Abstract
Objective
Up to 60% of patients with metastatic, castration-resistant prostate cancer (mCRPC) treated with 177Lu prostate-specific membrane antigen (PSMA) radioligand therapy (RLT) achieves a partial biochemical response with a decrease of > 50% in prostate-specific antigen (PSA) levels. The remaining fractions, however, do not respond to RLT. The aim of this explorative analysis was to identify pre-therapeutic factors for the prediction of response.
Methods
46 patients [age = 68 years (50–87)] with mCRPC who consecutively underwent RLT with 177Lu PSMA [median applied activity = 6 GBq (2.9–6.2)] were included and analysed retrospectively. The association of different clinical and laboratory factors and parameters from pre-therapeutic 68Ga PSMA positron emission tomography (PET) with the outcome of RLT was tested (Fisher’s test). Outcome was defined as PSA changes 8 weeks after second RLT [partial response (PR), PSA decrease > 50%; progressive disease (PD), PSA increase ≥ 25%; stable disease (SD), others]. Significant predictive factors were combined in a predictive score.
Results
30% showed a post-treatment PR (median 73% PSA decrease), 35% SD (median 17% PSA decrease) and 35% PD (median 42% PSA increase). Significant predictors for PD were alkaline phosphatase (ALP) > 135 U/l (p = 0.002), PSA > 200 ng/ml (p = 0.036), and maximum standardized uptake value (SUVmax) of the “hottest lesion” in pre-therapeutic PET < 45 (p = 0.005). The predictive score including PSA, ALP and SUVmax could separate 2 distinct groups of patients: ≤ 2 predictive factors (19% PD) and 3 predictive factors (90% PD).
Conclusion
The presented predictive score allowed a pre-therapeutic estimate of the expected response to 2 cycles of RLT. As our study was retrospective, prospective trials are needed for validation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer has the highest incidence and the third highest cancer related mortality for men in Europe [1]. In the last decade, several treatment options for patients with metastatic, castration-resistant prostate cancer (mCRPC) have become established, including abiraterone, enzalutamide, docetaxel and cabazitaxel [2]. More recently, radioligand therapy (RLT) with 177Lu prostate-specific membrane antigen (PSMA) was developed and has shown promising results with regard to prostate-specific antigen (PSA) response rates and radiographic responses [3]. Retrospective as well as early prospective data confirmed the favourable safety profile of RLT [4, 5].
Although up to 60% of the patients showed some degree of response to RLT, some do not benefit from this treatment [4, 5]. The presence of visceral metastases, alkaline phosphatase (ALP) ≥ 220 U/L [5], high platelet count, a regular need for analgesics [6] or elevated lactate dehydrogenase [7] has been identified as a negative predictor for the outcome, whereas ALP < 220 U/L, a cumulative injected activity ≥ 18.8 GBq [8], albumin ≥ 38.6 g/L, aspartate transaminase (AST) ≤ 24 U/L, haemoglobin ≥ 10.4 g/dL, absence of liver metastases [9], and higher standardized uptake value (SUV) mean or max in positron emission tomography (PET) with PSMA ligands have been reported to be associated with favourable outcomes [10]. However, the predictive value of these factors varies significantly between studies.
The aim of this study was first, to identify predictive factors for the outcome of RLT with 177Lu PSMA considering laboratory parameters, medical history and imaging obtained before the first RLT and second, to derive a predictive score which can simplify the selection of patients who are or are not likely to benefit from RLT.
Materials and methods
This single-institution retrospective study was approved by the institutional ethics review board (EA2/177/17). All patients signed a written informed consent form for the study.
Patients
Between 06/2015 and 12/2018, 91 patients with mCRPC consecutively underwent RLT with 177Lu PSMA in the department of nuclear medicine of our university hospital.
Inclusion criteria were: (1) at least two cycles of RLT with 177Lu PSMA, (2) 68Ga PSMA positron emission tomography (PET)/computed tomography (CT) examination in the same department ≤ 8 weeks prior to first cycle of RLT and (3) complete patient records including follow-up for at least 2 months after second cycle RLT. 45 patients were excluded for the following reasons: PSMA-PET prior to RLT in other facilities or non-PET imaging with PSMA ligands (n = 43) and absence of follow-up after RLT (n = 2), Thus, the final study population included 46 patients.
