Abstract
Purpose
We examined the prognostic significance of early changes in primary tumor SUV measured with Gallium-68-labeled prostate-specific membrane antigen positron emission tomography ([68Ga]Ga-PSMA-11-PET/CT) and serum PSA values after neoadjuvant androgen deprivation treatment (nADT) in high-risk prostate cancer (PCa) patients treated with definitive radiotherapy (RT).
Methods
The clinical data and SUV parameters of 71 PCa patients were reviewed retrospectively. The serum PSA and primary tumor SUV values were calculated before and after the start of ADT. Using univariable and multivariable analyses, the prognostic factors predicting biochemical disease free survival (bDFS) and prostate cancer specific survival (PCSS) were investigated. In addition, logistic regression analysis was used to identify predictors of biochemical failure (BF).
Results
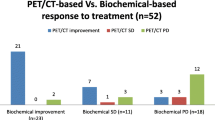
All but one patient responded with a 98.8% reduction in serum PSA (21.8 ng/mL vs. 0.3 ng/mL; p < 0.001), and 64 patients (91.1%) had a median 66.6% decrease in primary tumor SUV after ADT (13.2 vs. 4.8, p < 0.001). The primary tumor SUV response rate was significantly higher in patients with Gleason score (GS) of 7 than in patients with GS > 7 (59.5% vs. 40.5%; p = 0.04), and it was significantly lower in patients with inadequate treatment response than in those with complete (CR) or partial response (PR) (1.1% vs. 66.1%; p < 0.001). There was a strong and significant correlation (Spearman = 0.41, p < 0.001) and a high concordance (91.5%) between PSA response and SUV response after ADT. With a median follow-up time of 76.1 months, the 5-year bDFS and PCSS rates were 77.2% and 92.2%, respectively. Nineteen patients (26.7%) patients had recurrence at a median of 44.6 months after the completion of RT. In multivariate analysis, lymph node metastasis, GS greater than 7, and SD/PD after nADT were independent predictors of worse bDFS. However, no significant factor for PCSS was identified. In the multivariable logistic regression analysis, advanced age, GS of > 7 disease, lymph node metastasis, and SD or PD after nADT were independent predictors of BF.
Conclusion
These results imply that the metabolic response measured with [68Ga]Ga-PSMA-11-PET/CT after nADT could be used to predict progression in high-risk PCa patients treated with definitive RT.




Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Horwitz EM, Bae K, Hanks GE, Porter A, Grignon DJ, Brereton HD, et al. Ten-year follow-up of radiation therapy oncology group protocol 92–02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–504. https://doi.org/10.1200/jco.2007.14.9021.
Shipley WU, Lu JD, Pilepich MV, Heydon K, Roach M, Wolkov HB, et al. Effect of a short course of neoadjuvant hormonal therapy on the response to subsequent androgen suppression in prostate cancer patients with relapse after radiotherapy: a secondary analysis of the randomized protocol RTOG 86–10. Int J Radiat Oncol Biol Phys. 2002;54:1302–10. https://doi.org/10.1016/s0360-3016(02)03052-3.
Zilli T, Dal Pra A, Kountouri M, Miralbell R. Prognostic value of biochemical response to neoadjuvant androgen deprivation before external beam radiotherapy for prostate cancer: a systematic review of the literature. Cancer Treat Rev. 2016;46:35–41. https://doi.org/10.1016/j.ctrv.2016.03.016.
Kwak C, Jeong SJ, Park MS, Lee E, Lee SE. Prognostic significance of the nadir prostate specific antigen level after hormone therapy for prostate cancer. J Urol. 2002;168:995–1000. https://doi.org/10.1016/S0022-5347(05)64559-4.
Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395:1208–16. https://doi.org/10.1016/s0140-6736(20)30314-7.
Shagera QA, Artigas C, Karfis I, Critchi G, Chanza NM, Sideris S, et al. (68)Ga-PSMA PET/CT for response assessment and outcome prediction in metastatic prostate cancer patients treated with taxane-based chemotherapy. J Nucl Med. 2022;63:1191–8. https://doi.org/10.2967/jnumed.121.263006.
Zukotynski KA, Emmenegger U, Hotte S, Kapoor A, Fu W, Blackford AL, et al. Prospective, single-arm trial evaluating changes in uptake patterns on prostate-specific membrane antigen-targeted (18)F-DCFPyL PET/CT in patients with castration-resistant prostate cancer starting abiraterone or enzalutamide. J Nucl Med. 2021;62:1430–7. https://doi.org/10.2967/jnumed.120.259069.
Meller B, Bremmer F, Sahlmann CO, Hijazi S, Bouter C, Trojan L, et al. Alterations in androgen deprivation enhanced prostate-specific membrane antigen (PSMA) expression in prostate cancer cells as a target for diagnostics and therapy. EJNMMI Res. 2015;5:66. https://doi.org/10.1186/s13550-015-0145-8.
Hope TA, Truillet C, Ehman EC, Afshar-Oromieh A, Aggarwal R, Ryan CJ, et al. 68Ga-PSMA-11 PET imaging of response to androgen receptor inhibition: first human experience. J Nucl Med. 2017;58:81–4. https://doi.org/10.2967/jnumed.116.181800.
