Abstract
Purpose
In-vivo quantification of tumor uptake of 89-zirconium (89Zr)-labelled monoclonal antibodies (mAbs) with PET provides a potential tool in strategies to optimize tumor targeting and therapeutic efficacy. A specific challenge for 89Zr-immuno-PET is low tumor contrast. This is expected to result in interobserver variation in tumor delineation. Therefore, the aim of this study was to determine interobserver reproducibility of tumor uptake measures by tumor delineation on 89Zr-immuno-PET scans.
Methods
Data were obtained from previously published clinical studies performed with 89Zr-rituximab, 89Zr-cetuximab and 89Zr-trastuzumab. Tumor lesions on 89Zr-immuno-PET were identified as focal uptake exceeding local background by a nuclear medicine physician. Three observers independently manually delineated volumes of interest (VOI). Maximum, peak and mean standardized uptake values (SUVmax, SUVpeak and SUVmean) were used to quantify tumor uptake. Interobserver variability was expressed as the coefficient of variation (CoV). The performance of semi-automatic VOI delineation using 50% of background-corrected ACpeak was described.
Results
In total, 103 VOI were delineated (3–6 days post injection (D3-D6)). Tumor uptake (median, interquartile range) was 9.2 (5.2–12.6), 6.9 (4.0–9.6) and 5.5 (3.3–7.8) for SUVmax, SUVpeak and SUVmean. Interobserver variability was 0% (0–12), 0% (0–2) and 7% (5–14), respectively (n = 103). The success rate of the semi-automatic method was 45%. Inclusion of background was the main reason for failure of semi-automatic VOI.
Conclusions
This study shows that interobserver reproducibility of tumor uptake quantification on 89Zr-immuno-PET was excellent for SUVmax and SUVpeak using a standardized manual procedure for tumor segmentation. Semi-automatic delineation was not robust due to limited tumor contrast.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Therapy with monoclonal antibodies (mAbs) has greatly improved the outcome of cancer patients [1]. However, treatment failure due to the biology of the disease is a substantial problem. In addition to disease-related factors, therapy-related factors have been found to be responsible [2]. There is mainly information on pharmacokinetics in blood, whereas tumor targeting is crucial for mAb efficacy. Therefore, in-vivo quantification of antibody uptake in tumors is of interest in strategies to improve the efficacy of antibody treatment (e.g. using optimized pharmacokinetic models in early drug development to improve dosing schedules). PET imaging with zirconium-89 (89Zr)-labelled mAbs provides a non-invasive tool to visualize and quantify mAb tumor uptake [3], providing that biodistribution of the radiolabelled mAb represents that of the total mAb dose (radiolabelled and unlabelled). The number of clinical studies on 89Zr-labelled mAbs, also referred to as 89Zr-immuno-PET, increased in recent years [4]. Sources of measurement errors (including factors such as interobserver reproducibility of tumor uptake quantification and noise induced variability) should be known to define true biological differences. A standardized method of data acquisition and tumor uptake quantification forms the basis for obtaining experimental data that will allow such an understanding.
For quantification of tumor uptake, a volume of interest (VOI) is delineated. Subsequently, a tumor uptake measure is selected to characterize tumor uptake. Maximum (max) or peak standard uptake values (SUVmax and SUVpeak, respectively) provide information on a limited part of the tumor. Mean standardized uptake values (SUVmean) and total lesion uptake (TLU) serve to capture the entire lesion. In clinical studies, tumor uptake is quantified at a single (late) timepoint or at multiple timepoints. Additionally, quantification of tumor uptake at an early timepoint (D0) can be considered, for example, to estimate the blood volume fraction of the tumor.
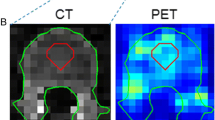
For imaging of mAbs, 89Zr is considered a suitable radioactive isotope due to its long half-life (t1/2 = 78.4 h), which matches the slow kinetics of large-sized proteins. Consequences of imaging with 89Zr are low positron abundance and relatively high radiation exposure, resulting in lower injected doses compared to 18F. Therefore, lower signal to noise ratios due to lower count rates may result in interobserver variability of tumor uptake quantification in 89Zr-immuno-PET. Other specific challenges for 89Zr-immuno-PET tumor delineation and quantification are relatively low, sometimes heterogeneous, tumor uptake (Fig. 1) and low (or even negative) contrast depending on tumor localization and background activity [5]. Therefore, the aim of this study was to determine interobserver reproducibility of tumor uptake values by manual delineation on 89Zr-immuno-PET.
