Abstract
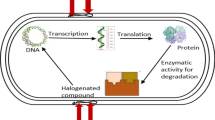
Bioremediation is a process wherein the decontamination strategies are designed so that a site could achieve the environmental abiotic and biotic parameters close to its baseline. In the process, the driving force is the available microbial genetic degradative capabilities, which are supported by required nutrients so that the desired expression of these capabilities could be exploited in favour of removal of pollutants. With genomics tools not only the available abilities could be estimated but their dynamic performance could also be established. These tools are now playing important role in bioprocess optimization, which not only derive the bio-stimulation plans but also could suggest possible genetic bio-augmentation options.

Similar content being viewed by others
References
Baltar F, Bayer B, Bednarsek N, Deppeler S et al (2019) Towards integrating evolution, metabolism, and climate change studies of marine ecosystems. Trends Ecol Evol. https://doi.org/10.1016/j.tree.2019.07.003
Lu M, Hedin LO (2019) Global plant–symbiont organization and emergence of biogeochemical cycles resolved by evolution-based trait modelling. Nat Ecol Evol 3:239. https://doi.org/10.1038/s41559-018-0759-0
Siles JA, Margesin R (2018) Insights into microbial communities mediating the bioremediation of hydrocarbon-contaminated soil from an Alpine former military site. Appl Microbiol Biotechnol 102:4409–4421. https://doi.org/10.1007/s00253-018-8932-6
Luo X, Bing H, Luo Z, Wang Y, Jin L (2019) Impacts of atmospheric particulate matter pollution on environmental biogeochemistry of trace metals in soil-plant system: a review. Environ Pollut. https://doi.org/10.1016/j.envpol.2019.113138
Kalia VC (2017) Mining metagenomes for novel bioactive molecules, Ch 01. In: Kalia VC, Shouche Y, Purohit HJ, Rahi P (eds) Mining of microbial wealth and metagenomics. Springer, Singapore, pp 1–10. https://doi.org/10.1007/978-981-10-5708-3_1
Kalia VC, Chauhan A, Bhattacharyya G (2003) Genomic databases yield novel bioplastic producers. Nat Biotechnol 21:845–846. https://doi.org/10.1038/nbt0803-845
Kalia VC, Lal S, Ghai R, Mandal M, Chauhan A (2003) Mining genomic databases to identify novel hydrogen producers. Trends Biotechnol 21:152–156. https://doi.org/10.1016/S0167-7799(03)00028-3
Porwal S, Lal S, Cheema S, Kalia VC (2009) Phylogeny in aid of the present and novel microbial lineages: diversity in Bacillus. PLoS ONE 4:e4438. https://doi.org/10.1371/journal.pone.0004438
Bhushan A, Joshi J, Shankar P, Kushwah J, Raju SC, Purohit HJ, Kalia VC (2013) Development of genomic tools for the identification of certain Pseudomonas up to species level. Indian J Microbiol 53:253–263. https://doi.org/10.1007/s12088-013-0412-1
Koul S, Kumar P, Kalia VC (2015) A unique genome wide approach to search novel markers for rapid identification of bacterial pathogens. J Mol Genet Med 9:194. https://doi.org/10.4172/1747-0862.1000194
Lal S, Raje DV, Cheema S, Kapley A, Purohit HJ, Kalia VC (2015) Investigating the phylogeny of hydrogen metabolism by comparative genomics: horizontal gene transfer. In: Kalia V (ed) Microbial factories. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2595-9_20
Koul S, Kalia VC (2016) Comparative genomics reveals biomarkers to identify Lactobacillus species. Indian J Microbiol 56:253–263. https://doi.org/10.1007/s12088-016-0605-5
Kalia VC, Kumar R, Koul S (2017) In silico analytical tools for phylogenetic and functional bacterial genomics, Ch 15. In: Arora G, Sajid A, Kalia VC (eds) Drug resistance in bacteria, fungi, malaria, and cancer. Springer, Basel, pp 339–356
