Abstract
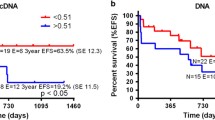
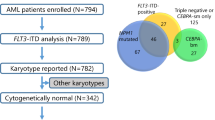
The prognostic value of WT1 mRNA expression in pediatric acute myeloid leukemia (AML) remains controversial. A sample of newly diagnosed (n = 158) AML patients from the Japanese Childhood AML Cooperative Treatment Protocol, AML 99, were simultaneously analyzed for WT1 expression, cytogenetic abnormalities and gene alterations (FLT3, KIT, MLL, and RAS). WT1 expression (including more than 2,500 copies/μgRNA) was detected in 122 of the 158 (77.8 %) initial diagnostic AML bone marrow samples (median 45,500 copies/μgRNA). Higher WT1 expression was detected in French American British (FAB)-M0, M3, M7 and lower expression in M4 and M5. Higher WT1 expression was detected in AML with inv(16), t(15;17) and Down syndrome and lower in AML with 11q23 abnormalities. Multivariate analyses demonstrated that FLT3-internal tandem duplication (ITD), KIT mutation, MLL-partial tandem duplication were correlated with poor prognosis; however, higher WT1 expression was not. FLT3-ITD was correlated with WT1 expression and prognosis. Furthermore, 74 WT1 expression after induction chemotherapy was analyzed. Higher WT1 expression after induction chemotherapy was significantly correlated with M1 or M2/M3 marrow, FLT3-ITD and poor prognosis. Multivariate analyses in 74 AML patients revealed that FLT3-ITD, MLL-PTD, and KIT mutations were associated with poor prognosis; however, NRAS Mutation, KRAS mutation and high WT1 expression (>10,000 copies/μgRNA) did not show poor prognosis. Our findings suggest that higher WT1 expression at diagnosis does not correlate with poor prognosis, but that WT1 expression after induction chemotherapy is considered to be a useful predictor of clinical outcome in pediatric AML.

Similar content being viewed by others
References
Raimondi SC, Chang MN, Ravindranath Y, et al. Chromosomal abnormalities in 478 children with acute myeloid leukemia: clinical characteristics and treatment outcome in a Cooperative Pediatric Oncology Group Study—POG8821. Blood. 1999;94:3707–16.
Webb DK, Harrison G, Stevens RF, et al. MRC Childhood Leukemia Working Party. Relationships between age at diagnosis, clinical features, and outcome of therapy in children treated in the Medical Research Council AML 10 and 12 trials for acute myeloid leukemia. Blood. 2001;98:1714–20.
Creutzig U, Ritter J, Zimmermann M, et al. Improved treatment results in high-risk pediatric acute myeloid leukemia patients after intensification with high-dose cytarabine and mitoxantrone: results of study acute myeloid leukemia-Berlin-Frankfurt-Munster 93. J Clin Oncol. 2001;19:2705–13.
Byrd JC, Mrozek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood. 2002;100:4325–36.
Rubnitz JE, Inaba H, Dahl G, Ribeiro RC, Bowman WP, Taub J, Pounds S, Razzouk BI, Lacayo NJ, Cao X, Meshinchi S, Degar B, Airewele G, Raimondi SC, Onciu M, Coustan-Smith E, Downing JR, Leung W, Pui CH, Campana D. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11:543–52.
Bergmann L, Miething C, Maurer U, et al. High levels of Wilms’ tumor gene (wt1) mRNA in acute myeloid leukemias are associated with a worse long-term outcome. Blood. 1997;90:1217–25.
Trka J, Kalinova M, Hrusak O, et al. Real-time quantitative PCR detection of WT1 gene expression in children with AML: prognostic significance, correlation with disease status and residual disease detection by flow cytometry. Leukemia. 2002;16:1381–9.
Garg M, Moore H, Tobal K, Liu Yin JA. Prognostic significance of quantitative analysis of WT1 gene transcripts by competitive reverse transcription polymerase chain reaction in acute leukaemia. Br J Haematol. 2003;123:49–59.
Barragan E, Cervera J, Bolufer P, et al. Prognostic implications of Wilms’ tumor gene (WT1) expression in patients with de novo acute myeloid leukemia. Haematologica. 2004;89:926–33.
Gaiger A, Schmid D, Heinze G, et al. Detection of the WT1 transcript by RT-PCR in complete remission has no prognostic relevance in de novo acute myeloid leukemia. Leukemia. 1998;12:1886–94.
Weisser M, Kern W, Rauhut S, et al. Prognostic impact of RT-PCR-based quantification of WT1 gene expression during MRD monitoring of acute myeloid leukemia. Leukemia. 2005;19:1416–23.