Challenges for 89Zr-immuno-PET tumor delineation and quantification. Example of 18F-FDG-PET (a) for a patient with a non-Hodgkin lymphoma showing intense tumor uptake (black arrow) and excellent contrast, while 89Zr-immuno-PET (b) with 89Zr-labelled-rituximab shows limited contrast for this tumor. Red arrows indicate uptake in blood vessels. Example of tumor delineation by two observers (observer 1 = blue line, observer 2 = black line) for 18F-FDG-PET (c) and 89Zr-immuno-PET (d). This example illustrates that excellent interobserver reproducibility (SUVmax = 10 for both observers) can be expected for 18F-FDG-PET, despite variability in tumor delineation. The limited tumor contrast for 89Zr-immuno-PET may result in substantial interobserver variability, even for SUVmax (a value of 2 and 3 for observers 1 and 2, respectively)
Materials and methods
Data inclusion
For this retrospective study, 89Zr-immuno-PET scans with corresponding 18F-FDG-PET scans were collected. Data were selected from previously published clinical studies with therapeutic mAbs: 89Zr-rituximab in patients with B cell lymphoma ([6]; Dutch Trial Register NTR 3392), 89Zr-cetuximab in patients with colorectal cancer ([5]; NCT01691391) and 89Zr-trastuzumab in patients with breast cancer ([7]; NCT01691391). These studies had been approved by the ethics committees (Medisch Ethische Toetsingscommissie VUmc and Medisch Ethische Toetsingscommissie UMC Groningen) and all subjects signed an informed consent. Data acquisition and visual assessment of tumor uptake was done locally: from the first two studies performed at the VUmc all subjects with visible tumor uptake were included, from the last study performed at the UMCG seven subjects were selected randomly. Scan data at 1 h (D0), 72 h (D3) and 144 h (D6) post injection (p.i.) for 89Zr- labelled rituximab and cetuximab and at 96 h (D4) p.i. for 89Zr-trastuzumab were included. See Table 1 for patient characteristics and 89Zr-immuno-PET scan details. 89Zr-rituximab and 89Zr-cetuximab PET scans were performed on a Philips Gemini TF-64 or Ingenuity TF-128 PET-CT scanner (Philips Healthcare, The Netherlands). A Siemens Biograph mCT64 PET-CT scanner (Siemens Healthcare, The Netherlands) was used for the 89Zr-trastuzumab-PET scans.
VOI delineation
All immuno-PET scans were acquired and reconstructed to conform to recommendations for multicenter harmonization of 89Zr-immuno-PET [8]. Visual assessment of immuno-PET scans was performed by an experienced nuclear medicine physician (OSH for 89Zr-rituximab and 89Zr-cetuximab, AHB for 89Zr-trastuzumab). Tumor uptake was defined as focal uptake exceeding local background. For visually positive tumor lesions, a screenshot indicating tumor localization on immuno-PET was obtained for tumor uptake quantification. Quantitative assessment of tumor uptake for all lesions was independently performed by three observers [1 data analyst (SP), 2 physician-researchers (FB, YJ)]. Tumor delineation for all VOI was performed using the ACCURATE software tool (developed in IDL version 8.4 (Harris Geospatial Solutions, Bloomfield, USA)) [9].
The observers recorded the analysis time per tumor lesion and VOI delineation method.
Manual tumor delineation on immuno-PET
The observers manually delineated tumor VOI on the immuno-PET scans (attenuation corrected image), using the low dose CT for anatomical reference (Fig. 2a). Adjustment of the following settings was allowed: zoom, contrast and orientation (coronal/axial/sagittal). Use of a threshold (upper or lower limit) or fixed size VOI was not allowed. For 89Zr-rituximab and 89Zr-cetuximab, tumors were manually delineated on both the D3 and D6 scans, starting with the latest time point. On D0, no tumor uptake was visible, therefore the VOI delineated on D6 were imported to the D0 scan. Observers could manually adjust localization of the VOI to optimize matching of the anatomical position of the tumor lesion on the D0 scan.
