Abstract
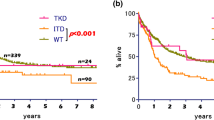
Acute myeloid leukemia harboring internal tandem duplication of FMS-like tyrosine kinase 3 (AMLFLT3-ITD) is associated with poor prognosis. We evaluated the results of the AML-05 study, in which all AMLFLT3-ITD patients were assigned to receive hematopoietic stem cell transplantation (HSCT) in the first remission (1CR). We also investigated the effects of additional genetic alterations on FLT3-ITD. The 5-year overall survival (OS) and event-free survival (EFS) rates among the 47 AMLFLT3-ITD patients were 42.2 and 36.8%, respectively. The 5-year disease-free survival rate among 29 patients without induction failure was 58.4%. We defined the allelic ratio (AR) of FLT3-ITD to WT > 0.7 as high. Significant differences were found in OS (AR-high, 20% vs. AR-low, 66%, p < 0.001) and EFS (13 vs. 50%, p = 0.004). All five patients with concurrent NPM1 mutations survived, while seven of eight patients who expressed the NUP98-NSD1 chimera failed to achieve 1CR and died. Multivariate analysis revealed that AR > 0.7 and expression of the NUP98-NSD1 chimera strongly impacted OS and EFS. Although all the AMLFLT3-ITD patients received HSCT at 1CR, the treatment outcome of AMLFLT3-ITD patients did not improve compared with those in a previous study. Heterogeneity was observed among AMLFLT3-ITD patients.



Similar content being viewed by others
Abbreviations
- FLT3 :
-
fms-related tyrosine kinase 3
- ITD:
-
Internal tandem duplication
- HSCT:
-
Hematopoietic stem cell transplantation
- CR:
-
Complete remission
- OS:
-
Overall survival
- DFS:
-
Disease-free survival
- EFS:
-
Event-free survival
- AR:
-
Allelic ratio
References
Meshinchi S, Woods WG, Stirewalt DL, Sweetser DA, Buckley JD, Tjoa TK, et al. Prevalence and prognostic significance of Flt3 internal tandem duplication in pediatric acute myeloid leukemia. Blood. 2001;97:89–94.
Zwaan CM, Meshinchi S, Radich JP, Veerman AJ, Huismans DR, Munske L, et al. FLT3 internal tandem duplication in 234 children with acute myeloid leukemia: prognostic significance and relation to cellular drug resistance. Blood. 2003;102:2387–94.
Staffas A, Kanduri M, Hovland R, Rosenquist R, Ommen HB, Abrahamsson J, et al. Presence of FLT3-ITD and high BAALC expression are independent prognostic markers in childhood acute myeloid leukemia. Blood. 2011;118:5905–13.
Shimada A, Taki T, Tabuchi K, Taketani T, Hanada R, Tawa A, et al. Tandem duplications of MLL and FLT3 are correlated with poor prognoses in pediatric acute myeloid leukemia: a study of the Japanese childhood AML Cooperative Study Group. Pediatr Blood Cancer. 2008;50:264–9.
Meshinchi S, Alonzo TA, Stirewalt DL, Zwaan M, Zimmerman M, Reinhardt D, et al. Clinical implications of FLT3 mutations in pediatric AML. Blood. 2006;108:3654–61.
Gale RE, Green C, Allen C, Mead AJ, Burnett AK, Hills RK, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008;111:2776–84.
Pratcorona M, Brunet S, Nomdedéu J, Ribera JM, Tormo M, Duarte R, et al. Favorable outcome of patients with acute myeloid leukemia harboring a low-allelic burden FLT3-ITD mutation and concomitant NPM1 mutation: relevance to post-remission therapy. Blood. 2013;121:2734–8.
Linch DC, Hills RK, Burnett AK, Khwaja A, Gale RE. Impact of FLT3(ITD) mutant allele level on relapse risk in intermediate-risk acute myeloid leukemia. Blood. 2014;124:273–6.
Koszarska M, Meggyesi N, Bors A, Batai A, Csacsovszki O, Lehoczky E, et al. Medium-sized FLT3 internal tandem duplications confer worse prognosis than short and long duplications in a non-elderly acute myeloid leukemia cohort. Leuk Lymphoma. 2014;55:1510–7.
Schlenk RF, Kayser S, Bullinger L, Kobbe G, Casper J, Ringhoffer M, et al. Differential impact of allelic ratio and insertion site in FLT3-ITD positive AML with respect to allogeneic hematopoietic stem cell transplantation. Blood. 2014;124:3441–9.
Shiba N, Ichikawa H, Taki T, Park MJ, Jo A, Mitani S, et al. NUP98-NSD1 gene fusion and its related gene expression signature are strongly associated with a poor prognosis in pediatric acute myeloid leukemia. Genes Chromosom Cancer. 2013;52:683–93.
Hollink IH, van den Heuvel-Eibrink MM, Arentsen-Peters ST, Pratcorona M, Abbas S, Kuipers JE, et al. NUP98/NSD1 characterizes a novel poor prognostic group in acute myeloid leukemia with a distinct HOX gene expression pattern. Blood. 2011;118:3645–56.
Ostronoff F, Othus M, Gerbing RB, Loken MR, Raimondi SC, Hirsch BA, et al. NUP98/NSD1 and FLT3/ITD coexpression is more prevalent in younger AML patients and leads to induction failure: a COG and SWOG report. Blood. 2014;124:2400–7.
Sano H, Shimada A, Tabuchi K, Taki T, Murata C, Park MJ, et al. WT1 mutation in pediatric patients with acute myeloid leukemia: a report from the Japanese Childhood AML Cooperative Study Group. Int J Hematol. 2013;98:437–45.