Patients records were assessed for the medical history including age at the time point of RLT, time between first diagnosis and RLT, initial Gleason score, initial D’Amico risk classification [11], presence of distant metastases at the time of initial diagnosis, treatment before RLT and type of metastases at time of RLT. Baseline laboratory tests including liver, renal and hematologic function, electrolytes, PSA and testosterone levels were recorded. Imaging parameters from 68Ga PSMA PET/CT including maximum and mean standard uptake value (SUVmax and SUVmean) of the lesion with the highest tracer uptake and the visual tumor burden were also determined. Visual tumor burden was subdivided into three categories: low (number of focal lesions in PET/CT < 10), medium (number of lesions in PET/CT > 10 and < 30) and high (disseminated metastatic spread).
Treatment, imaging, laboratory and response assessment
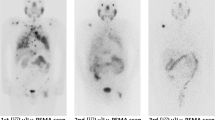
RLT with 177Lu PSMA and imaging with 68Ga PSMA PET/CT were performed according to consensus recommendations or as previously described [12, 13]. Laboratory parameters for liver, renal and hematologic function, electrolytes, PSA and testosterone levels were collected at each RLT cycle. In line with the German multicenter study for RLT with 177Lu PSMA [5], evaluation of the treatment response was based on the change in PSA levels between baseline at the first RLT cycle and two months after the second RLT cycle. Treatment response was defined according to Prostate Cancer Clinical Trials Working Group 2 and 3 as follows: partial response (PR) > 50% decline; progressive disease (PD) ≥ 25% increase; stable disease (SD) ≤ 50% decline and < 25% increase of PSA level [14, 15].
Statistical analysis
Statistical analyses were performed using SPSS 25 (IBM Corporation, Armonk, NY, USA). To evaluate the association between single factors and PD under RLT, receiver operating characteristic (ROC) curve analysis was performed for each factor. For factors with an area under the curve (AUC) > 0.65 or < 0.35 and a p value < 0.05, the optimal cut-off value was determined (Youden’s index) for binarization, and Fisher’s exact test for PD vs. SD/PR was performed. P values for each factor were corrected for multiple comparisons using the Benjamini–Hochberg procedure [16]. A p value < 0.05 was considered significant. The combined predictive score included all single factors with equal weighting that had shown significant association with PD in Fisher’s exact test. Additionally, all factors that were included in the predictive score, were included in a binary logistic regression analysis (PD vs. SD/PR) to examine their independent association with PD.
Results
Patient, treatment and response data
The median patient age was 68 years (range 50–87). The median PSA value before RLT was 79 ng/ml (range 4–3133). Patient characteristics including previous treatments and relevant baseline parameters are summarized in Table 1.
Overall, 117 treatment cycles were performed with a median activity of 6 GBq (range 2.9–6.2) 177Lu PSMA per cycle. 3 patients underwent four, 19 patients three and 24 patients underwent two RLT cycles.
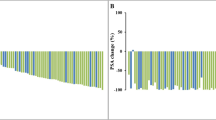
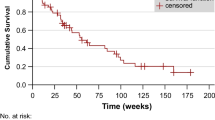
After the second RLT cycle, fourteen patients (30%) demonstrated PR with median PSA decrease of 73%, sixteen patients (35%) showed SD with median PSA decrease of 17%, and sixteen patients (35%) developed PD with median PSA increase of 42%. The median overall survival was 19.4 months (range 5.0–42.5) from the first RLT cycle.
Predictors of treatment response
Among all the analysed parameters, only PSA, ALP, SUVmax, SUVmean, therapy activity per RLT cycle and cumulative therapy activity fulfilled the predefined selection criteria as possible negative predictors. Results of the ROC analysis are summarized in Table 2. Although lower therapy activity per RLT cycle and lower cumulative therapy activity are significantly associated with PD in ROC curve analysis, neither parameter is included in the predictive score, since these parameters are unknown before the first RLT cycle and could therefore not be included as predictive factors into treatment decisions.
Table 3 shows the results of the Fisher’s exact test for PSA, ALP, SUVmax and SUVmean with their corresponding optimal cut-off values, sensitivity and specificity. All factors remained statistically significant after Benjamini–Hochberg adjustment. SUVmean was not considered in the predictive score because it did not show superior predictive value compared to the SUVmax while demonstrating lower inter-rater reliability due to the non-standardized measurement. Thus, the predictive score included baseline PSA > 200 ng/ml, ALP > 135 U/l, and SUVmax < 45, equally weighted.