Kumar R, Koul S, Kalia VC (2017) Exploiting bacterial genomes to develop biomarkers for identification, Ch 16. In: Arora G, Sajid A, Kalia VC (eds) Drug resistance in bacteria, fungi, malaria, and cancer. Springer, Cham, pp 357–370. https://doi.org/10.1007/978-3-319-48683-3_16
Agarwal L, Purohit HJ (2013) Genome sequence of Rhizobium lupini HPC(L) isolated from saline desert soil, Kutch (Gujarat). Genome Announc 1:e00071–122013. https://doi.org/10.1128/genomeA.00071-1
Purohit HJ, Raje DV, Kapley A, Padmanabhan P, Singh RN (2003) Genomics tools in environmental impact assessment. Environ Sci Technol 37:356A–363A. https://doi.org/10.1021/es032594m
Kapley A, Purohit HJ (2009) Genomic tools in bioremediation. Indian J Microbiol 49:108–113. https://doi.org/10.1007/s12088-009-0012-2
Paliwal V, Puranik S, Purohit HJ (2012) Integrated perspective for effective bioremediation. Appl Biochem Biotechnol 166:903–924. https://doi.org/10.1007/s12010-011-9479-5
Huang Y, Pan H, Wang Q, Ge Y, Liu W, Christie P (2019) Enrichment of the soil microbial community in the bioremediation of a petroleum-contaminated soil amended with rice straw or sawdust. Chemosphere 224:265–271. https://doi.org/10.1016/j.chemosphere.2019.02.148
Khardenavis AA, Vaidya AN, Kalia VC, Purohit HJ (2017) Recent advances in optimization of environmental bioprocesses. In: Purohit HJ, Kalia VC, Khardenavis AA, Vaidya AN (eds) Optimization and applicability of bioprocesses. Springer, Singapore, pp 1–10. https://doi.org/10.1007/978-981-10-5511-9_1
Kalia VC (2017) The dawn of the era of bioactive compounds, Ch 01. In: Kalia VC, Saini AK (eds) Metabolic engineering for bioactive compounds. Springer, Singapore, pp 3–10. https://doi.org/10.1007/978-981-10-5511-9_1
Kalia VC (2015) Microbes: factories for bioproducts. In: Kalia VC (ed) Microbial factories: volume 1: biofuels and waste treatment. Springer, New York, pp 1–5. https://doi.org/10.1007/978-81-322-2598-0_1
Huma N, Shankar P, Kushwah J, Bhushan A, Joshi J, Mukherjee T, Raju SC, Purohit HJ, Kalia VC (2011) Diversity and polymorphism in AHL-lactonase gene (aiiA) of Bacillus. J Microbiol Biotechnol 21:1001–1011. https://doi.org/10.4014/jmb.1105.05056
Kalia VC (2014) In search of versatile organisms for quorum sensing inhibitors: acyl homoserine lactones (AHL)-acylase and AHL-lactonase. FEMS Microbiol Lett 359:143. https://doi.org/10.1111/1574-6968.1258512
Kumar P, Pant DC, Mehariya S, Sharma R, Kansal A, Kalia VC (2014) Ecobiotechnological strategy to enhance efficiency of bioconversion of wastes into hydrogen and methane. Indian J Microbiol 54:262–267. https://doi.org/10.1007/s12088-014-0467-7
Kumar P, Singh M, Mehariya S, Patel SKS, Lee JK, Kalia VC (2014) Ecobiotechnological approach for exploiting the abilities of Bacillus to produce co-polymer of polyhydroxyalkanoate. Indian J Microbiol 54:151–157. https://doi.org/10.1007/s12088-014-0457-9
Kumar T, Singh M, Purohit HJ, Kalia VC (2009) Potential of Bacillus sp. to produce polyhydroxybutyrate from biowaste. J Appl Microbiol 106:2017–2023. https://doi.org/10.1111/j.1365-2672.2009.04160.x
Patel SKS, Kumar P, Kalia VC (2012) Enhancing biological hydrogen production through complementary microbial metabolisms. Int J Hydrogen Energy 37:10590–10603. https://doi.org/10.1016/j.ijhydene.2012.04.045
Patel SKS, Kumar P, Mehariya S, Purohit HJ, Lee JK, Kalia VC (2014) Enhancement in hydrogen production by co-cultures of Bacillus and Enterobacter. Int J Hydrogen Energy 39:14663–14668. https://doi.org/10.1016/j.ijhydene.2014.07.084
Patel SKS, Kumar P, Singh S, Lee JK, Kalia VC (2015) Integrative approach to produce hydrogen and polyhydroxybutyrate from biowaste using defined bacterial cultures. Bioresour Technol 176:136–141. https://doi.org/10.1016/j.biortech.2014.11.029
Patel SKS, Kumar P, Singh S, Lee JK, Kalia VC (2015) Integrative approach for hydrogen and polyhydroxybutyrate production, Ch 05. In: Kalia VC (ed) Microbial factories: volume 1: biofuels and waste treatment. Springer, New York, pp 73–85