Kayser S, Schlenk RF, Londono MC, Breitenbuecher F, Wittke K, Du J, et al. Insertion of FLT3 internal tandem duplication in the tyrosine kinase domain-1 is associated with resistance to chemotherapy and inferior outcome. Blood. 2009;114:2386–92.
Tomizawa D, Tawa A, Watanabe T, Saito AM, Kudo K, Taga T, et al. Appropriate dose reduction in induction therapy is essential for the treatment of infants with acute myeloid leukemia: a report from the Japanese Pediatric Leukemia/Lymphoma Study Group. Int J Hematol. 2013;98:578–88.
Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377:454–64.
Larrosa-Garcia M, Baer MR. FLT3 inhibitors in acute myeloid leukemia: current status and future directions. Mol Cancer Ther. 2017;16:991–1001.
Rautenberg C, Nachtkamp K, Dienst A, Schmidt PV, Heyn C, Kondakci M, Germing U, Haas R, Kobbe G, Schroeder T. Sorafenib and azacitidine as salvage therapy for relapse of FLT3-ITD mutated AML after allo-SCT. Eur J Haematol. 2017;98:348–54.
Brunner AM, Li S, Fathi AT, Wadleigh M, Ho VT, Collier K, et al. Haematopoietic cell transplantation with and without sorafenib maintenance for patients with FLT3-ITD acute myeloid leukaemia in first complete remission. Br J Haematol. 2016;175:496–504.
Iwasaki Y, Nishiuchi R, Aoe M, Takahashi T, Watanabe H, Tokorotani C, et al. Positive minimal residual disease of FLT3-ITD before hematopoietic stem cell transplantation resulted in a poor prognosis of an acute myeloid leukemia. Acta Med Okayama. 2017;71:79–83.
Shimada A, Taki T, Koga D, Tabuchi K, Tawa A, Hanada R, et al. High WT1 mRNA expression after induction chemotherapy and FLT3-ITD have prognostic impact in pediatric acute myeloid leukemia: a study of the Japanese Childhood AML Cooperative Study Group. Int J Hematol. 2012;96:469–76.
Sano H, Shimada A, Taki T, Murata C, Park MJ, Sotomatsu M, Tabuchi K, Tawa A, Kobayashi R, Horibe K, Tsuchida M, Hanada R, Tsukimoto I, Hayashi Y. RAS mutations are frequent in FAB type M4 and M5 of acute myeloid leukemia, and related to late relapse: a study of the Japanese Childhood AML Cooperative Study Group. Int J Hematol. 2012;95:509–15.
Hirade T, Abe M, Onishi C, Taketani T, Yamaguchi S, Fukuda S. Internal tandem duplication of FLT3 deregulates proliferation and differentiation and confers resistance to the FLT3 inhibitor AC220 by Up-regulating RUNX1 expression in hematopoietic cells. Int J Hematol. 2016;103:95–106.
Tian X, Xu Y, Yin J, Tian H, Chen S, Wu D, Sun A. TET2 gene mutation is unfavorable prognostic factor in cytogenetically normal acute myeloid leukemia patients with NPM1 + and FLT3-ITD− mutations. Int J Hematol. 2014;100:96–104.
Grunwald MR, Levis MJ. FLT3 inhibitors for acute myeloid leukemia: a review of their efficacy and mechanisms of resistance. Int J Hematol. 2013;97:683–94.
Acknowledgements
We thank all doctors involved for participating in the JPLSG AML-05 study. This work was supported by a Grant for Clinical Cancer Research and a Grant-in-Aid for Cancer Research from the Ministry of Health, Labour, and Welfare of Japan, and Japan Agency for Medical Research and Development (AMED). We thank Ryan Chastain-Gross, Ph.D., from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
Shimada A and Tomizawa D reviewed the data analysis and interpretation and were the main authors of the manuscript. Shimada A, Tawa A (principle investigator), Tomizawa D, Taga T, Iwamoto S, Terui K, Moritake H, Kinoshita A, Takahashi T, Nakayama H and Adachi S are the member of the AML committee and participated actively in the study conception and design of the AML-05 study. Iijima-Yamashita Y, Yamada M, Norio S, Hara Y, and Oki K performed the genetic analysis. Hayashi Y organized the genetic analysis. Koh K, Goto H, and Kosaka Y contributed to the recruitment of the patients. Kiyokawa N is responsible for specimen banking center. Saito AM and Horibe K are responsible for data center. Watanabe T and Tanaka S performed statistical analysis. Adachi S and Horibe K contributed to financial and administrative support of the AML-05 study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
This research was (partially) supported by the Practical Research for Innovative Cancer Control grant from the Japan Agency for Medical Research and Development, AMED.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12185_2017_2395_MOESM1_ESM.docx
Supplemental Figure 1 The clearance of bone marrow blasts after single induction course in complete remission (CR) vs. non-CR group. The median blast % dropped from 75.5% (29.4–93.9%) at BMA1 to 1.1% (0.2–17.2%) at BMA2 in the CR group vs. 85.8% (67.3–92.1%) at BMA1 to 30.6% (2.8–69.0%) at BMA2 in the non-CR group (p < 0.001) (DOCX 21 kb)
12185_2017_2395_MOESM2_ESM.docx
Supplemental Figure 2 Plot of receiver operating characteristic curve, depicting the allelic ratio (AR) of fms-related tyrosine kinase 3 (FLT3)-internal tandem duplication (ITD). We defined the cutoff value as 0.7 (DOCX 37 kb)
About this article
Cite this article
Shimada, A., Iijima-Yamashita, Y., Tawa, A. et al. Risk-stratified therapy for children with FLT3-ITD-positive acute myeloid leukemia: results from the JPLSG AML-05 study. Int J Hematol 107, 586–595 (2018). https://doi.org/10.1007/s12185-017-2395-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-017-2395-x