Abstract
To mitigate greenhouse gas emissions and improve energy supply security, there is an increasing effort toward the use of non-fossil energy sources. Crop residues have a great potential to be exploited as biomass for biogas production. However, due to their lignocellulosic structures they are difficult to degrade and do not reach competitive performance. A feasible option to mine these substrates is present in the forestomach of ruminants. Therefore, the aim of the present study was to use rumen microorganisms to improve anaerobic digestion (AD) of crop residues. For this purpose, hemp straw, mechanically pre-treated hemp fibers and shives, flax straw, flax shives, and aged and fresh rapeseed straw were evaluated using the rumen simulation technique. The AD of the substrates was divided into three batches. In two batches, hay was added as a control substrate. In summary, none of the analyzed substrates had an equivalent performance as the control hay, but pre-treated hemp fibers and shives had better AD parameters compared to all other alternative substrates, with the lowest pH (mean: 6.81), highest short chain fatty acid (20.0 mmol/day) and H2 production (25.6 mM) and highest degradability (25.2%). Flax straw had the second-best performance (6.81, 17.4 mmol/day, 20.6 mM and 22.2%, respectively), followed by fresh rapeseed straw, hemp straw, aged rapeseed straw and flax shives. Therefore, hemp fibers and shives demonstrated to be the most suitable substrates for AD. However, since pre-treatment can represent significant additional costs for biogas production, flax straw also demonstrated to be a good alternative.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the industrial revolution, a significant number of human activities has been driven by fossil fuels. These reserves are limited and have a harmful potential, since their combustion is associated with the emission of greenhouse gases to the atmosphere, which is directly associated with global warming [1, 2]. In order to improve energy supply security, there is an increasing effort toward the use of non-fossil energy sources [3, 4]. One alternative is biofuels (biodiesel, bioalcohol, biogas and biomethane) derived from various kinds of biomass. Biofuels are increasingly used in transportation, heat and power development [4]. The biomass used for biofuel production can include the conventional agricultural food-based crops, exploiting sugars, starch and vegetable oils as direct feedstocks to the conversion processes (so-called ‘first generation’ biofuel technologies), or non-food based biomass, such as agricultural residues (leaves, stalks, rice husk, straw, etc.), perennial grasses and animal waste (described as the ‘second generation’ biofuel technologies) [5, 6].
The majority of biofuel currently produced is first generation. The proteins, fats and simple sugars present in this biomass are converted within an economically reasonable retention time [1]. However, the use of such biomass is responsible for the competition with food production [7]. Therefore, there is a growing interest in the use of non-food-based biomass for energy production. In particular, crop residues (wheat straw, rice straw, corn straw, etc.) are ideal candidates to be used as raw materials for biofuel production, since they are often treated as a waste or by-product with no or low agricultural use, resulting in a high availability at low costs [8]. The use of such biomass may reduce competition among different forms of land use and contribute to effective waste management and could play a very significant future role for renewable energy production if processed anaerobically or with more complex technologies [9, 10].
Crop residues consist mainly of substrates rich in lignin, hemicellulose and cellulose, so called cellulose-based substrates (cbS), and are difficult to degrade [9]. Within the different types of biofuels that can be produced from cbS is the biogas, which can be directly applied as fuel for on-site heat, steam and electricity generation in industries or used in natural gas grids and as fuel in vehicles when refined [11]. Anaerobic digestion (AD) represents the most widely applied process for biogas production due to its high efficiency, operational flexibility and overall environmental benefits offered as compared to other biorefinery processes [12, 13]. However, the recalcitrant nature of this biomass limits the AD performance, requiring additional strategies such as physical, chemical or biological pre-treatments, as well as co-digestion in order to increase both the rates and the yields of biomethane production [9, 12]. Some pre-treatment methods have been demonstrated to induce the production of other toxic byproducts (furan aldehyde, organic acid and phenolic compounds) that can negatively affect AD [14, 15]. Moreover, those additional processes may increase costs for biogas production, decreasing its economic competitiveness with first generation biomass and fossil fuel [14, 16]. Therefore, second generation biofuels are still not a viable economic solution. In order to take them to a true commercial level extensive research and investment are required to improve the efficiency of cbS use and plant technology for biogas production, to anchor the principles of sustainability.
A feasible option to degrade these high-fiber and cellulosic substrates is present in the forestomach system of ruminants, which can be used as a model to improve methane yield in biogas plants [17, 18]. The digestive strategies of ruminants have been studied for years for implementation in industrial anaerobic reactors of biogas plants; however, no system has yet reached animal digestive performances [19, 20]. The rumen microbial consortium is a very effective, co-evolved ecosystem containing a wide range of enzymes and microorganisms involved in an efficient breakdown of cbS [21, 22]. Organic matter is fermented to short-chain fatty acids (SCFA), with carbon dioxide (CO2) and methane (CH4) produced as by-products [23]. In the rumen, methane is mainly formed via the hydrogenotrophic pathway from H2 and CO2 released during fermentation [24]. In the biogas plant, organic matter is also first hydrolysed (hydrolysis phase), then degraded into organic acids and alcohols (acidogenic phase), but then, these are mainly converted to acetate by acetogenic microorganisms (acetogenic phase) which serve as main substrate for methane production via the acetogenic pathway (methanogenic phase) [1]. Acetogenesis and methanogenesis are often physically separated from the hydrolysis/acidogenesis step to optimize performance [25]. By replacing animal manure, sewage sludge or organic waste which are still commonly used as starter cultures (inoculum) in biogas plants [12] with rumen contents in the first reactor (so-called DAUMEN system [26, 27]), hydrolysis of cbS and production of organic acids for acetogenesis and methane production can be enhanced.
