of the 21 kDa chaperone protein DnaK substrate binding domain: a preview of chaperone - protein interaction. Biochemistry 37:7929–7940
Wang L et al (2011) Molecular mechanism of the negative regulation of Smad1/5 protein by carboxyl terminus of Hsc70-interacting protein (CHIP). J Biol Chem 286:15883–15894. doi:10.1074/jbc.M110.201814
Wickner S, Maurizi MR, Gottesman S (1999) Posttranslational quality control: folding, refolding, and degrading proteins. Science (New York, NY) 286:1888–1893
Williamson DS et al (2009) Novel adenosine-derived inhibitors of 70 kDa heat shock protein, discovered through structure-based design. J Med Chem 52:1510–1513. doi:10.1021/jm801627a
Wisen S et al (2010) Binding of a small molecule at a protein-protein interface regulates the chaperone activity of hsp70-hsp40. ACS Chem Biol 5:611–622. doi:10.1021/cb1000422
Young JC, Agashe VR, Siegers K, Hartl FU (2004) Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol 5:781–791
Zhang Y, Zuiderweg ER (2004) The 70-kDa heat shock protein chaperone nucleotide-binding domain in solution unveiled as a molecular machine that can reorient its functional subdomains. Proc Natl Acad Sci U S A 101:10272–10277
Zhang H et al (2015) A bipartite interaction between Hsp70 and CHIP regulates ubiquitination of chaperoned client proteins. Structure (London, England: 1993) 23:472–482. doi:10.1016/j.str.2015.01.003
Zhu XT, Zhao X, Burkholder WF, Gragerov A, Ogata CM, Gottesman ME, Hendrickson WA (1996) Structural analysis of substrate binding by the molecular chaperone DnaK. Science (New York, NY) 272:1606–1614
Zhuravleva A, Gierasch LM (2015) Substrate-binding domain conformational dynamics mediate Hsp70 allostery. Proc Natl Acad Sci U S A 112:E2865–E2873. doi:10.1073/pnas.1506692112
Zuiderweg ER, Bertelsen EB, Rousaki A, Mayer MP, Gestwicki JE, Ahmad A (2013) Allostery in the Hsp70 chaperone proteins. Top Curr Chem 328:99–153. doi:10.1007/128_2012_323
Acknowledgements
This work was supported by NIH grant 5-R01-NS-059690-01-08.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zuiderweg, E.R.P., Hightower, L.E. & Gestwicki, J.E. The remarkable multivalency of the Hsp70 chaperones. Cell Stress and Chaperones 22, 173–189 (2017). https://doi.org/10.1007/s12192-017-0776-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-017-0776-y