Abstract
The dissolution of biomass into ionic liquids (ILs) has been shown to be a promising alternative biomass pretreatment technology, facilitating faster breakdown of cellulose through the disruption of lignin and the decrystallization of cellulose. Both biological and chemical catalysis have been employed to enhance the conversion of IL-treated biomass polysaccharides into monomeric sugars. However, biomass-dissolving ILs, sugar monomers, and smaller carbohydrate oligomers are all soluble in water. This reduces the overall sugar content in the recovered solid biomass and complicates the recovery and recycle of the IL. Near-complete recovery of the IL and the holocellulose is essential for an IL-based pretreatment technology to be economically feasible. To address this, a solvent extraction technique, based on the chemical affinity of boronates such as phenylboronic acid and naphthalene-2-boronic acid for sugars, was applied to the extraction of glucose, xylose, and cellobiose from aqueous mixtures of 1-ethyl-3-methylimidazolium acetate. It was shown that boronate complexes could extract up to 90% of mono- and disaccharides from aqueous IL solutions, 100% IL systems, and hydrolysates of corn stover containing IL. The use of boronate complexes shows significant potential as a way to recover sugars at several stages in ionic liquid biomass pretreatment processes, delivering a concentrated solution of fermentable sugars, minimizing toxic byproducts, and facilitating ionic liquid cleanup and recycle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, there has been a renewed interest and an increased effort toward the development of biofuels made from lignocellulosic biomass derived from agricultural wastes, forest residues, and dedicated energy crops [6, 11, 13]. One of the largest limitations facing the overall economic viability of this process is the slow and incomplete hydrolysis of biomass by cellulolytic enzymes into its component sugars [2, 11]. This recalcitrance necessitates the use of a “pretreatment” step to enhance the accessibility of these enzymes to the carbohydrate complexes present in biomass [11]. Most pretreatments are thermochemical processes that use combinations of high temperatures and pressures, and dilute acids or alkalis, to alter the structure of the biomass and increase surface accessibility. This typically necessitates the use of specialized equipment and high-energy inputs [14, 21, 34].
Ionic liquids (ILs) have received attention as an innovative class of solvents for chemical processing [28, 29]. They are known as environmentally friendly solvents primarily due to their low volatility [10] and their potential to be recycled. Recently, ILs have shown great promise for use in the pretreatment of biomass, with several classes of ILs being able to dissolve crystalline cellulose and biomass under relatively mild conditions. The resulting polysaccharides can be readily hydrolyzed using cellulolytic enzymes [4, 16, 17]. The IL 1-ethyl-3-methylimidazolium acetate (Fig. 1), abbreviated as [C2mim][OAc], has been found to be one of the most promising candidates for biomass pretreatment [27, 35].
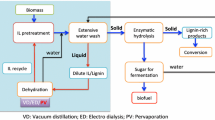
An IL pretreatment process typically involves the dissolution of biomass into the ionic liquid at an elevated temperature with stirring, followed by the addition of an anti-solvent that precipitates a fraction of the biomass from solution (Fig. 2). This precipitant can be water, ethanol, or a solvent with hydrogen bonding capacity that is added at volumetric ratios of approximately 3:1 precipitant/IL. Once the biomass has been precipitated, a solid–liquid separation is performed and the biomass is washed with water to remove any remaining IL prior to an enzymatic hydrolysis process step that yields a monosaccharide product stream suitable for fermentation. Washing is necessary as commercial enzyme mixtures are inhibited by residual IL [31].
The proposed IL deconstruction process contains two streams where the recovery of sugars from aqueous IL solutions would improve the overall process efficiency and help in the recycle of the IL (Fig. 2, stage 1 and stage 2). The most significant of these is the process stream resulting from washing the precipitated biomass (Fig. 2, stage B). The development of cellulases that are active in the presence of ILs [5, 15, 36] would enable the production of monomeric sugars without the need for solid–liquid separation and extensive washing after precipitant addition. This currently results in the dilution of the IL. As it is necessary to remove the precipitant from the wash stream so the IL can be reused, the reduction and possible elimination of washing would help avoid high-energy cost associated with distillation to recover the IL. Families of cellulases that are active at IL concentrations of up to 30% (v/v) have been recently reported [22, 31, 33], with some hyperthermophilic IL-tolerant enzymes exhibiting between 50% and 90% of their activity in the presence of 15% [C2mim][OAc] [5].