Among 46 patients, 36 showed ≤ 2 predictive factors and 10 showed 3 predictive factors. Among the 36 patients with ≤ 2 predictive factors, 29 patients (81%) had SD or PR compared to 7 patients (19%) with PD. In 10 patients with 3 predictive factors, 1 patient (10%) showed SD or PR while 9 patients (90%) developed PD (Fisher’s exact test, p < 0.001).
In binary logistic regression including PSA > 200 ng/ml, ALP > 135 U/l and SUVmax < 45, only SUVmax < 45 was significantly associated with PD vs. SD/PR [odds ratio (OR) 5.66; 95% confidence interval (CI) 1.03–31.0; p = 0.046), while PSA > 200 ng/ml (OR 3.3; 95% CI 0.6–18.4; p = 0.17) and ALP > 135 U/l (OR 3.9; 95% CI 0 7–20.7; p = 0.11] were both not significant.
Discussion
To the best of our knowledge, although there have been several previous reports on prognostic or predictive factors for RLT with 177Lu PSMA in patients with mCRPC [5,6,7,8,9,10], this is the first simple clinical score to predict the outcome after the first two cycles of 177Lu PSMA. The score combines laboratory (PSA, ALP) and imaging (SUVmax) parameters and can identify both patients likely to benefit from RLT and potential non-responders to RLT.
MCRPC patients with higher PSA levels have a higher likelihood to develop PD under treatment than patients with lower PSA levels. Especially, a high baseline serum PSA level of > 200 ng/ml seems to be a significant predictor of an unfavorable outcome. This is consistent with the results of former studies showing that high serum PSA levels correlate strongly with the risk of prostate cancer progression, not only in the initial stage of disease, but also in advanced disease [17].
In the present study, a high ALP of > 135 U/l was also defined as a significant predictive factor for unfavorable outcome after RLT. This is in line with previous studies showing a poor outcome after RLT with 177Lu PSMA in patients with elevated ALP [5, 8, 18]. A similar outcome has been also reported for patients treated with chemotherapy [19]. ALP is a marker for bone turnover [19], which has led to the hypothesis that a high ALP correlates with progressive bone metastases. However, the presence of bone metastases was not a potential predictive parameter in the current analysis because almost every patient (44/46 patients) showed bone metastases, regardless of outcome. The reason why visual extent of disease did not correlate with outcome, is probably that the extent of disease could also be affected by lymph node metastases (present in 39/46 patients), which do not result in an increased ALP.
Low baseline SUVmax (< 45) in 68Ga PSMA PET also suggested an unfavorable outcome after RLT in the current study, which persisted in multivariable binary logistic regression after adjustment for PSA and ALP. The SUVmax/mean denotes the maximum/mean uptake in a specified region of interest (e.g., a lesion) and is the most commonly used semi-quantitative parameter in PET. This may be explained by the requirement for a targeted therapy of a minimum level of PSMA expression on the cell surface to achieve adequate lutetium internalization into the cell. The SUVmax of a radiotracer has a significant role in the diagnosis and treatment selection for different types of tumors, e.g. lymphoma [20], head and neck squamous cell carcinoma [21], pancreatic tumor [22], neuroendocrine tumors [23] or even prostate cancer [24]. A recent pilot study on 14 prostate cancer patients showed that patients with a PSA response ≥ 50% after treatment with 177Lu PSMA had a significantly higher baseline SUVmax in a pre-therapeutic scan with 68Ga PSMA-PET (44 vs. 17) [10]. This study also reported significantly higher SUVmean in patients with PSA response ≥ 50% (10 vs. 6) [10]. Likewise, SUVmean in 68Ga PSMA-PET was a significant predictive factor of response in the present analysis. However, it was not included in the combined predictive score because of its high inter-rater variability and limited reproducibility [25, 26]. The current study used the SUVmax of a single “hottest” lesion as a predictor of PSA response. SUVmax can be obtained intuitively, conveniently and with presumably high interobserver agreement up to 100% [25], because minimal observer interaction is required to obtain the highest SUVmax among potentially dozens of (confluent) lesions without necessitating a specific volume of interest. However, this method does not account for potential inter-lesional heterogeneity of PSMA expression or 68Ga PSMA uptake [27], respectively. One may note that in the most relevant patient group with low SUVmax (i.e., higher risk for PD), a low SUVmax in the “hottest” lesion is per se representative of all lesions because any other lesion in the same patient has—by definition—an even lower uptake. Furthermore, inter-lesional SUV heterogeneity in these patients is limited because “adequate uptake of PSMA ligands on the basis of a pre-therapy imaging study” in every lesion is demanded for eligibility of these patients for RLT according to current guidelines [28].