Patel SKS, Lee JK, Kalia VC (2016) Integrative approach for producing hydrogen and polyhydroxyalkanoate from mixed wastes of biological origin. Indian J Microbiol 56:293–300. https://doi.org/10.1007/s12088-016-0595-3
Porwal S, Kumar T, Lal S, Rani A, Kumar S, Cheema S, Purohit HJ, Sharma R, Patel SKS, Kalia VC (2008) Hydrogen and polyhydroxybutyrate producing abilities of microbes from diverse habitats by dark fermentative process. Bioresour Technol 99:5444–5451. https://doi.org/10.1016/j.biortech.2007.11.011
Qureshi A, Prabu SK, Purohit HJ (2001) Isolation and characterization of Pseudomonas strain for utilization of 4-nitrophenol. Microbes Environ 16:49–52. https://doi.org/10.1264/jsme2.2001.49
Qureshi A, Purohit HJ (2002) Isolation of bacterial consortia for degradation of p-nitrophenol from agricultural soil. Ann Appl Biol 140:159–162. https://doi.org/10.1111/j.1744-7348.2002.tb00168.x
Narde G, Kapley A, Purohit HJ (2004) Isolation and characterization of Citrobacter strain HPC for broad range substrate specificity for chlorophenols. Curr Microbiol 48:419–423. https://doi.org/10.1007/s00284-003-4230-2
Qureshi A, Verma V, Kapley A, Purohit HJ (2007) Degradation of 4-nitroaniline by Stenotrophomonas strain HPC 135. Int J Biodeterior Biodegrad 60:215–218. https://doi.org/10.1016/j.ibiod.2007.03.004
Thangaraj K, Kapley A, Purohit HJ (2008) Characterization of diverse Acinetobacter isolates for utilization of multiple aromatic compounds. Bioresour Technol 99:2488–2494. https://doi.org/10.1016/j.biortech.2007.04.053
Sinha MM, Kapley A, Purohit HJ (2008) Study of biodiversity of Klebsiella sp. World J Microbiol Biotechnol 24:203–207. https://doi.org/10.1007/s11274-007-9457-9
Selvakumaran S, Kapley A, Kalia VC, Purohit HJ (2008) Phenotypic and phylogenic groups to evaluate the diversity of Citrobacter isolates from activated biomass of effluent treatment plants. Bioresour Technol 99:1189–1195. https://doi.org/10.1016/j.biortech.2007.02.021
Qureshi A, Kapley A, Purohit HJ (2012) Degradation of 2, 4, 6-Trinitrophenol (TNP) by Arthrobacter sp. HPC1223 isolated from effluent treatment plant. Indian J Microbiol 52:642–647. https://doi.org/10.1007/s12088-012-0288-5
Bera S, Roy AS, Mohanty K (2017) Biodegradation of phenol by a native mixed bacterial culture isolated from crude oil contaminated site. Int Biodeterior Biodegrad 121:107–113. https://doi.org/10.1016/j.ibiod.2017.04.002
Puranik S, Talkal R, Qureshi A, Khardenavis A, Kapley A, Purohit HJ (2013) Genome sequence of the pigment-producing bacterium Pseudogulbenkiania ferrooxidans, isolated from Loktak Lake. Genome Announc 1:e01115-13. https://doi.org/10.1128/genomeA.01115-13
Purohit HJ, Kapley A, Khardenavis A, Qureshi A, Dafale N (2016) Insights in waste management bioprocesses using genomic tools. Adv Appl Microbiol 97:121–170. https://doi.org/10.1016/bs.aambs.2016.09.002
Kapley A, Sagarkar S, Tanksale H, Sharma N, Qureshi A, Khardenavis A, Purohit HJ (2013) Genome sequence of Alcaligenes sp. strain HPC1271. Genome Announc 1:e00235-12. https://doi.org/10.1128/genomeA.00235-12
Kalia VC, Lal S, Chauhan A, Bhattacharyya G (2015) In silico reconstitution of novel routes for microbial plastic, Ch 19. In: Kalia VC (ed) Microbial factories. Springer, New Delhi, pp 299–315. https://doi.org/10.1007/978-81-322-2595-9_19
Phale PS, Paliwal V, Raju SC, Modak A, Purohit HJ (2013) Genome sequence of naphthalene-degrading soil bacterium Pseudomonas putida CSV86. Genome Announc 1:e00234-12. https://doi.org/10.1128/genomeA.00234-12
Kalia VC, Kumar P (2015) Genome wide search for biomarkers to diagnose Yersinia infections. Indian J Microbiol 55:366–374. https://doi.org/10.1007/s12088-015-0552-6