An alternative IL-based process, using chemical catalysis rather than enzymatic hydrolysis to break down cellulose, is a second avenue through which ionic liquids could be used to produce monomeric sugars (Fig. 3). Homogeneous catalysis, utilizing low concentrations (∼5%) of mineral acids such as HCl and H2SO4 in 1-butyl-3-methylimidazolium chloride ([C4mim]Cl) has been shown to effectively hydrolyze cellulose into its individual oligosaccharide components, with yields of up to 80% of the total reduced sugars [18, 19]. Sulfonate resins have also been used to catalyze the selective depolymerization of cellulose dissolved in [C4mim]Cl, producing lower degree of polymerization cellooligomers [23]. These chemical processes provide promising options for the hydrolysis and breakdown of lignocellulosic material into monosaccharides using the biomass-solvating capacity of ionic liquids.
Development of both IL-tolerant cellulases and chemical catalysis routes for the breakdown of biomass in ionic liquids would help increase the efficiency of the conversion of biomass into monosaccharides. However, both these processes rely on the removal of sugars from aqueous IL solution or from IL biomass liquor. Liquid–liquid extraction of sugars into organic phases has been used to recover up to 98% of sugars from aqueous solutions [30] and wood hydrolysates [12]. Through the formation of a complex with lipophilic–boronic acids, it is possible for sugars to pass into a water-immiscible organic phase consisting of a lipophilic quaternary alkyl amine salt (Aliquat 336™, Q+ in Scheme 2) and hexane [1, 12, 20, 30]. The mechanism of this complexation relies on the ionization of the boronic acid with OH− under basic conditions, resulting in the formation of a tetrahedral anion at the interface of the aqueous and organic phases (Scheme 1). The tetrahedral anion forms a complex with the cis-diols of pentose and hexose sugars (Scheme 2). For naphthalene-2-boronic acid (N2B), the most efficient removal of sugars occurs at pH levels between 11 and 12, above its pK a of ∼9. The negatively charged complex is then stabilized in the organic phase by the quaternary alkyl amine cation. The complexation reaction is reversible under acidic conditions and the sugar is recovered from the organic phase by stripping with a dilute acidic solution (Scheme 3). Figure 4 illustrates the overall process. In this study, the use of boronic acids to extract glucose, xylose, and cellobiose from aqueous IL solutions and from ionic liquid-only systems was investigated to determine the efficiency of sugar recovery in the presence of IL. Ionic liquid loss, and degradation was also investigated.
Formation of the N2B tetrahedral ion under basic conditions [1]
Tetrahedral boronate anion forms a complex with the cis-diol moiety of the carbohydrate. d-Glucose in the α-Furanose form is shown [1]. The Q+ is the quarternary amine Aliquat 336
Possible mechanism for the stripping of sugars from the tetrahedral boronate complex using HCl [1]
Materials and Experimental Methods
Ionic Liquid/Sugar Solutions
Ionic liquid/water solutions were prepared using specified volumes of 1-ethyl-3-methylimidazolium acetate (Sigma-Aldrich, BASF quality ≥90%) and 0.15 M NaHCO3 buffer, pH 11 (Mallinckrodt Chemicals, 99.7–100.3%). The 0.15 M NaHCO3 buffer was prepared by the dissolution of 1.26 g NaHCO3 buffer in 80 ml of water and adjustment of the pH to 11 with NaOH before adjusting the volume to 100 ml. The pH was measured using an ion-sensitive field effect transistor pH probe (IQ Scientific Instruments Inc., model IQ240). Experiments were conducted using 0% to 100% (v/v) [C2mim][OAc]/buffer containing 10 mM synthetic anhydrous d-glucose, xylose, or cellobiose (Sigma-Aldrich, >98%) in a total volume of 5 ml. Carbohydrate structures are shown in Fig. 5. For 100% IL systems, the correct mass of dry sugars was added. In addition to the 100% IL/sugar solution, the 100% IL/sugar solution was spiked with 5 µl of 10 N NaOH (VWR cat. no. VW3247-1) to test if additional hydroxide improved sugar extraction. The specified concentration of water in [C2mim][OAc] as received from the supplier was <0.2%, and so the addition of 5 μl of NaOH (0.1%) was treated as negligible. For all experimental trials in this study, the initial pH of the IL/water/sugar solutions was measured and buffered to pH 11–12.