To provide a better understanding on the microbial fermentation of different crop residues and their potential for anaerobic digestion in a rumen hydrolysis reactor, fermentation parameters of different crop residues were evaluated by using the rumen simulation technique (RUSITEC) [28]. The RUSITEC is a well-established model for long-term studies on rumen fermentation patterns and complex microbial interactions, highly similar to in vivo conditions [29] that can be used to determine the extent to which each substrate can be degraded by rumen microorganisms. The substrate with the best performance in the RUSITEC will consequently be used in a larger-scale setup of a rumen hydrolysis reactor combined with a UASB reactor for quantification of methane production.
Material and Methods
Ethics Statement
The two donor cows were housed at the Department for Physiology and Cell Biology, University of Veterinary Medicine, Hanover. The animals were kept and treated according to the guidelines of the German Animal Welfare Act. The Lower Saxony State Office for Consumer Protection and Food Safety (LAVES) approved the previous fistulation of the donor cows by the experiment number AZ 33.4–42505-04-13A373.
Substrates
The substrates analyzed were hemp straw (HSt), flax straw (FSt), fresh and aged rapeseed straw (FRSt and ARSt, respectively), which were cut into pieces of about 2 cm, and flax shives (FSh). Additionally, hemp pre-treated once with the BMS-Flaksy® device (HessenLeinen GmbH, Zierenberg, Germany) was used. This resulted in an uncomplete separation of fibers and shives, and the whole material was mixed for the trial (HF + Sh). This mechanical pre-treatment leads to the opening of the lignocellulosic structure of the substrate resulting in a larger specific surface area, thus making cellulose more accessible to hydrolysis [16].
Due to the great number of substrates to be analyzed, they were separated into three groups which were run separately. Hemp straw and flax substrates were analyzed in the first trial, while Flasky-treated hemp was analyzed in a second trial and the fresh and aged rapeseed in a third trial. The same hay which was fed to the cattle was added as a control substrate (named H1 and H2) in two of the trials, since it is a high-quality forage widely used as cattle feed and the rumen microbiota was adapted to it.
Rusitec Experiment
The present study is part of an in vitro experiment using a RUSITEC system [28] to observe effects of different substrates on fermentation parameters. Three fermenters were used for each substrate type (n = 3). At the beginning of the experiment, the fermenters were inoculated with mixed rumen contents collected in the morning (about 3 h after feeding) from two ruminal fistulated German Black-Pied cows, following the procedure described by Wetzels et al. [29]. Briefly, solid and liquid phases of the rumen were separated by gauze filtration. Fermenters were filled with the liquid phase, while 70 g of the solid phase was placed into a nylon mesh bag of 12 cm × 6.75 cm, pore size 50 µm (R712 Ankom Technologies, Gesellschaft für Analysentechnik, Salzwedel, Germany). Similar nylon bags were prepared and filled with 10 g dry matter of solid, coarsely chopped substrate. One bag with the solid rumen content and one with the substrate were then placed into the perforated inner vessel and inserted into the fermenter. The inner vessels were constantly moved up and down by an electric motor (6 times/min), to ensure the mix with the liquid phase. Fermentation vessels were kept in a water bath at 39 °C to imitate rumen conditions. After 24 h, the nylon bag containing the solid phase of the rumen was removed, rinsed with 40 ml of pre-warmed buffer solution for 1 min to dislodge the attached microorganisms and replaced by a substrate-filled one. The rinsing solution was returned to the fermenter. The nylon bags were replaced alternately with a new substrate bag every 24 h, such that the retention time for each nylon bag was 48 h. The fermentation vessels were continuously infused with a buffer solution, which mimics bovine saliva in pH and mineral composition [29]. The effluent was collected in conical glass flasks kept on ice. After the daily exchange of substrate bags, effluent flasks were flushed with nitrogen to maintain the anaerobic conditions.