Kalia VC, Kumar R, Kumar P, Koul S (2016) A genome-wide profiling strategy as an aid for searching unique identification biomarkers for Streptococcus. Indian J Microbiol 56:46–58. https://doi.org/10.1007/s12088-015-0561-5
Kumar R, Koul S, Kumar P, Kalia VC (2016) Searching biomarkers in the sequenced genomes of Staphylococcus for their rapid identification. Indian J Microbiol 56:64–71. https://doi.org/10.1007/s12088-016-0565-9
Tikariha H, Pal RR, Qureshi A, Kapley A, Purohit HJ (2016) In silico analysis for prediction of degradative capacity of Pseudomonas putida SF1. Gene 1119:30491–30497. https://doi.org/10.1016/j.gene.2016.06.028
Purohit HJ (2003) Biosensors as molecular tools for bioremediation. J Clean Prod 11:293–301. https://doi.org/10.1016/S0959-6526(02)00072-0
Gulhane M, Pandit P, Khardenavis AA, Purohit HJ (2017) Study of microbial community plasticity for anaerobic digestion of vegetable waste in plug flow bioreactor. Renew Energy 101:59–66. https://doi.org/10.1016/j.renene.2016.08.021
Moharikar A, Purohit HJ (2003) Specific ratio and survival of Pseudomonas CF600 as co-culture under DO limiting condition in cultivation. Int Biodeterior Biodegrad 52:255–260. https://doi.org/10.1016/S0964-8305(03)00114-8
Zhou W, Guo W, Zhou H, Chen X (2016) Phenol degradation by Sulfobacillus acidophilus TPY via the meta-pathway. Microbiol Res 190:37–45. https://doi.org/10.1016/j.micres.2016.05.005
Youness M, Sancelme M, Combourieu B, Besse-Hoggan P (2018) Identification of new metabolic pathways in the enantioselective fungicide tebuconazole biodegradation by Bacillus sp. 3B6. J Hazard Mater 351:160–168. https://doi.org/10.1016/j.jhazmat.2018.02.048
Lee Y, Lee Y, Jeon CO (2019) Biodegradation of naphthalene, BTEX, and aliphatic hydrocarbons by Paraburkholderia aromaticivorans BN5 isolated from petroleum-contaminated soil. Sci Rep. https://doi.org/10.1038/s41598-018-36165-x
Potts LD, Perez Calderon LJ, Gontikaki E, Keith L, Gubry-Rangin C, Anderson JA, Witte U (2018) Effect of spatial origin and hydrocarbon composition on bacterial consortia community structure and hydrocarbon biodegradation rates. FEMS Microbiol Ecol 94:fiy127. https://doi.org/10.1093/femsec/fiy127
Luo J, Wu L, Chen Y, Feng L, Cao J (2019) Integrated approach to enhance the anaerobic biodegradation of benz [α] anthracene: a high-molecule-weight polycyclic aromatic hydrocarbon in sludge by simultaneously improving the bioavailability and microbial activity. J Hazard Mater 365:322–330. https://doi.org/10.1016/j.jhazmat.2018.11.012
Patel SKS, Lee JK, Kalia VC (2017) Dark-fermentative biological hydrogen production from mixed biowastes using defined mixed cultures. Indian J Microbiol 57:171–176. https://doi.org/10.1007/s12088-017-0643-7
Patel SKS, Purohit HJ, Kalia VC (2010) Dark fermentative hydrogen production by defined mixed microbial cultures immobilized on ligno-cellulosic waste materials. Int J Hydrogen Energy 35:10674–10681. https://doi.org/10.1016/j.ijhydene.2010.03.025
Patel SKS, Ray S, Prakash J, Wee JH, Kim SY, Lee JK, Kalia VC (2019) Co-digestion of biowastes to enhance biological hydrogen process by defined mixed bacterial cultures. Indian J Microbiol 59:154–160. https://doi.org/10.1007/s12088-018-00777-8
Kapley A, Prasad S, Purohit HJ (2007) Changes in microbial diversity in fed-batch reactor operation with wastewater containing nitroaromtic residues. Bioresour Technol 98:2479–2484. https://doi.org/10.1016/j.biortech.2006.09.012
Kapley A, Tolamare A, Purohit HJ (2001) Role of oxygen in partial utilization of phenol by Pseudomonas CF600 in continuous culture. World J Microbiol Biotechnol 17:801–804. https://doi.org/10.1023/A:1013526001972