Organic Phase
The organic phase consisted of 150 mM Aliquat 336™ (Sigma-Aldrich, ρ/ρ w = 0.884 g/cm3) and 70 mM boronic acid. Napthelene-2-boronic acid (Frontier Scientific, 97%, batch 14973) or phenylborinic acid (Fluka Analytical, ≥97%) were dissolved in a 85:15 (v/v) solution of n-hexane and 1-octanol.
Stripping Solution
An aqueous solution of 0.5 M hydrochloric acid was used to strip the sugars from the loaded organic solution.
Quantitation of Sugars
Sugar concentrations were measured using HPAEC with pulsed amperometric detection on a Dionex DX600 equipped with a Dionex Carbopac PA-20 analytical column (3 × 150 mm) and a Carbopac PA-20 guard column (3 × 30 mm; Dionex, Sunnyvale, CA, USA). Eluent flow rate was 0.4 ml/min and the temperature was 30°C. A gradient consisting of a 12-min elution with 14 mM NaOH followed by a 5-min ramp to 450 mM NaOH for 20 min, returning to the original NaOH concentration of 14 mM for 10 min prior to the next injection, was employed. External standards covering the range between 6.25 and 125 μM glucose, xylose, and cellobiose were used to generate calibration curves from which concentrations were determined. Figure 6 displays a typical chromatogram used to determine carbohydrate concentrations.
Preparation of Hydrolysate Solutions
Pretreatment
A total of 300 mg of corn stover (4.8% moisture content) was mixed with 9.7 g of [C2mim][OAc] (as received, <0.2% moisture specified) in a 30-ml test tube. The contents were stirred with magnetic stirring in an oil bath at 120°C. After 3 h, 20 ml of hot water was slowly added to the mixture with vortexing to precipitate the dissolved biomass. The resulting slurry was washed with 4 × 40 ml of water to remove the ionic liquid and resuspended in 50 mM sodium acetate buffer, pH 4.8.
Enzymatic Hydrolysis
A cellulase cocktail consisting of a Novozymes cellulase complex, NS50013 (70 FPU/g), and ß-glucosidase, NS50010 (250 CBU/g), was prepared according to the manufacturers instructions. An enzyme loading of 5% NS50013 and 0.5% NS50010 (wt enzyme/wt glucan) was added to the recovered biomass, and shaken at 250 rpm for 72 hours at 50°C. The hydrolysate was then filter-sterilized and stored at 4°C until used. The concentration of glucose was 200 mM.
Preparation of IL/hydrolysate Solutions
Solutions containing 6.8 and 20.4 mM glucose were prepared using the hydrolysate and spiked with 5%, 10%, and 15% (v/v) [C2mim][OAc] with the volume made up with 150 mM of the NaHCO3 buffer, pH 11.