Sampling and Sample Analysis
The pH values and redox potential of the liquid phase were measure daily using a pH and a redox electrode (Polyplast pH Sensors, Polyplast ORP Sensors, Hamilton Bonaduz AG, Bonaduz, Switzerland) during the whole experiment, to ensure adequate environmental conditions for microbial survival and anaerobic fermentation. Likewise, effluent volumes were measured daily. After an equilibration phase of 7 days, during which the microbiome adapts to the substrate, effluent samples were collected daily for the next 7 days of experiment for the analysis of NH3-N and SCFA concentrations. The NH3-N concentrations were determined photometrically at 546 nm in a spectrometer (DU 640, Beckman Coulter GmbH, Krefeld, Germany) as described by Riede et al. [30]. Analysis of SCFA concentrations was performed by gas chromatography. Shortly, 1.5 ml of the effluent sample was centrifuged at 40,000 g for 20 min at 4 °C. From each supernatant fraction, 1 ml of fluid was acidified by adding 0.1 ml 98% formic acid, and the sample was incubated for 10 min for protein precipitation. After that, the sample was centrifuged again at 40,000 g for 10 min at 4 °C. The supernatant was than filled into glass vials for GC analysis. The GC analyses were conducted on a Shimadzu GC-2025 system (Shimadzu, Kyoto, Japan) equipped with a flame ionization detector and a split injector with SH-Stabilwax-DA column (30 m × 0.32 mm × 0.50 µm). Nitrogen was used as carrier gas. The following temperature program was used: stepwise rise in temperature from 100 to 240 °C (15 °C/min) and hold for 2.66 min. Fatty acid was identified and quantified by comparison of their retention times and area under the curve with fatty acid standards, also prepared with formic acid (Sigma-Aldrich, Munich, Germany). Production rates of SCFA were calculated by multiplying SCFA concentration with daily effluent volume. Substrate residues from the feed bags were collected and dried at 65 °C for 48 h. To determine degradation, dried substrates were incinerated in a muffle furnace at 600 °C for 24 h.
Hydrogen production was calculated using the formula by Wang et al. [31] derived from the equations from Demeyer [32]:
To determine the chemical composition of the substrates used, the crude fibers (Xfi), N-free extracts (Nfe), acid detergent fibers (ADF), neutral detergent fibers (NDF), crude ash (XA), crude protein (XP) and crude fat (XF) were analyzed by Weender analysis (Institute of Animal Nutrition, University of Veterinary Medicine Hannover, Germany). For this a representative sample was collected from the undigested substrates. For the digested substrates the dried residues from the feed bags were pooled per treatment to gain enough material for the analysis. The degradation of the chemical fractions was calculated based on the mass of the fraction analyzed in the digested samples compared to the undigested ones. The missing samples are due to the lack of enough material to perform all analyses.
Statistical Evaluation of the Rusitec Results
The results of the Rusitec tests were statistically evaluated by a two-factorial analysis of variance. For this purpose, the software GraphPad Prism 9 was used. Data were assessed for normal distribution of residuals by applying the Kolmogorov–Smirnov test. Two-way analysis of variance (ANOVA) for repeated measurements was applied to detect effects of Time, Treatment or interactions of Time × Treatment. In case of significant interaction, Tukey post-test was used to identify significant differences among treatments within time-points and between time-points within treatment. In case of significant effects of treatment without interaction, Tukey post-test was applied to detect differences among treatments. Significance levels were set at p < 0.05, p < 0.01 and p < 0.001. The presented graphs were created using GraphPad Prism 9.
Results
In general, all pH values were within the range of 6.8 to 7.2. No significant changes in pH were observed for any of the substrates along the course of the experiment. Therefore, pH is presented as the mean value along the 7 days of sampling, for each substrate (Fig. 1). Significant differences were observed among the different substrates (p < 0.0001) in the following order: flax shives (FSh) > aged rapeseed straw (ARSt) > hemp straw (HSt) > flax straw (FSt) > fresh rapeseed straw (FRSt) > hemp fibers and shives (HF + Sh) > hay (H1 and H2). The pH value of both hay groups did not differ.
Mean values of 7 days of consecutive pH measurements for fresh rapeseed straw (FRSt), aged rapeseed straw (ARSt), hemp straw (HSt), hemp fibers and shives (HF + Sh), flax shives (FSh), flax straw (FSt) and hay (H1 and H2). Different letters indicate significant differences among the substrates. Data are presented as mean + SD
The redox potential varied significantly along the course of the experiment for some substrates (effect of time p = 0.0076), such as ARSt and FSh. For ARSt, the redox potential decreased from − 243 mV on day 7 to − 292 mV on day 14, while the opposite occurred for FSh with an increase from − 269 mV on day 7 to − 210 mV on day 13. For all other substrates, no significant differences occurred along the time. However, the observed variations on ARSt and FSh are likely to have a negligible biological relevance.
The redox potential differed significantly among substrates (p < 0.0001, Fig. 2). In general, values ranged from − 221 to − 329 mV and with the exception of the FRSt, all other substrates exhibited a more positive redox potential than the control hay. Hemp had the most positive redox potential (− 221 mV and − 238 mV for HF + Sh and HSt, respectively), followed by FSt, ARSt and FRSt with the lowest value (− 329 mV). Treating hemp with the retting device significantly increased its redox potential, compared to the non-treated straw.
Mean redox potential [mV] of fresh rapeseed straw (FRSt), aged rapeseed straw (ARSt), hemp straw (HSt), hemp fibers and shives (HF + Sh), flax shives (FSh), flax straw (FSt) and hay (H1 and H2). Different letters indicate significant differences among substrates. Data are presented as box and whiskers
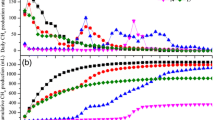
Again, no significant changes on the total daily production of SCFA occurred along the timeline of the experiment, but the SCFA production rates of the different substrates were significantly different from each other (p < 0.0001, Fig. 3), except for ARSt and FSh that had similar values. The H2 had a significantly greater SCFA production rate compared to H1, and both had higher production rates compared to all other analyzed substrates. Treating hemp with the retting device resulted in a higher SCFA production compared to the untreated samples and resulted in treated hemp samples to have the highest SCFA of all test substrates. The amount of SCFA produced by HF + Sh was followed by FSt, FRSt, HSt and ARSt. FSh produced significantly less SCFA compared to the FSt sample, and together with ARSt, they produced significantly less SCFA compared to all other samples.