Narde G, Purohit HJ (2002) Growth phase dependent substrate utilization by Pseudomonas strain PH1. Appl Biochem Microbiol 38:653–657. https://doi.org/10.1023/A:1020730712191
Moharikar A, Purohit HJ (2003) Induction of phenol utilization in Pseudomonas CF600 grown under varying nitrogen levels. J Basic Microbiol 43:56–61. https://doi.org/10.1002/jobm.200390005
Khardenavis AA, Kapley A, Purohit HJ (2010) Salicylic acid mediated enhanced biological treatment of wastewater. Appl Biochem Biotechnol 160:704–718. https://doi.org/10.1007/s12010-009-8538-7
Kalia VC, Purohit HJ (2011) Quenching the quorum sensing system: potential antibacterial drug targets. Crit Rev Microbiol 37:121–140. https://doi.org/10.3109/1040841X.2010.532479
Wilén BM, Liébana R, Persson F, Modin O, Hermansson M (2018) The mechanisms of granulation of activated sludge in wastewater treatment, its optimization, and impact on effluent quality. Appl Microbiol Biotechnol 102:5005–5020. https://doi.org/10.1007/s00253-018-8990-9
Liébana R, Modin O, Persson F, Wilén BM (2018) Integration of aerobic granular sludge and membrane bioreactors for wastewater treatment. Crit Rev Biotechnol 38:801–816. https://doi.org/10.1080/07388551.2017.1414140
Kapley A, Purohit HJ (2001) Tracking of phenol degrading genotype. Environ Sci Pollut Res 8:89–90. https://doi.org/10.1007/BF02987299
Moharikar A, Kapley A, Purohit HJ (2003) Detection of dioxygenase genes presents in various activated sludges. Environ Sci Pollut Res 10:373–378. https://doi.org/10.1065/espr2003.07.164
Kapley A, Lampel K, Purohit HJ (2001) Rapid detection of Salmonella in water samples by multiplex PCR. Water Environ Res 73:461–465. https://doi.org/10.2175/106143001X139515
Kapley A, Lampel K, Purohit HJ (2000) Thermocycling steps and optimization of multiplex PCR. Biotechnol Lett 22:1913–1918. https://doi.org/10.1023/A:1026748202071
Kapley A, Lampel K, Purohit HJ (2000) Development of duplex PCR for Salmonella and Vibrio. World J Microbiol Biotechnol 16:457–458. https://doi.org/10.1023/A:1008924119825
Purohit HJ, Kapley A (2002) Microbial quality control of drinking water: PCR as an emerging option. Trends Biotechnol 20:325–326. https://doi.org/10.1016/S0167-7799(02)02019-X
Khardenavis AA, Kapley A, Purohit HJ (2008) Phenol mediated improved performance of active biomass for treatment of distillery wastewater. Int J Biodeterior Biodegrad 62:38–45. https://doi.org/10.1016/j.ibiod.2007.06.016
Selvakumaran S, Kapley A, Kashyap SM, Daginawala HF, Kalia VC, Purohit HJ (2011) Diversity of aromatic ring-hydroxylating dioxygenase gene in Citrobacter. Bioresour Technol 102:4600–4609. https://doi.org/10.1016/j.biortech.2011.01.011
Verma V, Raju SC, Kapley A, Kalia VC, Kanade GS, Daginawala HF, Purohit HJ (2011) Degradative potential of Stenotrophomonas strain HPC383 having genes homologous to dmp operon. Bioresour Technol 102:3227–3233. https://doi.org/10.1016/j.biortech.2010.11.016
Verma V, Raju SC, Kapley A, Kalia VC, Daginawala HF, Purohit HJ (2010) Evaluation of genetic and functional diversity of Stenotrophomonas isolates from diverse effluent treatment plants. Bioresour Technol 101:7744–7753. https://doi.org/10.1016/j.biortech.2010.05.014
Ghosh S, Qureshi A, Purohit HJ (2017) Enhanced expression of catechol 1,2 dioxygenase gene in biofilm forming Pseudomonas mendocina EGD-AQ5 under increasing benzoate stress. Int J Biodeterior Biodegrad 118:57–65. https://doi.org/10.1016/j.ibiod.2017.01.019
Bengtsson G, Törneman N, De Lipthay JR, Sørensen SJ (2013) Microbial diversity and PAH catabolic genes tracking spatial heterogeneity of PAH concentrations. Microb Ecol 65:91–100. https://doi.org/10.1007/s00248-012-0112-0
Machado LF, de Assis Leite DC, da Costa Rachid CTC, Paes JE, Martins EF, Peixoto RS, Rosado AS (2019) Tracking mangrove oil bioremediation approaches and bacterial diversity at different depths in an in situ mesocosms system. Front Microbiol. https://doi.org/10.3389/fmicb.2019.02107
