Abstract
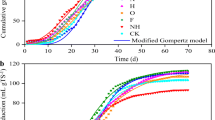
The anaerobic digestion efficiency and methane production of straw was limited by its complex composition and structure. In this study, rice straw (RS), cellulose, and hemicellulose were used as raw materials to study biogas production performance and changes in the volatile fatty acids (VFAs). Further, microbial communities and genetic functions were analyzed separately for each material. The biogas production potential of RS, cellulose, and hemicellulose was different, with cumulative biogas production of 283.75, 412.50, and 620.64 mL/(g·VS), respectively. The methane content of the biogas produced from cellulose and hemicellulose was approximately 10% higher than that produced from RS after the methane content stabilized. The accumulation of VFAs occurred in the early stage of anaerobic digestion in all materials, and the cumulative amount of VFAs in both cellulose and hemicellulose was relatively higher than that in RS, and the accumulation time was 12 and 14 days longer, respectively. When anaerobic digestion progressed to a stable stage, Clostridium was the dominant bacterial genus in all three anaerobic digestion systems, and the abundance of Ruminofilibacter was higher during anaerobic digestion of RS. Genetically, anaerobic digestion of all raw materials proceeded mainly via aceticlastic methanogenesis, with similar functional components. The different performance of anaerobic digestion of RS, cellulose, and hemicellulose mainly comes from the difference of composition of raw materials. Increasing the accessibility of cellulose and hemicellulose in RS feedstock by pretreatment is an effective way to improve the efficiency of anaerobic digestion. Since the similar microbial community structure will be acclimated during anaerobic digestion, there is no need to adjust the initial inoculum when the accessibility of cellulose and hemicellulose changes.







Similar content being viewed by others
Data Availability
All data of biogas and VFAs generated or analyzed during this study are included in this manuscript. The raw reads of 16 s RNA were deposited into the NCBI Sequence Read Archive (SRA) database (Accession Number: SRR12131890). The raw reads of metagenome were deposited into the NCBI Sequence Read Archive (SRA) database (Accession Number: SRPSRR12132972).
Abbreviations
- AD:
-
Anaerobic digestion
- RS:
-
Rice straw
- TS:
-
Total solids
- VS:
-
Volatile solids
- TC:
-
Total carbon
- TN:
-
Total nitrogen
- VFAs:
-
Volatile fatty acids
- TCD:
-
Thermal conductivity detector
- FID:
-
Flame ionization detector
- STAMP:
-
Statistical Analysis of Metagenomic Profiles
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- OTUs:
-
Operational taxonomic units
References
Li, K., Liu, R., Cui, S., Yu, Q., & Ma, R. (2018). Anaerobic co-digestion of animal manures with corn stover or apple pulp for enhanced biogas production. Renewable Energy, 118, 335–342.
Li, J., Wachemo, A. C., Yu, G., & Li, X. (2019). Enhanced anaerobic digestion performance of corn stalk pretreated with freezing-thawing and ammonia: An experimental and theoretical study. Journal of Cleaner Production, 247, 119112.
Liu, Y., Wachemo, A. C., Yuan, H., & Li, X. (2019). Anaerobic digestion performance and microbial community structure of corn stover in three-stage continuously stirred tank reactors. Bioresource Technology, 287, 121339.
McKendry, P. (2002). Energy production from biomass (part 1) overview of biomass. Bioresource Technology, 83, 37–46.
Houfani, A. A., Anders, N., Spiess, A. C., Baldrian, P., & Benallaoua, S. (2020). Insights from enzymatic degradation of cellulose and hemicellulose to fermentable sugars-a review. Biomass and Bioenergy, 134, 10581.
Behera, S., Arora, R., Nandhagopal, N., & Kumar, S. (2014). Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renewable and Sustainable Energy Reviews, 36, 91–106.
Jeffrey, G. A., & Saenger, W. (1994). Hydrogen binding in biological structure second printing. Springer.
Xiao, B., Sun, X. F., & Sun, R. (2001). Chemical, structural, and thermal characterizations of alkali-soluble lignins and hemicelluloses, and cellulose from maize stems, rye straw, and rice straw. Polymer Degradation and Stability, 74, 307–319.
Gao, L., Chen, S., & Zhang, D. (2017). Advances in modifying lignin structures for largely enhancing high-lignin biomass saccharification. Process Biochemistry, 57, 175–180.