Mean SCFA production (mmol d−1) of fresh rapeseed straw (FRSt), aged rapeseed straw (ARSt), hemp straw (HSt), hemp fibers and shives (HF + Sh), flax shives (FSh), flax straw (FSt) and hay (H1 and H2). Different letters indicate significant differences among substrates. Data are presented as mean + SD
Within the individual types of fatty acids produced, acetate was the one with the highest molar proportion for all substrates, representing around 60–70% of the total SCFA (Supplementary Fig. 1). Propionate was the fatty acid produced in second highest proportion in all substrates, representing 21–27% of the total SCFA. Butyrate varied between 4 and 11%. Valerate and isovalerate represented up to 2% of the total SCFA, while isobutyrate had the smallest proportion with up to 1% of the total SCFA.
Acetate production followed the very same pattern as observed for the total SCFA, with the highest production rate for hay and the lowest for ARSt and FSh (Table 1). Propionate followed a similar pattern compared to acetate and total SCFA, with the exception that propionate production of FSt and HF + Sh did not differ. Butyrate and valerate had similar pattern and were produced in high rates in H1 and H2, followed by HF + Sh. The FSt, HSt and FRSt had similar values and did not differ among each other but had significantly lower amounts of butyrate and valerate than the previously mentioned substrates and significantly higher amounts compared to ARSt and FSh. Isovalerate production was highest in H1 and H2, followed by FRSt. The FSt and HF + Sh had similar values, but significantly higher concentration compared to ARSt and HSt. The isovalerate concentration from FSh was below the detection limit. Similarly, isobutyrate could only be detected for hay, FSt and HF + Sh. The FSt and HF + Sh had similar values and both significantly lower than H1 and H2.
Acetate represented the major SCFA produced in all substrates. Since H2 is a by-product of the production of acetate and butyrate, the H2 production followed the same patterns as observed for the SCFA production. Therefore, within the substrates analyzed, HF + Sh exhibited the highest H2 production after the controls H1 and H2, followed by FSt and FRSt (Fig. 4). The FSh and ARSt had the lowest H2 production among substrates.
Calculated net H2 [mM] produced during AD of fresh rapeseed straw (FRSt); aged rapeseed straw (ARSt); hemp straw (HSt); hemp fibers and shives (HF + Sh); flax shives (FSh); flax straw (FSt) and hay (H1 and H2). Different letters indicate significant differences among substrates. Data are presented as mean + SD
The concentration of NH3-N remained relatively stable (Time p > 0.05) for each substrate but significantly differed among the substrates (p < 0.0001, Fig. 5). H1 had the highest NH3-N production with 9.78 mmol/l, nearly two times higher compared to 4.67 mmol/l from H2. ARSt and FRSt had comparable NH3-N concentrations to H2, followed by FSh, HSt and FSt. HF + Sh had the lowest NH3-N concentration of all substrates.
Ammonia-N [mmol l−1] concentration during AD of fresh rapeseed straw (FRSt), aged rapeseed straw (ARSt), hemp straw (HSt), hemp fibers and shives (HF + Sh), flax shives (FSh), flax straw (FSt) and hay (H1 and H2). Different letters indicate significant differences among samples. Data are presented as mean + SD
The degradation of organic matter of the substrates after 48 h of incubation did not change along the time of the experiment, but it differed among the different types of substrates (p < 0.0001, Fig. 6), except for FRSt and HSt which had a similar degradation. All substrates were less degradable compared to both controls hay, but FSt exhibited the highest degradation of all analyzed substrates, followed by FRSt, HSt and FSh. The ARSt had the lowest degradation rate of all substrates. Treating HSt with the retting device significantly affected degradability. While the degradation of HSt after 48 h in the Rusitec was 14.63%, HF + Sh of treated hemp had a better performance reaching 25.22%.
Degradation of organic matter (%) after 48 h of incubation of fresh rapeseed straw (FRSt), aged rapeseed straw (ARSt), hemp straw (HSt), hemp fibers and shives (HF + Sh), flax shives (FSh), flax straw (FSt) and hay (H1 and H2). Different letters indicate significant differences among samples. Data are presented as mean + SD
The substrate composition within the analyzed substrates differed (Table 2). ARSt had the highest XA and lowest Xfi of all test substrates, FRSt had the second highest XA and second lowest Xfi. Therefore, XA and Xfi were the main components affected by the storage/harvesting time. The other four test substrates (HSt, HF + Sh, FSh, FSt) were quite similar in chemical composition. All of the test substrates analyzed had higher amounts of Xfi, ADF and NDF, compared to the control hays (H1, H2), demonstrating the recalcitrant properties of those substrates.
Despite similar ADF and NDF amounts, the degradation of those components varied among the substrates. FSh had a low Xfi, ADF and NDF degradation, while HF + Sh and FSt had a much higher degradation of those components (Table 3). None of the substrates reached a comparable degradation to the control hays.