Purohit HJ, Kalia VC, More R (2018) Soft computing for biological systems. Integrative biotechnology series. Springer, Berlin. https://doi.org/10.1007/978-981-10-7455-4
Raje DV, Purohit HJ, Singh RN (2002) Distinguishing features of 16S rDNA gene for five dominating bacterial genus observed in bioremediation data. J Comput Biol 9:819–829. https://doi.org/10.1089/10665270260518290
Purohit HJ, Raje DV, Kapley A (2003) Identification of signature and primers specific to genus Pseudomonas using mismatched patterns of 16s rDNA sequences. BMC Bioinform 4:19. https://doi.org/10.1186/1471-2105-4-19
Liskiewicz M, Purohit HJ, Raje DV (2004) Relation of residues in the variable regions of 16S rDNA and their relevance to genus specificity. In: Jonassen I, Kim J (eds) Algorithms in bioinformatics. WABI 2004. Lecture notes in computer science, vol 3240, pp 362–373. https://doi.org/10.1007/978-3-540-30219-3_31
Iyer DS, Raje DV, Purohit HJ, Gupta A, Singh RN (2004) CAGCAG—the most consistent repeating pattern in evolution of small sub-unit of rRNA gene sequences. Curr Sci 87:494–499
Raje DV, Purohit HJ, Lijnzaad P, Singh RN (2006) Statistical analysis of counts and spacing of consistent repeating patterns in a set of homologous DNA sequences. Curr Sci 91:00113891
Qureshi A, Mohan M, Kapley A, Purohit HJ (2009) In-situ bioremediation of organochlorine pesticide contaminated microcosm soil and evaluation by gene probe. Pest Manage Sci 65:798–804. https://doi.org/10.1002/ps.1757
Raje DV, Purohit HJ, Badhe YP, Tambe SS, Kulkarni BD (2010) Self-Organizing maps: a tool to ascertain taxonomic relatedness based on features derived from 16S rDNA sequences. J Biosci 35:617–627. https://doi.org/10.1007/s12038-010-0070-y
More R, Purohit HJ (2016) The identification of discriminating patterns from 16S rRNA gene to generate signature for Bacillus genus. J Comput Biol 23:651–661. https://doi.org/10.1089/cmb.2016.0002
Purohit HJ, Kapley A, Moharikar A, Narde G (2003) A novel approach for extraction of PCR-compatible DNA from activated sludge samples collected from different biological effluent treatment plants. J Microbiol Methods 52:315–323. https://doi.org/10.1016/S0167-7012(02)00185-9
Albertsen M, Karst SM, Ziegler AS, Kirkegaard RH, Nielsen PH (2015) Back to basics–the influence of DNA extraction and primer choice on phylogenetic analysis of activated sludge communities. PLoS ONE 10:e0132783. https://doi.org/10.1371/journal.pone.0132783
Gupta P, Manjula A, Rajendhran J, Gunasekaran P, Vakhlu J (2017) Comparison of metagenomic DNA extraction methods for soil sediments of high elevation Puga hot spring in Ladakh, India to explore bacterial diversity. Geomicrobiol J 34:289–299. https://doi.org/10.1080/01490451.2015.1128995
Emaus MN, Clark KD, Hinners P, Anderson JL (2018) Preconcentration of DNA using magnetic ionic liquids that are compatible with real-time PCR for rapid nucleic acid quantification. Anal Bioanal Chem 410:4135–4144. https://doi.org/10.1007/s00216-018-1092-9
Moharikar A, Purohit HJ, Kumar R (2005) Microbial population dynamics at effluent treatment plants. J Environ Monitor 7:552–558. https://doi.org/10.1039/B406576J
Dafale N, Agrawal L, Kapley A, Meshram S, Purohit HJ, Wate S (2010) Selection of indicator bacteria based on screening of 16S rDNA metagenomic library from a two-stage anoxic–oxic bioreactor system degrading azo dyes. Bioresour Technol 101:476–484. https://doi.org/10.1016/j.biortech.2009.08.006
Kapley A, Thierry B, Purohit HJ (2007) Eubacterial diversity of activated biomass from a CETP. Res Microbiol 158:494–500. https://doi.org/10.1016/j.resmic.2007.04.004