Li, W., Khalid, H., Zhu, Z., Zhang, R., Liu, G., Chen, C., & Thorin, E. (2018). Methane production through anaerobic digestion: Participation and digestion characteristics of cellulose, hemicellulose and lignin. Applied Energy, 226, 1219–1228.
Lai, C., Tu, M., Xia, C., Shi, Z., Sun, S., Yong, Q., & Yu, S. (2017). Lignin alkylation enhances enzymatic hydrolysis of lignocellulosic biomass. Energy & Fuels, 31(11), 12317–12326.
Hjorth, M., Gränitz, K., Adamsen, A. P. S., & Møller, H. B. (2011). Extrusion as a pretreatment to increase biogas production. Bioresource Technology, 102(8), 4989–4994.
Liu, C. M., Wachemo, A. C., Yuan, H. R., Zou, D. X., Liu, Y. P., Zhang, L., Pang, Y. Z., & Li, X. J. (2018). Evaluation of methane yield using acidogenic effluent of NaOH pretreated corn stover in anaerobic digestion. Renewable Energy, 116, 224–233.
Li, L., Peng, X., Wang, X., & Wu, D. (2018). Anaerobic digestion of food waste: A review focusing on process stability. Bioresource Technology, 248(Pt A), 20–28.
Boe, K., Batstone, D. J., Steyer, J.-P., & Angelidaki, I. (2010). State indicators for monitoring the anaerobic digestion process. Water Research, 44(20), 5973–5980.
Chen, D., Zuo, X., Li, J., Wang, X., & Liu, J. (2020). Carbon migration and metagenomic characteristics during anaerobic digestion of rice straw. Biotechnology for Biofuels, 13, 130.
Cai, Y., Hua, B., Gao, L., Hu, Y., Yuan, X., Cui, Z., Zhu, W., & Wang, X. (2017). Effects of adding trace elements on rice straw anaerobic mono-digestion: Focus on changes in microbial communities using high-throughput sequencing. Bioresource Technology, 239, 454–463.
Azman, S., Khadem, A. F., Lier, J. B. V., Zeeman, G., & Plugge, C. M. (2015). Presence and role of anaerobic hydrolytic microbes in conversion of lignocellulosic biomass for biogas production. Critical Reviews in Environmental Science and Technology, 45(23), 2523–2564.
Meng, L., Xie, L., Kinh, C. T., Suenaga, T., Hori, T., Riya, S., Terada, A., & Hosomi, M. (2018). Influence of feedstock-to-inoculum ratio on performance and microbial community succession during solid-state thermophilic anaerobic co-digestion of pig urine and rice straw. Bioresource Technology, 252, 127–133.
Zhang, W., Li, L., Wang, X., Xing, W., Li, R., Yang, T., & Lv, D. (2020). Role of trace elements in anaerobic digestion of food waste: Process stability, recovery from volatile fatty acid inhibition and microbial community dynamics. Bioresource Technology, 315, 123796.
Xing, L., Yang, S., Yin, Q., Xie, S., Strong, P. J., & Wu, G. (2017). Effects of carbon source on methanogenic activities and pathways incorporating metagenomic analysis of microbial community. Bioresource Technology, 244(Pt 1), 982–988.
Guan, R., Li, X., Wachemo, A. C., Yuan, H., Zuo, X., & Gu, J. (2018). Enhancing anaerobic digestion performance and degradation of lignocellulosic components of rice straw by combined biological and chemical pretreatment. Science of the Total Environment, 637–638, 9–17.
Zuo, X., Yuan, H., Wachemo, A. C., Wang, X., Zhang, L., Li, J., Wen, H., Wang, J., & Li, X. (2019). The relationships among sCOD, VFAs, microbial community, and biogas production during anaerobic digestion of rice straw pretreated with ammonia. Chinese Journal of Chemical Engineering, 28(1), 286–292.
Nopharatana, A., Pullammanappallil, P. C., & Clarke, W. P. (2007). Kinetics and dynamic modelling of batch anaerobic digestion of municipal solid waste in a stirred reactor. Waste Management, 27(5), 595–603.
Frommhagen, M., Sforza, S., Westphal, A. H., Visser, J., Hinz, S. W. A., Koetsier, M. J., van Berkel, W. J. H., Gruppen, H., & Kabel, M. A. (2015). Discovery of the combined oxidative cleavage of plant xylan and cellulose by a new fungal polysaccharide monooxygenase. Biotechnology for Biofuels, 8, 101.