Discussion
The RUSITEC system is an in vitro technology that has been considered as a valuable tool to investigate the potential of different substrates to be used in anaerobic fermentation for biogas production. The aim of the present study was to investigate the decomposition parameters of different crop residues, with and without mechanical pre-treatment, that may represent a potential second generation biomass for large-scale biogas production when combined with a new forestomach reactor. For this, AD parameters were evaluated for six different substrates: aged rapeseed straw, fresh rapeseed straw, hemp straw, hemp fibers + shives, flax straw and flax shives. Hay was used as control.
During the experiment, pH fluctuated between 6.8 and 7.2 for all samples. Ruminal pH is particularly driven by the amount of fermentable carbohydrates, which are converted to SCFA and decrease pH. The level of SCFAs indicates the efficiency of hydrolytic and acidogenic processes; therefore, higher SCFA concentrations reflect a greater amount of fermented substrate by rumen microorganisms [33, 34]. In the present study, HF + Sh had the lowest pH, highest SCFA production and OM degradation, demonstrating to be the most suitable substrate for AD by rumen microbiota. Similarly, FSt had the second best AD performance, being also a good alternative to be used for AD, followed by FRSt. On contrary, FSh had the highest pH of all samples, which was above the normal biological range for the rumen (5.6–7.0), the lowest SCFA production and the second lowest degradation of OM, therefore, demonstrating to be a poor substrate for AD. Likewise, ARSt and HSt also had pH values above the normal biological range, and low SCFA production and OM degradation. Within the different SCFA produced during fermentation, acetate proportions are a good indicator for ruminal methane production, since acetate production is associated with the release of H2, which can be used by hydrogenotrophic methanogens to form methane [31, 35]. Moreover, the produced acetate can further be used for methanogenesis by acetoclastic methanogens in a second-step reactor. In this context, the retted HF + Sh had the highest acetate and H2 production, significantly higher than the untreated HSt, therefore demonstrating a great potential for both first-step (rumen reactor) and second-step (e.g. UASB reactor) methane production.
The amount of previous data on fermentability of these substrates by ruminal microorganisms is limited and mostly restricted to digestibility data derived from feeding experiments or in situ degradation. Inclusion of hemp stems in an oaten chuff diet in sheep increased apparent digestibility of OM, CP, NDF and ADF and acetate and butyrate concentrations in the rumen [36], underlining the ability of the rumen microorganisms to degrade hemp straw effectively despite its high fiber content. For flax straw an organic matter digestibility of 33.9% was reported in an in vitro assay [37], while lambs were able to digest 49% of flax straw organic matter [38]. In a comparison of barley straw, canola straw, timothy and alfalfa hay, in situ NDF and ADF digestibility of canola straw reached about 27% after 48 h which was considerably lower than for the other substrates [39]. Although a quantitative comparison is not possible due to different experimental conditions, these reports indicate a better rumen fermentability of hemp straw and flax straw compared to rapeseed straw.
Anaerobic digestion of these substrates with manure or sludge microbiota has mainly been studied in batch cultures with methane production as main parameter (biomethane potential test, BMP). In these experiments hemp fibers performed better than shives, with untreated stem being intermediate [40]. Therefore, mixing the high hemicellulosic fibers with the lignin rich shives can significantly increase anaerobic digestion performance of shives residues, while it may still provide a better performance than the untreated straw once the hemicellulosic structures of fibers are much more exposed to microbial degradation after retting [41]. Here, the whole material was used (fiber content: 30.4%); an increase of the fiber proportion should further increase AD performance but would require further retting and again result in the accumulation of shives as a waste material. Flax and rapeseed straw did not reach the same methane production in BMP experiments compared to hemp [42,43,44,45]. However, in contrast to our study, where fresh rapeseed straw performed slightly worse than flax and aged rapeseed straw performed poor, rapeseed seemed to perform a bit better in the mentioned reports; however, as the absolute values varied from study to study and the inoculums for the batch cultures differed, this difference could also be linked to the inoculum.
The differences in AD performance of the substrates investigated in the present study can be correlated with their chemical properties. Despite similar proportions of fiber fractions between all test substrates, the degradation of those fractions varied. Again, HF + Sh had the highest Xfi and ADF degradation of all analyzed substrates, quite superior compared to the values obtained for the untreated straw, while FSh had the lowest degradation. The ADF is the least digestible plant components, including cellulose and lignin. Therefore, higher degradation of such fraction in HF + Sh might have been decisive for its better AD performance. In addition, the higher XP and ADF degradation of HF + Sh compared to the untreated straw corroborates the expectation that the physical treatment would result in a larger surface area exposed to microbial attachment, thus enhancing the AD process.
Structural components, such as lignin content, can also affect other important parameter for AD of the substrates, the NH3-N concentration. The NH3-N concentration is dependent on the degradation of crude protein and the synthesis of microbial biomass [46]. Therefore, the content of crude protein together with the arrangement of such structural components in the substrate might limit cell-wall protein degradation decreasing NH3-N production. In the present experiment, the control hay had the highest NH3-N concentration of all substrates, which can also be explained by its highest XP concentration and its high degradability. Within the tested substrates, rapeseed straw, both fresh and aged, had higher NH3-N concentration during AD, which is also in accordance with its high XP concentration, although information regarding the degradability of the protein could not be obtained. Nevertheless, except for H1, values varied between 2.47 and 4.67 mmol l−1, which is within the range reported to favor bacterial growth [33, 47]; therefore, none of the substrates tested in the present study had inhibition patterns that could negatively affect AD.