Khardenavis AA, Kapley A, Purohit HJ (2007) Simultaneous nitrification and denitrification by diverse Diaphorobacter sp. Appl Microbiol Biotechnol 77:403–409. https://doi.org/10.1007/s00253-007-1176-5
Rani A, Porwal S, Sharma R, Kapley A, Purohit HJ, Kalia VC (2008) Assessment of microbial diversity in effluent treatment plants by culture dependent and culture independent approaches. Bioresour Technol 99:7098–7107. https://doi.org/10.1016/j.biortech.2008.01.003
Prajapati VS, Purohit HJ, Raje DV, Parmar N, Patel AB, Jones OA, Joshi CG (2016) The effect of a high-roughage diet on the metabolism of aromatic compounds by rumen microbes: a metagenomic study using Mehsani buffalo (Bubalus bubalis). Appl Microbiol Biotechnol 100:1319–1331. https://doi.org/10.1007/s00253-015-7239-0
Parmar K, Dafale N, Pal R, Tikariha H, Purohit HJ (2018) An insight into phage diversity at environmental habitats using comparative metagenomics approach. Curr Microbiol 75:132–141. https://doi.org/10.1007/s00284-017-1357-0
Talkal R, Tikariha H, Purohit HJ (2018) An approach to in silico dissection of bacterial intelligence through selective genomic tools. Indian J Microbiol 58:278–286. https://doi.org/10.1007/s12088-018-0726-0
Tikariha H, Purohit HJ (2019) Different dimensions in microbial community adaptation and function. Indian J Microbiol 59:387–390. https://doi.org/10.1007/s12088-019-00813-1
Mohan SV, Agarwal L, Mohanakrishna G, Srikanth S, Kapley A, Purohit HJ, Sharma PN (2011) Firmicutes with iron dependent hydrogenase drive hydrogen production in anaerobic bioreactor using distillery wastewater. Int J Hydrogen Energy 36:8234–8342. https://doi.org/10.1016/j.ijhydene.2011.04.021
Pandit P, Gulhane M, Khardenavis AA, Purohit HJ (2016) Mining of hemicellulose and lignin degrading genes from differentially enriched methane producing microbial. Bioresour Technol 216:923–930. https://doi.org/10.1016/j.biortech.2016.06.021
More RP, Mitra S, Raju SC, Kapley A, Purohit HJ (2014) Mining and assessment of catabolic pathways in the metagenome of a common effluent treatment plant to induce the degradative capacity of biomass. Bioresour Technol 153:137–146. https://doi.org/10.1016/j.biortech.2013.11.065
Gulhane M, Khardenavis AA, Karia S, Pandit P, Kanade GS, Lokhande S, Vaidya A, Purohit HJ (2016) Biomethanation of vegetable market waste in an anaerobic baffled reactor: effect of effluent recirculation and carbon mass balance analysis. Bioresour Technol 215:100–109. https://doi.org/10.1016/j.biortech.2016.04.039
Atuanya EI, Purohit HJ, Chakrabarty T (2000) Anaerobic and aerobic biodegradation of chlorophenols using UASB and ASG bioreactors. World J Microbiol Biotechnol 16:95–98. https://doi.org/10.1023/A:1008957229070
Marican A, Durán-Lara EF (2018) A review on pesticide removal through different processes. Environ Sci Pollut Res 25:2051–2064. https://doi.org/10.1007/s11356-017-0796-2
Jaiswal S, Singh DK, Shukla P (2019) Gene editing and systems biology tools for pesticide bioremediation: a review. Front Microbiol. https://doi.org/10.3389/fmicb.2019.00087
Kalia VC, Purohit HJ (2008) Microbial diversity and genomics in aid of bioenergy. J Ind Microbiol Biotechnol 35:403–419. https://doi.org/10.1007/s10295-007-0300-y
Khardenavis AA, Kapley A, Purohit HJ (2009) Processing of poultry feathers by alkaline keratin hydrolyzing enzyme from Serratia sp. HPC 1383. Waste Manag 29:1409–1415. https://doi.org/10.1016/j.wasman.2008.10.009
Patel SKS, Singh M, Kumar P, Purohit HJ, Kalia VC (2012) Exploitation of defined bacterial cultures for production of hydrogen and polyhydroxybutyrate from pea-shells. Biomass Bioenergy 36:218–225. https://doi.org/10.1016/j.biortech.2014.11.029
Fuke P, Pal RR, Khardenavis AA, Purohit HJ (2018) In silico characterization of broad range proteases produced by Serratia marcescens EGD-HP20. J Basic Microbiol 58:492–500. https://doi.org/10.1002/jobm.201700474