The practice of limiting assessment to a single lesion is in line with the PET Response Criteria in Solid Tumors [29] criteria, where only the “hottest single tumor lesion” is defined as the “target lesion”.
Not all potential imaging parameters were examined in our study, e.g. textural heterogeneity was recently postulated as a predictive factor in 177Lu PSMA treatment [30]. Our goal was however, to derive a predictive score that is simple and intuitive for clinical use. Hence, only parameters which are determined routinely and quickly before treatment were analyzed and included.
Clear limitations of this study are the retrospective design and small sample size, which may explain why predictive factors reported by other studies [5,6,7,8,9,10], e.g. blood count parameters or visceral metastases, were not significant in our analyses. Moreover, multivariable binary logistic regression did not prove the independent predictive value of PSA and ALP in addition to SUVmax—despite previous studies demonstrating their importance in the context of 177Lu PSMA treatment [5, 8, 9]. The observed odds ratios of approximately 3 to 4 and p values of < 0.2 in multivariable analysis for PSA and ALP may indicate that there is true independent value in both factors, but the power of the current multivariable analysis was insufficient to prove it, due to the small sample size. Consequently, our results are only hypothesis-generating and need to be confirmed in further, larger studies.
Conclusion
In this study, a score for easy clinical use to predict the treatment response to 2 cycles of RLT with 177Lu PSMA based on pretreatment PSA, ALP, and SUVmax of the “hottest lesion” in 68Ga PSMA-PET is presented, so that response rates of RLT can be increased and the rate of unnecessary adverse events can be reduced. This is a hypothesis-generating study, and further trials in independent, larger cohorts will be needed to test and validate this predictive score.
References
Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–87.
Crawford ED, Higano CS, Shore ND, Hussain M, Petrylak DP. Treating patients with metastatic castration resistant prostate cancer: a comprehensive review of available therapies. J Urol. 2015;194(6):1537–47.
Yadav MP, Ballal S, Bal C, Sahoo RK, Damle NA, Tripathi M, et al. Efficacy and safety of 177Lu-PSMA-617 radioligand therapy in metastatic castration-resistant prostate cancer patients. Clin Nucl Med. 2020;45(1):19–31.
Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19(6):825–33.
Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schafers M, Essler M, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58(1):85–90.
Ferdinandus J, Eppard E, Gaertner FC, Kurpig S, Fimmers R, Yordanova A, et al. Predictors of response to radioligand therapy of metastatic castrate-resistant prostate cancer with 177Lu-PSMA-617. J Nucl Med. 2017;58(2):312–9.
Heck MM, Tauber R, Schwaiger S, Retz M, D’Alessandria C, Maurer T, et al. Treatment outcome, toxicity, and predictive factors for radioligand therapy with (177)Lu-PSMA-I&T in metastatic castration-resistant prostate cancer. Eur Urol. 2018;75:920.
Rahbar K, Boegemann M, Yordanova A, Eveslage M, Schafers M, Essler M, et al. PSMA targeted radioligandtherapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur J Nucl Med Mol Imaging. 2018;45(1):12–9.
Ahmadzadehfar H, Schlolaut S, Fimmers R, Yordanova A, Hirzebruch S, Schlenkhoff C, et al. Predictors of overall survival in metastatic castration-resistant prostate cancer patients receiving [(177)Lu]Lu-PSMA-617 radioligand therapy. Oncotarget. 2017;8(61):103108–16.
Emmett L, Crumbaker M, Ho B, Willowson K, Eu P, Ratnayake L, et al. Results of a prospective phase 2 pilot trial of (177)Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer including imaging predictors of treatment response and patterns of progression. Clin Genitourin Cancer. 2019;17(1):15–22.
D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–74.
Fendler WP, Kratochwil C, Ahmadzadehfar H, Rahbar K, Baum RP, Schmidt M, et al. 177Lu-PSMA-617 therapy, dosimetry and follow-up in patients with metastatic castration-resistant prostate cancer. Nuklearmedizin. 2016;55(3):123–8.