Extraction and Stripping of Sugars
The extraction of sugars was adapted from the method of Griffin and Shu [12]. Extraction experiments were conducted separately for each sugar (glucose, xylose, and cellobiose) and for the IL/hydrolysate solution. Equal volumes of IL/water sugar solutions (0–100% IL) and organic phase were vigorously mixed at 1,400 rpm, 25°C, for 2 hours (Eppendorf Thermomixer). Tubes were then transferred to a centrifuge (Eppendorf centrifuge 5434) and spun at 13,000 rpm for 5 min to separate the two phases. Samples of the IL/water phase were then analyzed using high-performance anion exchange chromatography (HPAEC) to determine the quantity of sugars transferred into the organic phase. Positive controls of known initial starting sugar solutions (10 mM) were used to validate the experimental protocol, with <5% variation observed. Stripping sugar experiments were conducted by taking the loaded organic phase and vigorously mixing it with an equal volume of stripping solution (0.5 M HCl, mixing for 30 min and spinning down in centrifuge for 5 min, 13,000 rpm). Samples of the aqueous stripping solution were analyzed using HPAEC to determine the quantity of sugars recovered from the loaded organic phase. All experiments were performed at 25°C. All trials were conducted in triplicate. The percent extracted was defined as the percentage of sugar, on a molar basis, initially present in the IL or IL/water phase that is transferred into the organic phase:
Results and Discussion
Extraction of Sugars
The results of all extraction trials are presented in Figs. 7, 8, 9, 10, 11, 12, 13, and 14. Error bars represent one standard deviation above and below the mean. For all experiments in this study, the pH of the IL/water/sugar solution before and after extraction did not change. For both N2B and phenylboronic acid (PBA), a higher percentage in terms of extraction and recovery of glucose than xylose for IL/water solutions from 0% to 50% IL was observed (Figs. 7 and 8). Of the two boronic acids, N2B had a higher affinity for xylose than PBA (Fig. 10). For glucose, a clear trend was inconclusive in the region from 0% to 30% IL, but N2B had greater extraction percentages than PBA in 40% and 50% IL (Fig. 9). Negligible amounts of cellobiose were extracted by PBA or N2B in the 0% to 50% IL region (Table 1).
Phenylboronic acid (PBA) extraction of glucose and xylose from aqueous [C2mim][OAc] solutions. PBA concentration was 70 mM in organic phase and initial sugar concentration in the IL/water phase before extraction was 10 mM and at pH 11. All extraction trials in this study were at ambient temperature 25°C
Phenylboronic acid extraction of glucose, xylose, and cellobiose in 100% IL and 100% IL spiked with NaOH. The latter solution was spiked with NaOH by adding 5 μl 10 N NaOH to the 5-ml volume of the IL/sugar mixture before extraction. The specified concentration of water in [C2mim][OAc] as received from the supplier was <0.2%. The addition of 5 μl of NaOH represents approximately 0.1% increase in water content and is treated as negligible. PBA concentration was 70 mM in organic phase and initial sugar concentration in the IL phase before extraction was 10 mM
Extraction efficiency of glucose, xylose, and cellobiose in 100% [C2Mim][OAc] and 100% [C2Mim][OAc] spiked with 5 μl 10 N NaOH to the 5-ml volume of the IL/sugar mixture before extraction. Napthalene-2-boronic acid concentration was 70 mM in the organic phase and initial sugar concentration in the IL phase before extraction was 10 mM
Percentage extraction of glucose from pretreated corn stover hydrolysate in the presence of [C2mim][OAc]. Two different (6.8 and 20.4 mM) initial hydrolysate/glucose concentrations were tested. The organic phase consisted of 70 mM boronic acid and 150 mM Aliquat 336 in hexane/octanol (85:15). IL/hydrolysate solutions were at pH 11
For IL/water solutions of 60–90% IL, small amounts of white precipitate were observed. While not tested, this is most likely sodium bicarbonate precipitating at high salt concentrations. The extraction of the sugars using both N2B and PBA at these ionic liquid concentrations became highly variable, with negligible amounts of glucose, xylose, and cellobiose extracted (Figs. 7 and 8 and Table 1). Evaporation was considered and rejected as a potential source of this variability through the use of positive controls using known sugar concentrations (10 mM). Under these high salt loadings, it is unknown what the actual activity of [OH−] ions are and how they influence the association constant (K a-acid) of the tetrahedral boronate anion (Scheme 1) and the association constant of the tetrahedral anion and the sugar diols (K eq in Scheme 2). Literature suggests that upon the formation of boronate ester, the pK a decreases compared to the boronic acid itself, resulting in an overall decrease in the pH of the solution [32]. In our study, the pH was not observed to decrease, and it is unknown to what extent the ions in [C2mim][OAc] are interacting with [H+] or [OH−] in solution. These factors may be the source of the wide variation in the extent of extraction. Further investigation is needed to fully understand these results.