VOI delineation methods for 89Zr-immuno-PET. Manual tumor delineation on immuno-PET (a) using the low dose CT (left panel), attenuation corrected 89Zr-cetuximab-PET on D6 (middle panel) with tumor lesion indicated by the red arrow and example VOI on 89Zr-cetuximab-PET shown in green (right panel). Manual tumor delineation on immuno-PET after reviewing the corresponding 18F-FDG-PET (b) the original manually delineated VOI shown in green on the 89Zr-trastuzumab-PET on D4 (left panel), reviewing the 18F-FDG-PET scan with tumor lesion indicated by the red arrow (middle panel) and adapting the original VOI after reviewing the 18F-FDG-PET scan; the FDG adapted tumor VOI shown on 89Zr-trastuzumab-PET is in green (right panel). Semi-automatic delineation (c) with the attenuation corrected 89Zr-rituximab-PET on D6 (left panel), the mask delineated on the 89Zr-rituximab-PET shown in orange (middle panel) and the semi-automatic VOI (50% of ACpeak, mask restricted) on the 89Zr-rituximab-PET shown in green (right panel). This semi-automatic VOI was accepted by the observer, as it contains tumor and no other structures or background
For all VOI, max, peak and mean activity concentrations (AC in Bq/mL) were derived and converted to standardized uptake values (SUV), by correcting for body weight and injected dose (ID). In addition, delineated volume (mL) and TLU (defined as ACmean * volume, in %ID) were obtained.
Manual tumor delineation on immuno-PET after viewing the 18F-FDG-PET
In order to support delineation of the tumor, the observers had access to the corresponding 18F-FDG-PET and could adapt the original manually delineated VOI if necessary (for example, by creating a smaller or larger VOI, or changing the position of the VOI) (Fig. 2b). This procedure was performed on scans with visible tumor uptake (D3, D4, D6). The number of VOIs that were adapted after viewing the 18F-FDG-PET was obtained.
Semi-automatic VOI delineation
Finally, we investigated the feasibility of a mask-restricted semi-automatic VOI delineation method. Each observer, for every tumor lesion, manually delineated a mask, which is a VOI including the tumor, excluding non-tumor structures (e.g. nearby blood vessels) on the immuno-PET scan. Subsequently, the semi-automatic VOI was generated including all voxels with a value ≥50% of background-corrected ACpeak within the mask (Fig. 2c). The semi-automatic isocontour was defined as 0.5 * (peak value + average background value). The background region was determined with a region growing algorithm of the tumor border, expanding three voxels away from the border of the tumor in all three dimensions [10]. The observers rated the semi-automatic VOI and accepted the VOI if it contained the tumor and no other structures or background. The number of tumor lesions for which the semi-automatic VOI was accepted by all observers was obtained.
Eligibility criteria for VOI delineation
Quantification of lesions with low tumor uptake and/or high background uptake (e.g. lesions with low contrast and/or nearby presence of blood vessels or elevated healthy tissue uptake) is difficult, due to the intrinsically low signal to noise ratios in 89Zr-immuno-PET. To ensure that quantification is only reported when delineation is feasible, a method to determine eligibility for VOI delineation was explored. Criteria were selected based on the potential for incorporation in a standardized workflow for tumor identification by a nuclear medicine physician, followed by tumor delineation by a data-analyst.
When measurement variability for SUVmax was >0, VOI were assessed for apparent insufficient tumor contrast for manual tumor delineation.
Based on this assessment VOI were deemed ineligible for quantification, according to the following criteria:
-
1.
A different structure was delineated by at least one observer.
-
2.
The voxel with maximum intensity was located at the border of the VOI, of at least one observer.
Interobserver variability and reliability were analyzed for the entire group of VOI, as well as for the subset of VOI eligible for quantification.