Datta, R., Kelkar, A., Baraniya, D., Molaei, A., Moulick, A., Meena, R. S., & Formanek, P. (2017). Enzymatic degradation of lignin in soil: A review. Sustainability, 9(7), 1163.
Feofilova, E. P., & Mysyakina, I. S. (2016). Lignin: Chemical structure, biodegradation, and practical application (a review). Applied Biochemistry and Microbiology, 52(6), 573–581.
Zhao, C., Yan, H., Liu, Y., Huang, Y., Zhang, R., Chen, C., & Liu, G. (2016). Bio-energy conversion performance, biodegradability, and kinetic analysis of different fruit residues during discontinuous anaerobic digestion. Waste Management, 52, 295–301.
Wang, X., Cheng, S., Li, Z., Men, Y., & Wu, J. (2020). Impacts of cellulase and amylase on enzymatic hydrolysis and methane production in the anaerobic digestion of corn straw. Sustainability, 12(13), 5453.
Rajagopal, R., Massé, D. I., & Singh, G. (2013). A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresource Technology, 143, 632–641.
Lin, J., Zuo, J., Gan, L., Li, P., Liu, F., Wang, K., Chen, L., & Gan, H. (2011). Effects of mixture ratio on anaerobic co-digestion with fruit and vegetable waste and food waste of China. Journal of Environmental Sciences, 23(8), 1403–1408.
Yuan, H., Guan, R., Wachemo, A. C., Zhu, C., Zou, D., Li, Y., Liu, Y., Zuo, X., & Li, X. (2019). Enhancing methane production of excess sludge and dewatered sludge with combined low frequency CaO-ultrasonic pretreatment. Bioresource Technology, 273, 425–430.
Lee, J., Kim, J. R., Jeong, S., Cho, J., & Kim, J. Y. (2017). Long-term performance of anaerobic digestion for crop residues containing heavy metals and response of microbial communities. Waste Management, 59, 498–507.
Ju, X., Engelhard, M., & Zhang, X. (2013). An advanced understanding of the specific effects of xylan and surface lignin contents on enzymatic hydrolysis of lignocellulosic biomass. Bioresource Technology, 132, 137–145.
Chen, S., Liu, G., Zhang, R., Qin, B., & Luo, Y. (2012). Development of the microbial electrolysis desalination and chemical-production cell for desalination as well as acid and alkali productions. Environmental Science and Technology, 46(4), 2467–2472.
Lay, J.-J., Li, Y.-Y., & Noike, T. (1997). Influences of pH and moisture content on themethane production in high-solids sludge digestion. Water Research, 31, 1518–1524.
Capson-Tojo, G., Ruiz, D., Rouez, M., Crest, M., Steyer, J.-P., Bernet, N., Delgenès, J.-P., & Escudié, R. (2017). Accumulation of propionic acid during consecutive batch anaerobic digestion of commercial food waste. Bioresource Technology, 245(Pt A), 724–733.
Pullammanappallil, P. C., Chynoweth, D. P., Lyberatos, G., & Svoronos, S. A. (2001). Stable performance of anaerobic digestion in the presence of a high concentration of propionic acid. Bioresource Technology, 78, 165–169.
Liu, C., Wachemo, A. C., Tong, H., Shi, S., Zhang, L., Yuan, H., & Li, X. (2018). Biogas production and microbial community properties during anaerobic digestion of corn stover at different temperatures. Bioresource Technology, 261, 93–103.
Song, L., Song, Y., Li, D., Liu, R., & Niu, Q. (2019). The auto fluorescence characteristics, specific activity, and microbial community structure in batch tests of mono-chicken manure digestion. Waste Management, 83, 57–67.
Zou, H., Chen, Y., Shi, J., Zhao, T., Yu, Q., Yu, S., Shi, D., Chai, H., Gu, L., He, Q., & Ai, H. (2018). Mesophilic anaerobic co-digestion of residual sludge with different lignocellulosic wastes in the batch digester. Bioresource Technology, 268, 371–381.
Feng, X., Wang, Y., Zubin, R., & Wang, F. (2019). Core metabolic features and hot origin of Bathyarchaeota. Engineering, 5(3), 498–504.
Lazar, C. S., Baker, B. J., Seitz, K., Hyde, A. S., Dick, G. J., Hinrichs, K. U., & Teske, A. P. (2016). Genomic evidence for distinct carbon substrate preferences and ecological niches of Bathyarchaeota in estuarine sediments. Environmental Microbiology, 18(4), 1200–1211.