Hoberück S, Löck S, Winzer R, Zöphel K, Froehner M, Fedders D, et al. [(68)Ga]Ga-PSMA-11 PET before and after initial long-term androgen deprivation in patients with newly diagnosed prostate cancer: a retrospective single-center study. EJNMMI Res. 2020;10:135. https://doi.org/10.1186/s13550-020-00723-0.
Afshar-Oromieh A, Debus N, Uhrig M, Hope TA, Evans MJ, Holland-Letz T, et al. Impact of long-term androgen deprivation therapy on PSMA ligand PET/CT in patients with castration-sensitive prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:2045–54. https://doi.org/10.1007/s00259-018-4079-z.
Onal C, Guler OC, Torun N, Reyhan M, Yapar AF. The effect of androgen deprivation therapy on (68)Ga-PSMA tracer uptake in non-metastatic prostate cancer patients. Eur J Nucl Med Mol Imaging. 2020;47:632–41. https://doi.org/10.1007/s00259-019-04581-4.
D’Amico AV, Whittington R, Schultz D, Malkowicz SB, Tomaszewski JE, Wein A. Outcome based staging for clinically localized adenocarcinoma of the prostate. J Urol. 1997;158:1422–6.
Onal C, Sonmez S, Erbay G, Guler OC, Arslan G. Simultaneous integrated boost to intraprostatic lesions using different energy levels of intensity-modulated radiotherapy and volumetric-arc therapy. Br J Radiol. 2014;87:20130617. https://doi.org/10.1259/bjr.20130617.
Harris VA, Staffurth J, Naismith O, Esmail A, Gulliford S, Khoo V, et al. Consensus guidelines and contouring atlas for pelvic node delineation in prostate and pelvic node intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2015;92:874–83. https://doi.org/10.1016/j.ijrobp.2015.03.021.
Onal C, Ozyigit G, Guler OC, Hurmuz P, Torun N, Tuncel M, et al. Role of 68-Ga-PSMA-PET/CT in pelvic radiotherapy field definitions for lymph node coverage in prostate cancer patients. Radiother Oncol. 2020;151:222–7. https://doi.org/10.1016/j.radonc.2020.08.021.
Roach M 3rd, Hanks G, Thames H Jr, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–74. https://doi.org/10.1016/j.ijrobp.2006.04.029.
Onal C, Torun N, Oymak E, Guler OC, Reyhan M, Yapar AF. Retrospective correlation of (68)ga-psma uptake with clinical parameters in prostate cancer patients undergoing definitive radiotherapy. Ann Nucl Med. 2020;34:388–96. https://doi.org/10.1007/s12149-020-01462-x.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S-S150. https://doi.org/10.2967/jnumed.108.057307.
Zelefsky MJ, Lyass O, Fuks Z, Wolfe T, Burman C, Ling CC, et al. Predictors of improved outcome for patients with localized prostate cancer treated with neoadjuvant androgen ablation therapy and three-dimensional conformal radiotherapy. J Clin Oncol. 1998;16:3380–5. https://doi.org/10.1200/JCO.1998.16.10.3380.
Mitchell DM, McAleese J, Park RM, Stewart DP, Stranex S, Eakin RL, et al. Failure to achieve a PSA level <or=1 ng/mL after neoadjuvant LHRHa therapy predicts for lower biochemical control rate and overall survival in localized prostate cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2007;69:1467–71. https://doi.org/10.1016/j.ijrobp.2007.05.008.
Foo M, Lavieri M, Pickles T. Impact of neoadjuvant prostate-specific antigen kinetics on biochemical failure and prostate cancer mortality: results from a prospective patient database. Int J Radiat Oncol Biol Phys. 2013;85:385–92. https://doi.org/10.1016/j.ijrobp.2012.04.009.
Zelefsky MJ, Gomez DR, Polkinghorn WR, Pei X, Kollmeier M. Biochemical response to androgen deprivation therapy before external beam radiation therapy predicts long-term prostate cancer survival outcomes. Int J Radiat Oncol Biol Phys. 2013;86:529–33. https://doi.org/10.1016/j.ijrobp.2013.02.004.
Zilli T, Jorcano S, Escude L, Linero D, Rouzaud M, Dubouloz A, et al. Hypofractionated external beam radiotherapy to boost the prostate with >/=85 Gy/equivalent dose for patients with localised disease at high risk of lymph node involvement: feasibility, tolerance and outcome. Clin Oncol (R Coll Radiol). 2014;26:316–22. https://doi.org/10.1016/j.clon.2014.02.014.
di Sant’Agnese PA. Neuroendocrine differentiation in carcinoma of the prostate. Diagnostic, prognostic, and therapeutic implications. Cancer. 1992;70:254–68. https://doi.org/10.1002/1097-0142(19920701)70:1+<254::aid-cncr2820701312>3.0.co;2-e
Jiborn T, Bjartell A, Abrahamsson PA. Neuroendocrine differentiation in prostatic carcinoma during hormonal treatment. Urology. 1998;51:585–9. https://doi.org/10.1016/s0090-4295(97)00684-5.