Interobserver reproducibility
Interobserver reproducibility for manual tumor delineation on immuno-PET was assessed by an agreement parameter (standard error of measurement (SEM)) as well as a reliability parameter (ICC; [11]). As we expected that the interobserver variability between lesions within a single patient was equal or higher than between patients, we performed a VOI-based analysis.
Interobserver variability
The agreement parameter reflects the measurement error due to interobserver variability [11]. For every tumor lesion, three values (value1, value2 and value3) were obtained from observers 1, 2 and 3, respectively. Absolute interobserver variability was calculated as:
where SD is the standard deviation.
SEM was calculated for each individual tumor lesion and has the same unit as the uptake measure (SUVmax, SUVpeak and SUVmean, dimensionless; volume in mL; TLU in %ID).
Relative interobserver variability was calculated as:
where CoV (%) is the coefficient of variation.
When all observers measure the exact same tumor uptake, SEM and CoV equal 0.
Correlation of absolute and relative variability with tumor uptake was assessed. For a group of n VOI, the interobserver variability is given as the median (interquartile range).
Reliability
A reliability parameter was used to assess whether differences in tumor uptake between lesions can be distinguished, despite measurement error due to interobserver variability. A two-way random model with absolute agreement (single measure) was used to obtain the ICC and 95% confidence interval. This means that the three observers in our study were considered as a random sample of all possible observers, and the systematic differences between the observers were included in the measurement error as we were interested in absolute agreement between the observers.
Reliability, expressed as ICC, was calculated as:
where σ2obs is the systematic part, and σ2error is the random part of the measurement error, while σ2lesion is the true variance between tumor lesions. ICC calculations were performed in SPSS, version 22.
Statistical analysis
For comparison of interobserver variability between two groups, Wilcoxon matched-pairs signed rank test was used for paired data (e.g. SUVmean on D3 and D6 for the same tumor lesions). For comparison of median CoV between multiple groups, a one-way ANOVA (non-parametric) was performed, using Friedman test with Dunn’s multiple correction to compare median CoV for paired data (SUVmean, SUVmax and SUVpeak for the same tumor lesions). For all statistical tests, a p value <0.05 was considered statistically significant. Statistical tests were performed in GraphPad Prism, version 6.02.
Results
VOI delineation
In total, 103 VOI were manually delineated by each observer. The number of VOI was not evenly distributed over the patients (Table 1). The range in interobserver variability (SEM for SUVpeak) for all VOI combined was 0 to 2.3 (median 0.4, n = 103). The range in interobserver variability between VOI within a single patient was 0 to 2.3 (median 0.6, n = 22) for patient 2 (89Zr-rituximab at D6). Interobserver variability (SEM) at D6 for the remaining five 89Zr-rituximab patients ranged from 0.1 to 1.4 (median 0.3, n = 8).
Thus, as interobserver variability was higher within a single patient than between patients, a VOI-based analysis was performed.
Manual delineation on 89Zr-immuno-PET required a median time of 2 min (range 1–5 min). Viewing of the 18F-FDG-PET /adaption of the original VOI required an additional time of 1 min (range 1–30 min). The semi-automatic procedure required 1 min (range 1–5 min).
All observers reported difficulties to distinguish the borders of some tumor lesions on immuno-PET, especially if the tumor was in proximity to other structures with high uptake, e.g. a blood vessel. Viewing the corresponding 18F-FDG-PET did not resolve this issue, as the localization and borders of the tumor lesions on immuno-PET were still not fully clear when viewing both the immuno-PET and the 18F-FDG-PET. After viewing the corresponding 18F-FDG-PET, 25% of the VOI were adapted by at least one observer (Table 2).
Semi-automatically generated VOI were accepted by all three observers in 45% of all VOI (Table 2). Inclusion of background was the main reason for failure of semi-automatic VOI.
Eligibility criteria for VOI delineation
Measurement variability for SUVmax was >0 in 25% (26/103) of the manually delineated VOI.
In 4% of the cases (4/103) a different structure (e.g. another tumor lesion) was delineated by at least one observer (2/32 (D6) for 89Zr-rituximab; 1/10 (D6) for 89Zr-cetuximab and 1/28 (D4) for 89Zr-trastuzumab). In 15% of the cases (15/103), the voxel with the maximum intensity was located at the border of the VOI (3/26 (D3) and 2/30 (D6) for 89Zr-rituximab; 5/7 (D3) and 1/9 (D6) for 89Zr-cetuximab and 4/27 (D4) for 89Zr-trastuzumab).