Conclusion
The main objective of the present work was to evaluate the potential of different crop residues for anaerobic digestion by rumen microorganisms to select suitable substrates to be further used as energy source for biogas production and repowering of existing agricultural biogas plants. A hemp fiber and shives mix obtained by retting exhibited the best anaerobic digestion performance, followed by flax straw. These substrates are candidates for further testing of methane production in an up-scaled two-stage biogas system.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Chandra R, Takeuchi H, Hasegawa T (2012) Methane production from lignocellulosic agricultural crop wastes: a review in context to second generation of biofuel production. Renew Sustain Energy Rev 16(3):1462–1476. https://doi.org/10.1016/j.rser.2011.11.035
IPCC (2022) Climate change 2022: mitigation of climate change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. [P.R. Shukla, J. Skea, R. Slade, A. Al Khourdajie, R. van Diemen, D. McCollum, M. Pathak, S. Some, P. Vyas, R. Fradera, M. Belkacemi, A. Hasija, G. Lisboa, S. Luz, J. Malley, (eds.)]. Cambridge, UK and New York, NY, USA. https://doi.org/10.1017/9781009157926
Prade T, Svensson S-E, Mattsson JE (2012) Energy balances for biogas and solid biofuel production from industrial hemp. Biomass Bioenerg 40:36–52. https://doi.org/10.1016/j.biombioe.2012.01.045
Malode SJ, Prabhu KK, Mascarenhas RJ, Shetti NP, Aminabhavi TM (2021) Recent advances and viability in biofuel production. Energy Convers Manag: X 10:100070. https://doi.org/10.1016/j.ecmx.2020.100070
Karatzos S, van Dyk JS, McMillan JD, Saddler J (2017) Drop-in biofuel production via conventional (lipid/fatty acid) and advanced (biomass) routes Part I. Biofuels, Bioprod Bioref 11(2):344–362. https://doi.org/10.1002/bbb.1746
Zucaro A, Fiorentino G, Ulgiati S (2020) Chapter 8—Constraints, impacts and benefits of lignocellulose conversion pathways to liquid biofuels and biochemicals. In: Yousuf A, Pirozzi 497 D, Sannino F (eds) Lignocellulosic biomass to liquid biofuels. Academic press, pp 249–282. https://doi.org/10.1016/B978-0-12-815936-1.00008-3
Muscat A, De Olde E, de Boer IJ, Ripoll-Bosch R (2020) The battle for biomass: a systematic review of food-feed-fuel competition. Glob Food Sec 25:100330. https://doi.org/10.1016/j.gfs.2019.100330
Meyer A, Ehimen E, Holm-Nielsen J (2018) Future European biogas: animal manure, straw and grass potentials for a sustainable European biogas production. Biomass Bioenerg 111:154–164. https://doi.org/10.1016/j.biombioe.2017.05.013
Vu HP, Nguyen LN, Vu MT, Johir MAH, McLaughlan R, Nghiem LD (2020) A comprehensive review on the framework to valorise lignocellulosic biomass as biorefinery feedstocks. Sci Total Environ 743:140630. https://doi.org/10.1016/j.scitotenv.2020.140630
Kamperidou V, Terzopoulou P (2021) Anaerobic digestion of lignocellulosic waste materials. Sustainability 13(22):12810. https://doi.org/10.3390/su132212810
Shankar S (2017) Renewable and nonrenewable energy resources: bioenergy and biofuels. In: Singh R (eds) Principles and applications of environmental biotechnology for a sustainable future. 293–314. https://doi.org/10.1007/978-981-10-1866-4_9
Karki R, Chuenchart W, Surendra KC, Shrestha S, Raskin L, Sung S, Hashimoto A, Kumar Khanal S (2021) Anaerobic co-digestion: current status and perspectives. Bioresour Technol 330:125001. https://doi.org/10.1016/j.biortech.2021.125001
Sawatdeenarunat C, Surendra K, Takara D, Oechsner H, Khanal SK (2015) Anaerobic digestion of lignocellulosic biomass: challenges and opportunities. Bioresour Technol 178:178–186. https://doi.org/10.1016/j.biortech.2014.09.103
Croce S, Wei Q, D’Imporzano G, Dong R, Adani F (2016) Anaerobic digestion of straw and corn stover: the effect of biological process optimization and pre-treatment on total bio-methane yield and energy performance. Biotechnol Adv 34(8):1289–1304. https://doi.org/10.1016/j.biotechadv.2016.09.004
Kim D (2018) Physico-chemical conversion of lignocellulose: inhibitor effects and detoxification strategies: a mini review. Molecules 23(2):309. https://doi.org/10.3390/molecules23020309
Ariunbaatar J, Panico A, Esposito G, Pirozzi F, Lens PNL (2014) Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl Energy 123:143–156. https://doi.org/10.1016/j.apenergy.2014.02.035
Stopp P (2016) Biogasproduktion im technischen Maßstab basierend auf dem Vormagensystem der Wiederkäuer. Dissertation, Leibniz University Hannover (Germany)
