Abstract
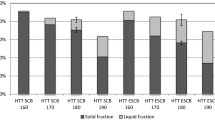
This paper describes an improved process for bioethanol production using a recently developed combined extrusion–saccharification technology. Blue agave bagasse (BAB) was pretreated via a thermo-mechano-chemical process (co-rotational twin-screw, reactive extrusion) to increase the availability of cellulose and hemicellulose for enzymatic saccharification. Then, several commercial enzyme preparations, boosted with accessory enzymes (exoglucanase, endoglucanase, hemicellulase, xylanase, and β-glucosidase), were tested with extruded BAB at 5 % consistency in a stirred vessel. The enzyme blend that produced the highest saccharification yield was evaluated at different BAB consistencies. The obtained concentration of sugars increased up to 69.5 g/L (73 % yield) when a 20 % BAB mixture was used. When the enzyme blend was fed into the extruder and with a residence time of 2 min, the yield reached 15 % of the maximum theoretical of C6 sugars along this step. This extruded and pre-saccharified BAB was further hydrolyzed and used for fermentation. The pre-saccharification step significantly enhanced cellulose degradation and ethanol production. Our results indicate that the enzymatic saccharification of BAB, coupled with reactive extrusion, produces an excellent substrate for bioethanol production.







Similar content being viewed by others
References
Yang B, Wyman CE (2008) Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod Biorefin 2:26–40
Saucedo-Luna J, Castro-Montoya AJ, Martínez-Pacheco MM, Sosa-Aguirre CR, Campos-García J (2011) Efficient chemical and enzymatic saccharification of the lignocellulosic residue from Agave tequilana bagasse to produce ethanol by Pichia caribbica. J Ind Microbiol Biotechnol 38:725–732
Vandenbossche V, Brault J, Vilarem G, Hernández- Meléndez O, Vivaldo-Lima E, Hernández-Luna M, Bárzana E, Duque A, Manzanares P, Ballesteros M, Mata J, Castellón E, Rigal L (2014) A new lignocellulosic biomass deconstruction process combining thermo-mechano-chemical action and bio-catalytic enzymatic hydrolysis in a twin-screw extruder. Ind Crop Prod 55:258–266
Singh R, Shukla A, Tiwari A, Srivastava M (2014) A review on delignification of lignocellulosic biomass for enhancement of ethanol production potential. Renew Sust Energ Rev 32:713–728
Hernández-Salas JM, Villa-Ramírez MS, Veloz-Rendón JS, Rivera-Hernández KN, González RA, Plascencia-Espinosa MA, Trejo-Estrada SR (2009) Comparative hydrolysis and fermentation of sugarcane and agave bagasse. Bioresour Technol 100:1238–1245
Perez-Pimienta JA, López-Ortega MG, Chavez-Carvayar JA, Varanasi P, Stavila V, Chen G, Singh S, Simmons BA (2015) Characterization of agave bagasse as a function of ionic liquid pretreatment. Biomass Bioenerg 75:180–188
Caspeta L, Caro-Bermúdez MA, Ponce-Noyola T, Martínez A (2014) Enzymatic hydrolysis at high-solids loading for the conversion of agave bagasse to fuel ethanol. Appl Energy 113:277–286
Maurya DP, Singla A (2015) An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech 5:597–609
Lee SH, Teramoto Y, Tanaka N, Endo T (2007) Improvement of enzymatic saccharification of woody biomass by nano-fibrillation using extruder. In: The 57th Annual Meeting of The Japan Wood Research Society
Pilli TD, Severini C, Baiano A, Derossi A, Arhaliass A, Legrand J (2005) Effects of operating conditions on oil loss and properties of products obtained by co-rotating twin-screw extrusion of fatty meal: preliminary study. J Food Eng 70:109–116
Thymi S, Krokida MK, Pappa A, Maroulis ZB (2005) Structural properties of extruded corn starch. J Food Eng 68:519–526
Mosier NS, Wyman CE, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96:673–686
Balat M, Balat H (2008) Progress in bioethanol processing. Prog Energy Combust Sci 34:551–573
Mosier NS, Ladisch CM, Ladisch M (2002) Characterization of acid catalytic domains for cellulose hydrolysis and glucose degradation. Biotechnol Bioeng 79:610–618
Rodrigues AM, Ostergaard Haven M, Lindedam J, Felby C, Gama M (2015) Celluclast and Cellic CTec2: saccharification/fermentation of wheat straw, solid–liquid partition and potential of enzyme recycling by alkaline washing. Enzym Microb Technol 79:70–77
Marcos M, García-Cubero MT, González-Benito G, Coca M, Bolado S, Lucas S (2013) Optimization of the enzymatic hydrolysis conditions of steam-exploded wheat straw for maximum glucose and xylose recovery. J Chem Technol Biotechnol 88:237–246