Application of eligibility criteria resulted in exclusion of 19 VOI, as tumor contrast was apparently insufficient for correct VOI delineation.
Interobserver reproducibility
Interobserver variability
Relative interobserver variability (CoV) was not correlated with tumor uptake (SUVmean) (Fig. 3). Therefore, interobserver variability is reported as a relative value per individual VOI and per datagroup (e.g. timepoint, mAb) (Table 3). For all VOI combined (n = 103), interobserver variability was 0% (0–2) for SUVmax, 0% (0–12) for SUVpeak and 7% (5–14) for SUVmean. Manual delineation resulted in an interobserver variability of 35% (21–49) for delineated volume and 30% (17–44) for TLU.
Absolute and relative interobserver variability as a function of tumor uptake. a Absolute variability (SEM) per individual VOI as a function of tumor uptake (SUVmean), Spearman correlation coefficient is 0.47 and p < 0.0001 (n = 103). b Relative variability (CoV) per individual VOI as a function of tumor uptake (SUVmean). Spearman correlation coefficient is −0.16 and p = 0.10 (n = 103). SUVmean is presented as the average value for observers 1, 2 and 3
There was no difference in interobserver variability for VOI delineated at D3 or D6 for 89Zr-rituximab (6 vs 8%, p = 0.38, n = 26). To obtain tumor uptake at D0 (without visible tumor contrast), a different technique was applied (importing VOI delineated at D6 to the D0 scan). Using this method, interobserver variability for SUVmean at D0 was 13% (8–28) for 89Zr-rituximab and 10% (5–27) for 89Zr-cetuximab (Supplemental Table 1).
Interobserver variability did not change after viewing the corresponding 18F-FDG-PET (p = 0.62, n = 25 VOI adapted by at least 1 observer).
VOI eligible for quantification (n = 84) showed higher tumor uptake (median SUVpeak of 7.6 vs 3.8, p < 0.001) and lower interobserver variability (SUVpeak, 0 vs 17%, p < 0.001) compared to ineligible VOI (n = 19).
Reliability
ICC data are presented in Table 4. For eligible VOI, ICC values for SUVmax, SUVpeak and SUVmean were ≥ 0.90 for 89Zr-rituximab (D3, D6) and 89Zr-cetuximab (D6). For 89Zr-trastuzumab, ICC values ≥ 0.82 were obtained. For volume and TLU ICC values were > 0.66 for all mAbs. In addition, ICC values for all combinations of two observers were calculated (Supplemental Table 2).
Discussion
Interobserver reproducibility for tumor uptake measures was investigated, as knowledge of measurement error is required for future clinical application of 89Zr-immuno-PET. Interobserver reproducibility was excellent for SUVmax and SUVpeak (variability of 0%) and very reasonable for SUVmean (variability of 7%), especially considering the lower signal to noise ratios for 89Zr-immuno-PET compared to 18F-FDG-PET. For example, interobserver variability of 14% for SUVmean has been reported for manual tumor delineation of pulmonary lesions on 18F-FDG-PET [12].
For 89Zr-immuno-PET, this is the first study to report interobserver reproducibility of tumor uptake measures. Several factors should be considered to determine to which extent these results are generalizable. Interobserver reproducibility was determined for three different 89Zr-labelled mAbs (rituximab, cetuximab and trastuzumab), at different time points (D3, D4, D6) and different injected doses (74 MBq for 89Zr-rituximab vs 37 MBq for 89Zr-trastuzumab and 89Zr-cetuximab). This study was not designed to assess how these factors individually impact interobserver variability. Instead, the results obtained reflect a broad range of uptake characteristics, which can be used as a general estimate of the measurement error due to interobserver variability in VOI delineation. Future, larger studies can focus on factors that influence tumor contrast (e.g. tumor localization, differences in uptake characteristics between mAbs).