Miyawaki S, Hatsumi N, Tamaki T, Naoe T, Ozawa K, Kitamura K, Karasuno T, Mitani K, Kodera Y, Yamagami T, Koga D. Prognostic potential of detection of WT1mRNA level in peripheral blood in adult acute myeloid leukemia. Leuk Lymphoma. 2010;51:1855–61.
Lapillonne H, Renneville A, Auvrignon A, et al. High WT1 expression after induction therapy predicts high risk of relapse and death in pediatric acute myeloid leukemia. J Clin Oncol. 2006;24:1507–15.
Cilloni D, Renneville A, Hermitte F, Hills RK, Daly S, Jovanovic JV, Gottardi E, Fava M, Schnittger S, Weiss T, Izzo B, Nomdedeu J, van der Heijden A, van der Reijden BA, Jansen JH, van der Velden VH, Ommen H, Preudhomme C, Saglio G, Grimwade D. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European LeukemiaNet study. J Clin Oncol. 2009;27:5195–201.
Noronha SA, Farrar JE, Alonzo TA, Gerbing RB, Lacayo NJ, Dahl GV, Ravindranath Y, Arceci RJ, Loeb DM. WT1 expression at diagnosis does not predict survival in pediatric AML: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2009;53:1136–9.
Keilholz U, Menssen HD, Gaiger A, et al. Wilms’ tumour gene 1 (WT1) in human neoplasia. Leukemia. 2005;19:1318–23.
Yang L, Han Y, Saurez Saiz F, Minden MD. A tumor suppressor and oncogene: the WT1 story. Leukemia. 2007;21:868–76.
Meshinchi S, Stirewalt DL, Alonzo TA, et al. Activating mutations of RTK/ras signal transduction pathway in pediatric acute myeloid leukemia. Blood. 2003;102:1474–9.
Zwaan CM, Meshinchi S, Radich JP, et al. FLT3 internal tandem duplication in 234 children with acute myeloid leukemia: prognostic significance and relation to cellular drug resistance. Blood. 2003;102:2387–94.
Dohner K, Tobis K, Ulrich R, et al. Prognostic significance of partial tandem duplications of the MLL gene in adult patients 16 to 60 years old with acute myeloid leukemia and normal cytogenetics: a study of the Acute Myeloid Leukemia Study Group Ulm. J Clin Oncol. 2002;20:3254–61.
Shimada A, Taki T, Tabuchi K, Taketani T, Hanada R, Tawa A, Tsuchida M, Horibe K, Tsukimoto I, Hayashi Y. Tandem duplications of MLL and FLT3 are correlated with poor prognoses in pediatric acute myeloid leukemia: a study of the Japanese childhood AML Cooperative Study Group. Pediatr Blood Cancer. 2008;50:264–9.
Care RS, Valk PJ, Goodeve AC, et al. Incidence and prognosis of c-KIT and FLT3 mutations in core binding factor (CBF) acute myeloid leukaemias. Br J Haematol. 2003;121:775–7.
Shimada A, Taki T, Tabuchi K, et al. KIT mutations, and not FLT3 internal tandem duplication, are strongly associated with a poor prognosis in pediatric acute myeloid leukemia with t(8;21): a study of the Japanese Childhood AML Cooperative Study Group. Blood. 2006;107:1806–9.
Bacher U, Haferlach T, Schoch C, Kern W, Schnittger S. Implications of NRAS mutations in AML: a study of 2502 patients. Blood. 2006;107:3847–53.
Spassov BV, Stoimenov AS, Balatzenko GN, Genova ML, Peichev DB, Konstantinov SM. Wilms’ tumor protein and FLT3-internal tandem duplication expression in patients with de novo acute myeloid leukemia. Hematology. 2011;16:37–42.
Becker H, Marcucci G, Maharry K, Radmacher MD, Mrózek K, Margeson D, Whitman SP, Paschka P, Holland KB, Schwind S, Wu YZ, Powell BL, Carter TH, Kolitz JE, Wetzler M, Carroll AJ, Baer MR, Moore JO, Caligiuri MA, Larson RA, Bloomfield CD. Mutations of the Wilms tumor 1 gene (WT1) in older patients with primary cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Blood. 2010;116:788–92.
Ho PA, Zeng R, Alonzo TA, Gerbing RB, Miller KL, Pollard JA, Stirewalt DL, Heerema NA, Raimondi SC, Hirsch B, Franklin JL, Lange B, Meshinchi S. Prevalence and prognostic implications of WT1 mutations in pediatric acute myeloid leukemia (AML): a report from the Children’s Oncology Group. Blood. 2010;116:702–10.