In aqueous solutions, it has been reported that while the binding constants of boronic acid–carbohydrate complexes are buffer-independent [3, 26], the stability of the boronate ester is pH- and solvent-dependent [24, 32]. Springsteen and Wang [26] reported association constants (K eq) in aqueous systems for d-glucose and d-xylose to be 4.6 and 14 M−1 respectively, at pH 7.4. K eq is a function of the two equilibrium constants (K eq-tet and K eq-trig) for the tetrahedral and trigonal forms of the boronic acid with the sugar diols. These authors also reported that K eq increased with increasing pH for the d-glucose complex with phosphate buffer. At pH values of 7.0, 7.4, 8.0, and 8.5, the K eq values were 2.0, 4.6, 7.2, and 11 M−1, respectively, and the optimal binding pH for the boronate ester was not always above the pK a of the boronate species (the authors reported that the binding constants were at a maximum around pH 7) [26]. K eq values were not reported for pH above 9. In our high IL concentration systems, how the [C2mim][OAc] species affect the binding affinity and equilibrium constants between the boronic acids and carbohydrates was undetermined and is the subject of further study.
The results obtained in 100% IL-sugar solutions were unexpected. In both 100% [C2mim][OAc] and spiked (addition of NaOH) 100% [C2mim][OAc], not only were all three sugars extracted to a significant amount (>60% extracted in PBA), cellobiose was also extracted to a greater extent than either xylose or glucose (over 80% in Figs. 11 and 12). According to Scheme 1, hydroxide ions are required for the conversion of boronates to the tetrahedral anion formation. However, in 100% IL solutions, no buffer was present to allow this transformation. Hence, the boronic acids alone, without conversion to the tetrahedral form, possibly formed a stable species with the sugars in the organic phase. In addition, 100% [C2mim][OAc] solutions that were spiked with NaOH showed higher degrees of extraction when using N2B (Fig. 12). While this may imply that the N2B is forming a tetrahedral anion with the addition of OH- to enhance the extraction of sugars, the same trend was not observed when extracting with PBA (Fig. 11). A more in-depth investigation of the structure of the boronic acid-sugar complex under this condition is required before conclusions can be drawn about the mechanism of extraction in this 100% IL case.
The possible degradation of the ionic liquid during the experiment was investigated, as it has been reported that the under basic conditions the acid-labile hydrogen on the C2 position of the imidazole is lost, resulting in the formation of N-heterocyclic carbenes. 1HMR and FTIR showed that no carbene product was formed under the extraction conditions (see Electronic supplementary material, Figs. S1–S4).
Extraction of Glucose from Pretreated Corn Stover
For both N2B and PBA systems, extraction of 80–90% of the original glucose contained in the hydrolysate was achieved (Fig. 13). Within experimental error, there was no impact on the extraction efficiency with increasing concentration of [C2mim][OAc] (5–15%, v/v). A more concentrated hydrolysate solution (20.4 mM glucose vs. 6.8 mM glucose) was extracted in parallel to test the capacity of the two extraction systems. The concentration of boronic acids was kept constant at 70 mM. As seen in Fig. 13, the percentage extraction may be dependent on the boronic acid concentration in the organic phase, with ~50% of the original 20.4 mM glucose in the hydrolysate being extracted, compared to the ~80% of the 6.8 mM glucose. While the maximum theoretical loading of sugars in the organic phase is unknown, this reduction in extraction efficiency implies that the 70 mM boronic acid system may be approaching its theoretical limit.
Recovery of Glucose from Corn Stover Hydrolysates
The recovery of glucose from corn stover hydrolysate samples reached up to 97%. Samples extracted with N2B showed the highest percent recovery compared to samples treated with PBA (where the percentage recovery is defined in Eq. 2 as the percentage of sugars in the organic phase that is transferred to the stripped solution). Samples treated with PBA had a decrease in percentage recovery with increasing IL content:
Alternatively, samples extracted with N2B recovered 90–97% of glucose from the loaded organic phase regardless of IL content (Fig. 14). Therefore, this stripping technique can provide a process to deliver fermentable sugars extracted from pretreated hydrolysate solutions efficiently.