Cheng, J., Li, H., Zhang, J., Ding, L., Ye, Q., & Lin, R. (2019). Enhanced dark hydrogen fermentation of Enterobacter aerogenes/HoxEFUYH with carbon cloth. International Journal of Hydrogen Energy, 44(7), 3560–3568.
Merlino, G., Rizzi, A., Schievano, A., Tenca, A., Scaglia, B., Oberti, R., Adani, F., & Daffonchio, D. (2013). Microbial community structure and dynamics in two-stage vs single-stage thermophilic anaerobic digestion of mixed swine slurry and market bio-waste. Water Research, 47(6), 1983–1995.
Cho, H. U., Kim, Y. M., & Park, J. M. (2018). Changes in microbial communities during volatile fatty acid production from cyanobacterial biomass harvested from a cyanobacterial bloom in a river. Chemosphere, 202, 306–311.
Abe, K., Ueki, A., Ohtaki, Y., Kaku, N., Watanabe, K., & Ueki, K. (2012). Anaerocella delicata gen. nov., sp. nov., a strictly anaerobic bacterium in the phylum Bacteroidetes isolated from a methanogenic reactor of cattle farms. Journal of General & Applied Microbiology, 58(6), 405–412.
Buhlmann, C. H., Mickan, B. S., Jenkins, S. N., Tait, S., Kahandawala, T. K. A., & Bahri, P. A. (2019). Ammonia stress on a resilient mesophilic anaerobic inoculum: Methane production, microbial community, and putative metabolic pathways. Bioresource Technology, 275, 70–77.
Smith, K. S., & Ingram-Smith, C. (2007). Methanosaeta, the forgotten methanogen? Trends in Microbiology, 15(4), 150–155.
Chellapandi, P., Bharathi, M., Sangavai, C., & Prathiviraj, R. (2018). Methanobacterium formicicum as a target rumen methanogen for the development of new methane mitigation interventions: A review. Veterinary and Animal Science, 6, 86–94.
Dridi, B., Fardeau, M. L., Ollivier, B., Raoult, D., & Drancourt, M. (2012). Methanomassiliicoccus luminyensis gen. nov., sp. nov., a methanogenic archaeon isolated from human faeces. International Journal of Systematic and Evolutionary Microbiology, 62(Pt 8), 1902–1907.
Li, N., He, J., Yan, H., Chen, S., & Dai, X. (2017). Pathways in bacterial and archaeal communities dictated by ammonium stress in a high solid anaerobic digester with dewatered sludge. Bioresource Technology, 241, 95–102.
Pore, S. D., Engineer, A., Dagar, S. S., & Dhakephalkar, P. K. (2019). Meta-omics based analyses of microbiome involved in biomethanation of rice straw in a thermophilic anaerobic bioreactor under optimized conditions. Bioresource Technology, 279, 25–33.
Conrad, R. (2020). Importance of hydrogenotrophic, aceticlastic and methylotrophic methanogenesis for methane production in terrestrial, aquatic and other anoxic environments: A mini review. Pedosphere, 30(1), 25–39.
Du, M., Liu, X., Wang, D., Yang, Q., Duan, A., Chen, H., Liu, Y., Wang, Q., & Ni, B. J. (2021). Understanding the fate and impact of capsaicin in anaerobic co-digestion of food waste and waste activated sludge. Water Research, 188, 116539.
Feng, K., Wang, Q., Li, H., Du, X., & Zhang, Y. (2021). Microbial mechanism of enhancing methane production from anaerobic digestion of food waste via phase separation and pH control. Journal of Environmental Management, 288, 112460.
Acknowledgements
Microbial community structure and metagenome analysis were performed using the free online platform of Majorbio Cloud Platform (www.majorbio.com).
Funding
The project was funded by the National Natural Science Foundation of China [21808010] and the National Key R&D Program of China [2018YFE0111000].
Author information
Authors and Affiliations
Contributions
Xiaoyu Zuo initiated the project and was responsible for coordinating the entire study and its funding. Ke Peng and Rui He completed most of the experiments. Jie Liu assisted in completing the experiment and analyzing the experimental data and wrote the manuscript. Luyao Yang and Rufei Liu modified the grammar of the article appropriately.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, J., Zuo, X., Peng, K. et al. Biogas and Volatile Fatty Acid Production During Anaerobic Digestion of Straw, Cellulose, and Hemicellulose with Analysis of Microbial Communities and Functions. Appl Biochem Biotechnol 194, 762–782 (2022). https://doi.org/10.1007/s12010-021-03675-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03675-w