Kim HG, Son HJ, Jeong MJ, Sim SJ, Park DJ, Yang JK, Yoo SB, Yeo JK, Karigar CS, Choi MS (2011) Enzymatic saccharification of Salix viminalis cv. Q683 biomass for bioethanol production. J For Sci 27:143–149
Ovando LS, Waliszewski KN, Pardio VT (2005) The effect of hydration time and ethanol concentration on the rate of hydrolysis of extracted vanilla beans by commercial cellulase preparation. Int J Food Sci Technol 40:1011–1018
Cornejo-Mazón M, Hernández-Sanchez H, Gutiérrez-López GF, Dorantes-Alvarez L, Cortés-Sanchez AJ, Jiménez-Aparicio A, Gimeno-Seco M, Moreno A, Jaramillo-Flores ME (2012) Production and partial characterization of an exopolysaccharide from Ustilago maydis in submerged culture. Afr J Biotechnol 11:7079–7087
Lamsal B, Yoo J, Brijwani K, Alavi S (2010) Extrusion as a thermo-mechanical pre-treatment for lignocellulosic ethanol. Biomass Bioenerg 34:1703–1710
Segal L, Creely JJ, Martin AE, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29:786–794
Park S, Baker JO, Himmel ME, Parilla PA, Johnson DK (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels 3:1–10
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268
Bailey MJ, Biely P, Poutanen K (1992) Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol 23:257–270
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Zhu JY, Wang GS (2009) Specific surface to evaluate the efficiencies of milling and pretreatment of wood for enzymatic saccharification. Chem Eng Sci 64:474–485
Sun XF, Xu F, Sun RC, Fowler P, Baird MS (2005) Characteristics of degraded cellulose obtained from steam-exploded wheat straw. Carbohydr Res 340:97–106
Kumar R, Wyman CE (2009) Does change in accessibility with conversion depend on both the substrate and pretreatment technology? Bioresour Technol 100:4193–4202
Dinand E, Vignon M, Chanzy H, Heux L (2002) Mercerization of primary wall cellulose and its implication for the conversion of cellulose I to cellulose II. Cellulose 9:17–18
Gusakov AV, Salanovich TN, Antonov AI, Ustinov BB, Okunev ON, Burlingame R, Emalfarb M, Baez M, Sinitsyn AP (2007) Design of highly efficient cellulase mixtures for enzymatic hydrolysis of cellulose. Biotechnol Bioeng 1:1028–1038
Selig MJ, Knoshaug EP, Adney WS, Himmel ME, Decker SR (2008) Synergistic enhancement of cellobiohydrolase performance on pretreated corn stover by addition of xylanase and esterase activities. Bioresour Technol 99:4997–5005
Qin E (2010) High consistency enzymatic hydrolysis of lignocellulose. Dissertation, The University of British Columbia, Vancouver
Ioelovich M, Morag E (2012) Study of enzymatic hydrolysis of pretreated biomass at increased solids loading. Bioresources 7:4672–4682
Hodge DB, Karim MN, Schell DJ, MacMillan JD (2008) Soluble and insoluble solids contributions to high-solids enzymatic hydrolysis of lignocellulose. Bioresour Technol 99:8940–8948
Xiao Z, Zhang X, Gregg DJ, Saddler JN (2004) Effects of sugar inhibition on cellulases and beta-glucosidase during enzymatic hydrolysis of softwood substrates. Appl Biochem Biotechnol 115:1115–1126
Tengborg C, Galbe M, Zacchi G (2001) Reduced inhibition of enzymatic hydrolysis of steam-pretreated softwood. Enzym Microb Technol 28:835–838
Acknowledgments
This work was funded by the European Community, Seventh Framework Program, under grant agreement no. 227498 (BABETHANOL PROJECT). It is also part of a research project carried out through the Bioenergy Thematic Network (“Red Temática de Bioenergía”) with partial support from the Mexican Council for Science and Technology (CONACYT), grant no. 260457. The authors wish to thank Advanced Enzyme Technologies, Novozyme, Enmex, Genecore, and Lallemand for kindly supplying enzymes and yeast. We also thank PATRON Spirits Company and Consejo Regulador del Tequila (CRT) for the supply of raw BAB. Technical training and discussions with several members of the BABETHANOL team (INPT, CIEMAT, INSA, and VTT, mostly) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Montiel, C., Hernández-Meléndez, O., Vivaldo-Lima, E. et al. Enhanced Bioethanol Production from Blue Agave Bagasse in a Combined Extrusion–Saccharification Process. Bioenerg. Res. 9, 1005–1014 (2016). https://doi.org/10.1007/s12155-016-9747-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-016-9747-x