Conclusion
The extraction of mono- and disaccharides in ionic liquid-based systems using boronic acids has been shown to be an effective technology to provide fermentable sugars from pretreated lignocellulosic biomass. This boronic acid extraction technology has not been previously employed with ionic liquid systems and offers the possibility of efficiently recovering C5 and C6 sugars from ionic liquids. The complete extraction of cellobiose in 100% IL is also encouraging. Furthermore, there is the possibility that boronic acid membrane-based systems could be applied to pretreated biomass in future processing applications [7–9, 25]. These results demonstrate the potential of this approach to develop an IL pretreatment process technology with high overall sugar yields.
References
Aziz H, Kamaruddin A, Bakar M (2008) Process optimization studies on solvent extraction with naphthalene-2-boronic acid ion-pairing with trioctylmethylammonium chloride in sugar purification using design of experiments. Sep Purif Technol 60(2):190–197
Chandra RP, Bura R, Mabee WE, Berlin A, Pan X, Saddler JN (2007) Substrate pretreatment: the key to effective enzymatic hydrolysis of lignocellulosics? Adv Biochem Eng/Biotechnol 108:67–93
Conner J, Bulgrin V (1967) Equilibria between borate ion and some polyols in aqueous solution. J Inorg Nucl Chem 29(8):1953
Dadi AP, Varanasi S, Schall CA (2006) Enhancement of cellulose saccharification kinetics using an ionic liquid pretreatment step. Biotechnol Bioeng 95(5):904–910
Datta S, Holmes B, Park JI, Chen Z, Dibble DC, Hadi M et al. (2010) Ionic liquid tolerant hyperthermophilic cellulases for biomass pretreatment and hydrolysis. Green Chem 12:338–345
Demirbas A (2008) Biofuels sources, biofuel policy, biofuel economy and global biofuel projections. Energy Convers Manage 49(8):2106–2116
Di Luccio M, Smith B, Kida T, Borges C, Alves T (2000) Separation of fructose from a mixture of sugars using supported liquid membranes. J Membr Sci 174(2):217–224
Duggan P (2004) Fructose-permeable liquid membranes containing boronic acid carriers. Aust J Chem 57(4):291–299
Duggan PJ, Offermann DA (2009) Remarkably selective saccharide recognition by solid-supported peptide boronic acids. Tetrahedron 65(1):109–114
Earle M, Seddon K (2000) Ionic liquids. Green solvents for the future. Pure Appl Chem 72(7):1391–1398
Galbe M, Zacchi G (2007) Pretreatment of lignocellulosic materials for efficient bioethanol production. Adv Biochem Eng Biot 108:41–65
Griffin GJ, Shu L (2004) Solvent extraction and purification of sugars from hemicellulose hydrolysates using boronic acid carriers. J Chem Technol Biotechnol 79(5):505–511
Himmel ME, Ding S-Y, Johnson DK, Adney WS, Nimlos MR, Brady JW et al. (2007) Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315(5813):804–807
Hu G, Heitmann JA, Rojas OJ (2008) Feedstock pretreatment strategies for producing ethanol from wood, bark, and forest residues. BioResources 3(1):270–294
Kamiya N, Matsushita Y, Hanaki M, Nakashima K, Narita M, Goto M et al. (2008) Enzymatic in situ saccharification of cellulose in aqueous-ionic liquid media. Biotechnol Lett 30(6):1037–1040
Kilpelainen I, Xie H, King A, Granstrom M, Heikkinen S, Argyropoulos DS (2007) Dissolution of wood in ionic liquids. J Agric Food Chem 55(22):9142–9148
Li C, Knierim B, Manisseri C, Arora R, Scheller HV, Auer M et al. (2010) Comparison of dilute acid and ionic liquid pretreatment of switchgrass: biomass recalcitrance, delignification and enzymatic saccharification. Bioresour Technol 101:4900–4906