Bhujbal SK, Ghosh P, Vijay VK, Rathour R, Kumar M, Singh L, Kapley A (2022) Biotechnological potential of rumen microbiota for sustainable bioconversion of lignocellulosic waste to biofuels and value-added products. Sci Total Environ 814:152773. https://doi.org/10.1016/j.scitotenv.2021.152773
Liang J, Nabi M, Zhang P, Zhang G, Cai Y, Wang Q, Zhou Z, Ding Y (2020) Promising biological conversion of lignocellulosic biomass to renewable energy with rumen microorganisms: a comprehensive review. Renew Sustain Energy Rev 134:110335. https://doi.org/10.1016/j.rser.2020.110335
Bayané A, Guiot SR (2011) Animal digestive strategies versus anaerobic digestion bioprocesses for biogas production from lignocellulosic biomass. Rev Environ Sci Biotechnol 10(1):43–62. https://doi.org/10.1007/s11157-010-9209-4
Comtet-Marre S, Parisot N, Lepercq P, Chaucheyras-Durand F, Mosoni P, Peyretaillade E, Bayat AR, Shingfield KJ, Peyret P, Forano E (2017) Metatranscriptomics reveals the active bacterial and eukaryotic fibrolytic communities in the rumen of dairy cow fed a mixed diet. Front Microbiol 8:67. https://doi.org/10.3389/fmicb.2017.00067
Nagler M, Kozjek K, Etemadi M, Insam H, Podmirseg SM (2019) Simple yet effective: microbial and biotechnological benefits of rumen liquid addition to lignocellulose-degrading biogas plants. J Biotechnol 300:1–10. https://doi.org/10.1016/j.jbiotec.2019.05.004
Hales K, Lourenco J, Seidel DS, Koyun OY, Davis D, Welch C, Wells JE, Callaway TR (2020) The use of feedlot/cereal grains in improving feed efficiency and reducing by-products such as methane in ruminants. In: Improving rumen function. Burleigh Dodds Science Publishing, pp 693–726. https://doi.org/10.19103/AS.2020.0067.23
Morgavi D, Forano E, Martin C, Newbold CJ (2010) Microbial ecosystem and methanogenesis in ruminants. Animal 4(7):1024–1036. https://doi.org/10.1017/S1751731110000546
Caruso MC, Braghieri A, Capece A, Napolitano F, Romano P, Galgano F, Altieri G, Genovese F (2019) Recent updates on the use of agro-food waste for biogas production. Appl Sci 9(6):1217. https://doi.org/10.3390/app9061217
Stopp P, Weichgrebe D, Rosenwinkel K, Strecker M, Breves G, GbR AC (2009) DAUMEN-Energy “Design for separation and augmented methanisation of fibres substrates–contribution to sustainable biogas production. “In: Bayerische Landesanstaltung für Landwirtschaft (ed) Internationale Wissenschaftstagung Biogas Science. Volume 1, DS-Druck, Freising, pp 151–161
Weichgrebe D (2016) Nutzung des Vormagensystems der Wiederkäuer zur Erschliessung cellulosebasierter Substrate (cbS) als Energieträger zur Biogasproduktion: öffentlicher Schlussbericht zum Vorhaben: Berichtszeitraum: 01.09. 2012 bis 31.12. 2015. Leibniz Universität Hannover, Institut für Siedlungswasserwirtschaft und Tierärztliche Hochschule Hannover, Institut für Physiologie. https://www.fnr.de/ftp/pdf/berichte/22025211.pdf. Accessed 27th April 2023
Czerkawski JW, Breckenridge G (1977) Design and development of a long-term rumen simulation technique (Rusitec). Br J Nutr 38(3):371–384. https://doi.org/10.1079/bjn19770102
Wetzels SU, Eger M, Burmester M, Kreienbrock L, Abdulmawjood A, Pinior B, Wagner M, Breves G, Mann E (2018) The application of rumen simulation technique (RUSITEC) for studying dynamics of the bacterial community and metabolome in rumen fluid and the effects of a challenge with Clostridium perfringens. PLoS One 13(2):e0192256. https://doi.org/10.1371/journal.pone.0192256
Riede S, Boguhn J, Breves G (2013) Studies on potential effects of fumaric acid on rumen microbial fermentation, methane production and microbial community. Arch Anim Nutr 67(5):368–380. https://doi.org/10.1080/1745039x.2013.830518
Wang M, Sun X, Janssen P, Tang S, Tan Z (2014) Responses of methane production and fermentation pathways to the increased dissolved hydrogen concentration generated by eight substrates in in vitro ruminal cultures. Anim Feed Sci Technol 194:1–11. https://doi.org/10.1016/j.anifeedsci.2014.04.012
Demeyer D (1991) Quantitative aspects of microbial metabolism in the rumen and hindgut. In: Jouany JP (ed) Rumen microbial metabolism and ruminant digestion, Editions INRA, Paris, pp 217–237
Ebeid HM, Hassan F-u, Li M, Peng L, Peng K, Liang X, Yang C (2020) Camelina sativa L. oil mitigates enteric in vitro methane production, modulates ruminal fermentation, and ruminal bacterial diversity in buffaloes. Front Vet Sci 7:550. https://doi.org/10.3389/fvets.2020.00550
Purba RAP, Paengkoum S, Yuangklang C, Paengkoum P (2020) Flavonoids and their aromatic derivatives in Piper betle powder promote in vitro methane mitigation in a variety of diets. Ciênc Agrotec 44. https://doi.org/10.1590/1413-7054202044012420