Although ICC are reported, reliability is dependent on the range in tumor uptake and therefore not directly generalizable to other studies. In addition, tumor uptake and interobserver variability are influenced by the disproportionate high number of lesions in patient 2. Therefore, ICC values for this lesion-based analysis cannot be applied to determine whether we can reliably detect differences between patients.
Improved tumor contrast, in combination with a broad range in tumor uptake, is expected to result in improved interobserver reproducibility for all tumor uptake measures.
Another aspect to consider is that all observers used the same quantification software and a standardized operating procedure (no use of thresholds or fixed size VOI). Use of different software platforms without a standardized procedure may result in lower interobserver reproducibility. In addition, generalizability could be hampered if the three observers would have read the images in a systematically different way. In this study, there was no indication for such a systematic difference between the three observers.
These results suggest that interobserver agreement for SUVmean is sufficient to consider this uptake measure to quantify tumor uptake in a larger tumor area (opposed to only the maximum voxel or very small sample of the tumor as defined by SUVpeak). However, manual tumor delineation is a laborious task. As the concept of total lesion mAb uptake is of interest, the feasibility of semi-automatic VOI delineation was explored. For 18F-FDG-PET with perfect interobserver agreement for SUVmax [13] and higher tumor contrast, semi-automatic procedures are used to obtain SUVmean based on a semi-automatic method (e.g. with a threshold of 0.6 of SUVmax), total lesion glycolysis (TLG) and total metabolic tumor volume (TMTV) [14, 15]. For our datasets, the area included by the semi-automatic VOI was often too large, indicating low tumor to local background ratios, resulting in inclusion of background voxels in the semi-automatic VOI. For mAbs showing higher tumor contrast, as well as imaging with higher count statistics (due to, for example, higher injected doses or the availability of scanners with improved detection sensitivity or time of flight resolution), semi-automatic delineation may be feasible. Reduction of noise (e.g. by introduction of total body PET scanners) is the first step towards further improvement of tumor delineation procedures. Future studies into accuracy of tumor delineation should include ‘supervised’ delineation methods (semi-automatic procedures with a manual check) in which the optimal threshold is experimentally determined. If the success rate can thus be increased, this may lead to further development towards a robust automatic method, which is desired for clinical application.
As semi-automatic delineation was not feasible in our datasets, we explored eligibility criteria to improve standardization for manual tumor delineation, especially in case of limited tumor contrast.
In our study, 81% of the VOI (84 out of 103) were considered suitable for quantification. Based on these results, we recommend a two-step procedure to exclude lesions with insufficient tumor contrast for manual delineation: (1) verification of VOI delineation by a nuclear medicine physician to identify delineation of an incorrect structure due to limited tumor contrast, (2) exclusion of VOI with the voxel with the highest uptake located at the border of the VOI, indicating low tumor uptake and/or high background uptake.
These measures support optimal scan interpretation and standardization, which is an essential step towards potential clinical implementation of 89Zr-immuno-PET.
For this study, we performed a multicenter interobserver analysis for data that was originally obtained in single center studies. With this experience, the next step towards standardization of quantification for 89Zr-immuno-PET studies can be done in the context of a multicenter study [e.g. the IMPACT trials, (NCT02228954, NCT02117466 and NCT01957332)].
Reliable delineation of tumor uptake on 89Zr-immuno-PET allows future use as a non-invasive clinical tool to determine mAb concentrations in the tumor. Knowledge on in-vivo drug delivery of mAb-based therapy (including antibody-drug conjugates, bispecific mAbs and immune checkpoint inhibitors) is crucial to understand and predict efficacy of treatment.
Conclusion
This study shows that interobserver reproducibility of tumor uptake quantification on 89Zr-immuno-PET was excellent for SUVmax and SUVpeak using a standardized manual procedure for tumor segmentation. Semi-automatic delineation was not robust due to limited tumor contrast.
References
Oldham RK, Dillman RO. Monoclonal antibodies in cancer therapy: 25 years of progress. J Clin Oncol. 2008;26:1774–7.
Tout M, Casasnovas O, Meignan M, et al. Rituximab exposure is influenced by baseline metabolic tumor volume and predicts outcome of DLBCL patients: a lymphoma study association report. Blood. 2017;129(19):2616–23.