Li C, Wang Q, Zhao ZK (2008) Acid in ionic liquid: an efficient system for hydrolysis of lignocellulose. Green Chem 10(2):177–182
Li C, Zhao ZK (2007) Efficient acid-catalyzed hydrolysis of cellulose in ionic liquid. Adv Synth Catal 349(11–12):1847–1850
Matsumoto M, Ueba K, Kondo K (2005) Separation of sugar by solvent extraction with phenylboronic acid and trioctylmethylammonium chloride. Sep Purif Technol 43(3):269–274
Mosier N (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96(6):673–686
Pottkämper J, Barthen P, Ilmberger N, Schwaneberg U, Schenk A, Schulte M et al. (2009) Applying metagenomics for the identification of bacterial cellulases that are stable in ionic liquids. Green Chem 11(7):957
Rinaldi R, Palkovits R, Schüth F (2008) Depolymerization of cellulose using solid catalysts in ionic liquids. Angew Chem Int Ed 47(42):8047–8050
Sienkiewicz PA, Roberts DC (1980) Chemical affinity systems—I: pH dependence of boronic acid–diol affinity in aqueous solution. J Inorg Nucl Chem 42(11):1559–1575
Smith B (1996) Liquid membrane transport using boronic acid carriers. Supramol Chem 7(1):55–60
Springsteen G, Wang B (2002) A detailed examination of boronic acid–diol complexation. Tetrahedron 58(26):5291–5300
Sun N, Rahman M, Qin Y, Maxim ML, Rodríguez H, Rogers RD (2009) Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chem 11(5):646
Swatloski R, Rogers R, Holbrey J (2004) Dissolution and processing of cellulose using ionic liquids. US Patent 6,824,599, 1 Jan 2004
Swatloski R, Spear S, Holbrey J, Rogers R (2002) Dissolution of cellose with ionic liquids. J Am Chem Soc 124(18):4974–4975
Takeuchi M, Koumoto K, Goto M, Shinkai S (1996) Efficient glucoside extraction mediated by a boronic acid with an intramolecular quaternary ammonium ion. Tetrahedron 52(40):12931–12940
Turner MB, Spear SK, Huddleston JG, Holbrey JD, Rogers RD (2003) Ionic liquid salt-induced inactivation and unfolding of cellulase from Trichoderma reesei. Green Chem 5(4):443
Van Duin M, Peters JA, Kieboom APG, Van Bekkum H (1985) Studies on borate esters. II: Structure and stability of borate esters of polyhydroxycarboxylates and related polyols in aqueous alkaline media as studied by 11BNMR. Tetrahedron 41(16):3411–3421
Voget S, Steele HL, Streit WR (2006) Characterization of a metagenome-derived halotolerant cellulase. J Biotechnol 126(1):26–36
Yang B, Wyman CE (2008) Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuels Bioproducts and Biorefining 2(1):26–40
Zavrel M, Bross D, Funke M, Büchs J, Spiess AC (2009) High-throughput screening for ionic liquids dissolving (ligno-)cellulose. Bioresour Technol 100(9):2580–2587
Zhao H, Baker GA, Song Z, Olubajo O, Crittle T, Peters D (2008) Designing enzyme-compatible ionic liquids that can dissolve carbohydrates. Green Chem 10(6):696–705
Acknowledgments
We would like to thank Ozgul Persil Cetinkol and Andreia Michelle Smith for their contribution to the supplementary information. This work was part of the DOE Joint BioEnergy Institute (http://www.jbei.org) supported by the US Department of Energy, Office of Science, Office of Biological and Environmental Research, through contract DE-AC02-05CH11231 between Lawrence Berkeley National Laboratory and the US Department of Energy.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 170 kb)
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Brennan, T.C.R., Datta, S., Blanch, H.W. et al. Recovery of Sugars from Ionic Liquid Biomass Liquor by Solvent Extraction. Bioenerg. Res. 3, 123–133 (2010). https://doi.org/10.1007/s12155-010-9091-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-010-9091-5