Janssen PH (2010) Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim Feed Sci Technol 160(1–2):1–22. https://doi.org/10.1016/j.anifeedsci.2010.07.002
Krebs GL, De Rosa DW, White DM, Blake BL, Dods KC, May CD, Tai ZX, Clayton EH, Lynch EE (2021) Intake, nutrient digestibility, rumen parameters, growth rate, carcase characteristics and cannabinoid residues of sheep fed pelleted rations containing hemp (Cannabis sativa L.) stubble. Transl Anim Sci 5(4):txab213. https://doi.org/10.1093/tas/txab213
Mann M, Cohen R, Kernan J, Nicholson H, Christensen D, Smart M (1988) The feeding value of ammoniated flax straw, wheat straw and wheat chaff for beef cattle. Anim Feed Sci Technol 21(1):57–66. https://doi.org/10.1016/0377-8401(88)90019-3
Howard M, Cohen R, Kernan J (1991) Effects of ammoniation and supplementation with sweet clover hay on intake and digestibility of flax straw by sheep. Can J Anim Sci 71(2):599–602. https://doi.org/10.4141/cjas91-073
Griffith C, Ribeiro GO, Oba M, McAllister TA, Beauchemin KA (2017) Potential for improving fiber digestion in the rumen of cattle (Bos taurus) through microbial inoculation from bison (Bison bison): In situ fiber degradation. J Anim Sci 95(5):2156–2167. https://doi.org/10.2527/jas.2017.1403
Matassa S, Esposito G, Pirozzi F, Papirio S (2020) Exploring the biomethane potential of different industrial hemp (Cannabis sativa L.) biomass residues. Energies 13(13):3361. https://doi.org/10.3390/en13133361
Easson DL, Molloy R (1996) Retting—a key process in the production of high value fibre from flax. Outlook Agric 25(4):235–242. https://doi.org/10.1177/003072709602500405
Böske J, Wirth B, Garlipp F, Mumme J, Van den Weghe H (2014) Anaerobic digestion of horse dung mixed with different bedding materials in an upflow solid-state (UASS) reactor at mesophilic conditions. Bioresour Technol 158:111–118. https://doi.org/10.1016/j.biortech.2014.02.034
Elsayed M, Andres Y, Blel W (2022) Anaerobic co-digestion of linen, sugar beet pulp, and wheat straw with cow manure: effects of mixing ratio and transient change of co-substrate. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-021-02229-8
Gaballah ES, Abomohra AE-F, Xu C, Elsayed M, Abdelkader TK, Lin J, Yuan Q (2020) Enhancement of biogas production from rape straw using different co-pretreatment techniques and anaerobic co-digestion with cattle manure. Bioresour Technol 309:123311. https://doi.org/10.1016/j.biortech.2020.123311
Vivekanand V, Ryden P, Horn SJ, Tapp HS, Wellner N, Eijsink VG, Waldron KW (2012) Impact of steam explosion on biogas production from rape straw in relation to changes in chemical composition. Bioresour Technol 123:608–615. https://doi.org/10.1016/j.biortech.2012.06.088
Bach A, Calsamiglia S, Stern MD (2005) Nitrogen metabolism in the rumen. J Dairy Sci 88:E9–E21. https://doi.org/10.3168/jds.S0022-0302(05)73133-7
Terry SA, Ramos AF, Holman DB, McAllister TA, Breves G, Chaves AV (2018) Humic substances alter ammonia production and the microbial populations within a RUSITEC fed a mixed hay–concentrate diet. Front Microbiol 9:1410. https://doi.org/10.3389/fmicb.2018.01410
Acknowledgements
The authors would like to thank Janine Beiche for her help in performing the Rusitec experiments and Marion Burmester for her help in performing the Rusitec experiments and for performing laboratory analyses. We would like to thank Prof. Gerhard Breves who majorly contributed to the development of the DAUMEN system.
Funding
Open Access funding enabled and organized by Projekt DEAL. The presented research is part of the joint project DAUMEN 3.0, with the Institute for Sanitary Engineering and Waste Management of the Leibniz Universität Hannover and the Institute for Physiology and Cell Biology of the University of Veterinary Medicine Hannover and was supported by the German Federal Ministry of Food and Agriculture (BMEL) and Fachagentur Nachwachsende Rohstoffe e. V. (FNR). The funding supported the participation of LI (Grant No. 2220NR035A) and AF (Grant No. 2220NR035B) in the project.
Author information
Authors and Affiliations
Contributions
MB, DW and LI developed the concept of the project and designed the experiments. JH and LR performed the experiments and acquired and analyzed the data, AF evaluated the data and wrote the manuscript. MB revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Figueiredo, A.F., Brede, M., Heller, J. et al. Anaerobic Digestion of Hemp and Flax Straw and Shives and Rapeseed Straw by the Ruminal Microbiota. Bioenerg. Res. 17, 700–709 (2024). https://doi.org/10.1007/s12155-023-10667-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-023-10667-7