James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–77. https://doi.org/10.1016/S0140-6736(15)01037-5.
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–60. https://doi.org/10.1056/NEJMoa1704174.
Phillips R, Shi WY, Deek M, Radwan N, Lim SJ, Antonarakis ES, et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: the ORIOLE phase 2 randomized clinical trial. JAMA Oncol. 2020;6:650–9. https://doi.org/10.1001/jamaoncol.2020.0147.
Francolini G, Ganovelli M, Di Cataldo V, Detti B, Caini S, Loi M, et al. Early biochemical outcomes following PSMA guided approach for bIoCHEmical relapse after prostatectomy-PSICHE trial (NCT05022914): preliminary results. Clin Exp Metastasis. 2023;40:197–201. https://doi.org/10.1007/s10585-023-10204-y.
Ettala O, Malaspina S, Tuokkola T, Luoto P, Loyttyniemi E, Bostrom PJ, et al. Prospective study on the effect of short-term androgen deprivation therapy on PSMA uptake evaluated with (68)Ga-PSMA-11 PET/MRI in men with treatment-naive prostate cancer. Eur J Nucl Med Mol Imaging. 2020;47:665–73. https://doi.org/10.1007/s00259-019-04635-7.
Malaspina S, Ettala O, Tolvanen T, Rajander J, Eskola O, Bostrom PJ, et al. Flare on [(18)F]PSMA-1007 PET/CT after short-term androgen deprivation therapy and its correlation to FDG uptake: possible marker of tumor aggressiveness in treatment-naive metastatic prostate cancer patients. Eur J Nucl Med Mol Imaging. 2023;50:613–21. https://doi.org/10.1007/s00259-022-05970-y.
Aggarwal R, Wei X, Kim W, Small EJ, Ryan CJ, Carroll P, et al. Heterogeneous flare in prostate-specific membrane antigen positron emission tomography tracer uptake with initiation of androgen pathway blockade in metastatic prostate cancer. Eur Urol Oncol. 2018;1:78–82. https://doi.org/10.1016/j.euo.2018.03.010.
Emmett L, Yin C, Crumbaker M, Hruby G, Kneebone A, Epstein R, et al. Rapid modulation of PSMA expression by androgen deprivation: serial (68)Ga-PSMA-11 PET in men with hormone-sensitive and castrate-resistant prostate cancer commencing androgen blockade. J Nucl Med. 2019;60:950–4. https://doi.org/10.2967/jnumed.118.223099.
Han S, Woo S, Kim YI, Lee JL, Wibmer AG, Schoder H, et al. Concordance between response assessment using prostate-specific membrane antigen PET and serum prostate-specific antigen levels after systemic treatment in patients with metastatic castration resistant prostate cancer: a systematic review and meta-analysis. Diagnostics (Basel). 2021;11. https://doi.org/10.3390/diagnostics11040663
Fanti S, Goffin K, Hadaschik BA, Herrmann K, Maurer T, MacLennan S, et al. Consensus statements on PSMA PET/CT response assessment criteria in prostate cancer. Eur J Nucl Med Mol Imaging. 2021;48:469–76. https://doi.org/10.1007/s00259-020-04934-4.
Fendler WP, Eiber M, Beheshti M, Bomanji J, Calais J, Ceci F, et al. PSMA PET/CT: joint EANM procedure guideline/SNMMI procedure standard for prostate cancer imaging 2.0. Eur J Nucl Med Mol Imaging. 2023;50:1466–86. https://doi.org/10.1007/s00259-022-06089-w
Seifert R, Emmett L, Rowe SP, Herrmann K, Hadaschik B, Calais J, et al. Second version of the prostate cancer molecular imaging standardized evaluation framework including response evaluation for clinical trials (PROMISE V2). Eur Urol. 2023. https://doi.org/10.1016/j.eururo.2023.02.002.
Gafita A, Rauscher I, Fendler WP, Murthy V, Hui W, Armstrong WR, et al. Measuring response in metastatic castration-resistant prostate cancer using PSMA PET/CT: comparison of RECIST 1.1, aPCWG3, aPERCIST, PPP, and RECIP 1.0 criteria. Eur J Nucl Med Mol Imaging. 2022;49:4271–81. https://doi.org/10.1007/s00259-022-05882-x
Funding
This project is partially supported by supported by the Başkent University Research Fund.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Ozan Cem Guler, Nese Torun, and Mehmet Reyhan. The first draft of the manuscript was written by Cem Onal, Ezgi Oymak, and Mehmet Reyhan. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was conducted according to the guidelines of the Declaration of Helsinki. This study was approved by the Başkent University Institutional Review Board (Project no: KA19/45).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Onal, C., Guler, O.C., Torun, N. et al. The significance of metabolic response to neoadjuvant androgen deprivation therapy detected with [68Ga]Ga-PSMA-11-PET/CT in high-risk prostate cancer patients treated with definitive radiotherapy. Eur J Nucl Med Mol Imaging 50, 3755–3764 (2023). https://doi.org/10.1007/s00259-023-06321-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06321-1