Tsukimoto I, Tawa A, Hanada R, et al. Excellent outcome of risk stratified treatment for childhood acute myeloid leukemia-AML99 trial: for the Japanese Childhood AML Cooperative Study Group. Blood 2005:106:261a. Abstract 889.
Kobayashi R, Tawa A, Hanada R, Horibe K, Tsuchida M. Japanese childhood AML cooperative study group. Extramedullary infiltration at diagnosis and prognosis in children with acute myelogenous leukemia. Pediatr Blood Cancer. 2007;48:393–8.
Taketani T, Taki T, Sugita K, et al. FLT3 mutations in the activation loop of tyrosine kinase domain are frequently found in infant ALL with MLL rearrangements and pediatric ALL with hyperdiploidy. Blood. 2004;103:1085–8.
Yamamoto Y, Kiyoi H, Nakano Y, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434–9.
Jamal R, Taketani T, Taki T, et al. Coduplication of the MLL and FLT3 genes in patients with acute myeloid leukemia. Genes Chromosomes Cancer. 2001;31:187–90.
Schnittger S, Wormann B, Hiddemann W, et al. Partial tandem duplications of the MLL gene are detectable in peripheral blood and bone marrow of nearly all healthy donors. Blood. 1998;92:1728–34.
Sano H, Shimada A, Taki T, Murata C, Park MJ, Sotomatsu M, Tabuchi K, Tawa A, Kobayashi R, Horibe K, Tsuchida M, Hanada R, Tsukimoto I, Hayashi Y. RAS mutations are frequent in FAB type M4 and M5 of acute myeloid leukemia, and related to late relapse: a study of the Japanese Childhood AML Cooperative Study Group. Int J Hematol. 2012;95:509–15.
Sekiya M, Adachi M, Hinoda Y, Imai K, Yachi A. Downregulation of Wilms’ tumor gene (wt1) during myelomonocytic differentiation in HL60 cells. Blood. 1994;83:1876–82.
Nishida S, Hosen N, Shirakata T, et al. AML1-ETO rapidly induces acute myeloblastic leukemia in cooperation with the Wilms tumor gene, WT1. Blood. 2006;107:3303–12.
Hossain A, Nixon M, Kuo MT, Saunders GF. N-terminally truncated WT1 protein with oncogenic properties overexpressed in leukemia. J Biol Chem. 2006;281:28122–30.
Siehl JM, Reinwald M, Heufelder K, Menssen HD, Keilholz U, Thiel E. Expression of Wilms’ tumor gene 1 at different stages of acute myeloid leukemia and analysis of its major splice variants. Ann Hematol. 2004;83:745–50.
Ito K, Oji Y, Tatsumi N, et al. Antiapoptotic function of 17AA(+)WT1 (Wilms’ tumor gene) isoforms on the intrinsic apoptosis pathway. Oncogene. 2006;25:4217–29.
Acknowledgements
We would like to express our appreciation to all the doctors for their participation in the Japanese Childhood AML Cooperative Study Group.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported in part by a Grant-in-Aid for Cancer Research and a grant for Clinical Cancer Research and Research on Children and Families from the Ministry of Health, Labor and Welfare of Japan, and by a research grant for Gunma Prefectural Hospitals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12185_2012_1163_MOESM1_ESM.doc
Supplemental Figure 1. WT1 mRNA expression in 158 AML patients at time of initial diagnosis and 28 normal controls. A total of 122 out of 158 (77.8%) AML patients expressed more than the cut-off value of 2,500 copies/μgRNA. (DOC 40 kb)
12185_2012_1163_MOESM2_ESM.doc
Supplemental Figure 2. WT1 mRNA expression in 74 AML patients except for t(15;17) and Down syndrome at initial diagnosis and after 1st induction chemotherapy. A total of 58 out of 74 (78.4%) patients were WT1-positive at time of diagnosis (median, 18,000 μg/RNA) and 11 out of 74 (14.9%) still remained WT1-positive after induction chemotherapy (median, 215 μg/RNA). (DOC 45 kb)
About this article
Cite this article
Shimada, A., Taki, T., Koga, D. et al. High WT1 mRNA expression after induction chemotherapy and FLT3-ITD have prognostic impact in pediatric acute myeloid leukemia: a study of the Japanese Childhood AML Cooperative Study Group. Int J Hematol 96, 469–476 (2012). https://doi.org/10.1007/s12185-012-1163-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-012-1163-1