Lamberts LE, Williams SP, Terwisscha van Scheltinga AG, et al. Antibody positron emission tomography imaging in anticancer drug development. J Clin Oncol. 2015;33:1491–504.
Jauw YWS, Menke-van der Houven van Oordt CW, Hoekstra OS, et al. Immuno-positron emission tomography with zirconium-89-labelled monoclonal antibodies in oncology: what can we learn from initial clinical trials? Front Pharmacol. 2016;7:131.
Menke-van der Houven van Oordt CW, Gootjes EC, Huisman MC, et al. 89Zr-cetuximab PET imaging in patients with advanced colorectal cancer. Oncotarget. 2015;30:30384–93.
Jauw YWS, Zijlstra JM, de Jong D, et al. Performance of 89Zr-labelled-rituximab-PET as an imaging biomarker to assess CD20 targeting: a pilot study in patients with relapsed/refractory diffuse large B cell lymphoma. PLoS One. 2017;12:e0169828.
Bensch F, Brouwers AH, Lub-de Hooge MN, et al. 89Zr-trastuzumab PET supports clinical decision making in breast cancer patients, when HER2 status cannot be determined by standard work up. Eur J Nucl Med Mol Imaging. 2018;45(13):2300–6. https://doi.org/10.1007/s00259-018-4099-8.
Makris NE, Boellaard R, Visser EP, et al. Multicenter harmonization of 89Zr PET/CT performance. J Nucl Med. 2014;55:264–7.
Boellaard R. Quantitative on cology molecular analysis suite: ACCURATE. J Nucl Med. 2018;59:1753.
Frings V, de Langen AJ, Yaqub M, et al. Methodological considerations in quantification of 3′-deoxy-3′-[18F]fluorothymidine uptake measured with positron emission tomography in patients with non-small cell lung cancer. Mol Imaging Biol. 2014;16:136–45.
de Vet HC, Terwee CB, Knol DL, et al. When to use agreement versus reliability measures. J Clin Epidemiol. 2006;59:1033–9.
Huang YE, Chen CF, Huang YJ, et al. Interobserver variability among measurements of the maximum and mean standardized uptake values on (18)F-FDG PET/CT and measurements of tumor size on diagnostic CT in patients with pulmonary tumors. Acta Radiol. 2010;51:782–8.
Lee JR, Madsen MT, Bushnel D, et al. A threshold method to improve standardized uptake reproducibility. Nucl Med Commun. 2000;21:685–90.
Kanoun S, Tal I, Berriolo-Riedinger A, et al. Influence of software tool and methodological aspects of total metabolic tumor volume calculation on baseline [18F]FDG PET to predict survival in Hodgkin lymphoma. PLoS One. 2015;10:e0140830.
Boellaard R, Delgado-Bolton R, Oyen WJ, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54.
Acknowledgements
We thank Emma Mulder (Department of Radiology & Nuclear Medicine, VU University Medical Center, Amsterdam, The Netherlands) and Nikie Hoetjes (Department of Hematology, VU University Medical Center, Amsterdam, The Netherlands) for pilot project support and Maqsood Yaqub (Department of Radiology & Nuclear Medicine, VU University Medical Center, Amsterdam, The Netherlands) for the PET analysis software.
Funding
This research was financially supported by the Dutch Cancer Society (grant VU 2013–5839 to YJ).
Author information
Authors and Affiliations
Contributions
Study design/methodology: MH, RB, EdV, HdV, GvD, SZ, YJ.
Data acquisition: JZ, WM, EdV, CS.
Data analysis: OH, AB, SP, FB, YJ, HdV.
Writing manuscript: all co-authors.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology - General.
Electronic supplementary material
Supplemental Table 1
(DOCX 14 kb)
Supplemental Table 2
(DOCX 14 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jauw, Y.W.S., Bensch, F., Brouwers, A.H. et al. Interobserver reproducibility of tumor uptake quantification with 89Zr-immuno-PET: a multicenter analysis. Eur J Nucl Med Mol Imaging 46, 1840–1849 (2019). https://doi.org/10.1007/s00259-019-04377-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04377-6