Rogasch JM, Cash H, Zschaeck S, Elezkurtaj S, Brenner W, Hamm B, et al. Ga-68-PSMA PET/CT in treatment-naive patients with prostate cancer: which clinical parameters and risk stratification systems best predict PSMA-positive metastases? Prostate. 2018. https://doi.org/10.1002/pros.23685.
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer clinical trials working group. J Clin Oncol. 2008;26(7):1148–59.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34(12):1402–18.
Victor A, Elsasser A, Hommel G, Blettner M. Judging a plethora of p values: how to contend with the problem of multiple testing—part 10 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2010;107(4):50–6.
Stephan C, Ralla B, Jung K. Prostate-specific antigen and other serum and urine markers in prostate cancer. Biochim Biophys Acta. 2014;1846(1):99–112.
Brauer A, Grubert LS, Roll W, Schrader AJ, Schafers M, Bogemann M, et al. (177)Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44(10):1663–70.
Wajsman Z, Chu TM, Bross D, Saroff J, Murphy GP, Johnson DE, et al. Clinical significance of serum alkaline phosphatase isoenzyme levels in advanced prostatic carcinoma. J Urol. 1978;119(2):244–6.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–68.
Cacicedo J, Fernandez I, Del Hoyo O, Navarro A, Gomez-Iturriaga A, Pijoan JI, et al. Prognostic value of maximum standardized uptake value measured by pretreatment 18F-FDG PET/CT in locally advanced head and neck squamous cell carcinoma. Clin Transl Oncol. 2017;19(11):1337–49.
Pergolini I, Crippa S, Salgarello M, Belfiori G, Partelli S, Ruffo G, et al. SUVmax after (18)fluoro-deoxyglucose positron emission tomography/computed tomography: a tool to define treatment strategies in pancreatic cancer. Dig Liver Dis. 2018;50(1):84–90.
Kratochwil C, Stefanova M, Mavriopoulou E, Holland-Letz T, Dimitrakopoulou-Strauss A, Afshar-Oromieh A, et al. SUV of [68Ga]DOTATOC-PET/CT predicts response probability of PRRT in neuroendocrine tumors. Mol Imaging Biol. 2015;17(3):313–8.
Basha MAA, Hamed MAG, Hussein O, El-Diasty T, Abdelkhalek YI, Hussein YO, et al. (68)Ga-PSMA-11 PET/CT in newly diagnosed prostate cancer: diagnostic sensitivity and interobserver agreement. Abdom Radiol (NY). 2019;44(7):2545–56.
Huang YE, Chen CF, Huang YJ, Konda SD, Appelbaum DE, Pu Y. Interobserver variability among measurements of the maximum and mean standardized uptake values on (18)F-FDG PET/CT and measurements of tumor size on diagnostic CT in patients with pulmonary tumors. Acta Radiol. 2010;51(7):782–8.
Jauw YWS, Bensch F, Brouwers AH, Hoekstra OS, Zijlstra JM, Pieplenbosch S, et al. Interobserver reproducibility of tumor uptake quantification with (89)Zr-immuno-PET: a multicenter analysis. Eur J Nucl Med Mol Imaging. 2019;46(9):1840–9.
Mannweiler S, Amersdorfer P, Trajanoski S, Terrett JA, King D, Mehes G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol Oncol Res. 2009;15(2):167–72.
Kratochwil C, Fendler WP, Eiber M, Baum R, Bozkurt MF, Czernin J, et al. EANM procedure guidelines for radionuclide therapy with (177)Lu-labelled PSMA-ligands [(177)Lu-PSMA-RLT]. Eur J Nucl Med Mol Imaging. 2019;46(12):2536–44.
Jh O, Lodge MA, Wahl RL. Practical PERCIST: a simplified guide to PET response criteria in solid tumors 1.0. Radiology. 2016;280(2):576–84.
Khurshid Z, Ahmadzadehfar H, Gaertner FC, Papp L, Zsoter N, Essler M, et al. Role of textural heterogeneity parameters in patient selection for 177Lu-PSMA therapy via response prediction. Oncotarget. 2018;9(70):33312–21.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
No potential conflicts of interest relevant to this article exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, K., Schatka, I., Rogasch, J.M.M. et al. Explorative analysis of a score predicting the therapy response of patients with metastatic, castration resistant prostate cancer undergoing radioligand therapy with 177Lu-labeled prostate-specific membrane antigen. Ann Nucl Med 35, 314–320 (2021). https://doi.org/10.1007/s12149-020-01567-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-020-01567-3