Bohra V, Dafale N, Purohit HJ (2019) Understanding the alteration in rumen microbiome and CAZymes profile with diet and host through comparative metagenomic approach. Arch Microbiol. https://doi.org/10.1007/s00203-019-01706-z
Purohit HJ (2019) Aligning microbial biodiversity for valorization of biowastes: conception to perception. Indian J Microbiol. https://doi.org/10.1007/s12088-019-00826-w
Chhatre S, Purohit HJ, Shanker R, Khanna P (1996) Bacterial consortia for crude oil spill remediation. Water Sci Technol 34:187–193. https://doi.org/10.1007/s0273-1223(96)001713-5
Domde P, Kapley A, Purohit HJ (2007) Impact of bioaugmentation with consortium of bacteria on the remediation of wastewater containing hydrocarbons. Environ Sci Pollut Res 14:7–11. https://doi.org/10.1065/espr2006.11.358
Pal RR, Khardenavis AA, Purohit HJ (2015) Identification and monitoring of nitrification and denitrification genes in Klebsiella pneumoniae EGD-HP19-C for its ability to perform heterotrophic nitrification and aerobic denitrification. Funct Integr Genomics 15:63–76. https://doi.org/10.1007/s10142-014-0406-z
Paliwal V, Raju SC, Modak A, Phale PS, Purohit HJ (2014) Pseudomonas putida CSV86: A candidate genome for genetic bioaugmentation. PLoS ONE 9:e84000. https://doi.org/10.1371/journal.pone.0084000
Liu X, Selonen V, Steffen K, Surakka M et al (2019) Meat and bone meal as a novel biostimulation agent in hydrocarbon contaminated soils. Chemosphere 225:574–578. https://doi.org/10.1016/j.chemosphere.2019.03.053
Xu X, Zarecki R, Medina S et al (2019) Modeling microbial communities from atrazine contaminated soils promotes the development of biostimulation solutions. ISME J 13:494. https://doi.org/10.1038/s41396-018-0288-5
Dangelmayr MA, Figueroa LA, Williams KH, Long PE (2019) Characterizing organic carbon dynamics during biostimulation of a uranium contaminated field site. Biogeochemistry 143:117–132. https://doi.org/10.1007/s10533-019-00553-w
Ghosh S, Qureshi A, Purohit HJ (2019) d-Tryptophan governs biofilm formation rates and bacterial interaction in P. mendocina and S. aureus. J Biosci 44:3. https://doi.org/10.1007/s12038-018-9841-7
Sarkar J, Kazy SK, Gupta A et al (2016) Biostimulation of indigenous microbial community for bioremediation of petroleum refinery sludge. Front Microbiol 7:1407. https://doi.org/10.3389/fmicb.2016.01407
Roy A, Dutta A, Pal S et al (2018) Biostimulation and bioaugmentation of native microbial community accelerated bioremediation of oil refinery sludge. Bioresour Technol 253:22–32. https://doi.org/10.1016/j.biortech.2018.01.004
Cervantes-González E, Guevara-García MA, García-Mena J, Ovando-Medina VM (2019) Microbial diversity assessment of polychlorinated biphenyl–contaminated soils and the biostimulation and bioaugmentation processes. Environ Monit Assess 191:118. https://doi.org/10.1007/s10661-019-7227-4
Raimondo EE, Saez JM, Aparicio JD, Fuentes MS, Benimeli CS (2020) Coupling of bioaugmentation and biostimulation to improve lindane removal from different soil types. Chemosphere 238:124512. https://doi.org/10.1016/j.chemosphere.2019.124512
Wu M, Wu J, Zhang X, Ye X (2019) Effect of bioaugmentation and biostimulation on hydrocarbon degradation and microbial community composition in petroleum-contaminated loessal soil. Chemosphere 237:124456. https://doi.org/10.1016/j.chemosphere.2019.124456
Cycoń M, Mrozik A, Piotrowska-Seget Z (2017) Bioaugmentation as a strategy for the remediation of pesticide-polluted soil: a review. Chemosphere 172:52–71. https://doi.org/10.1016/j.chemosphere.2016.12.129
dos Santos JJ, Maranho LT (2018) Rhizospheric microorganisms as a solution for the recovery of soils contaminated by petroleum: a review. J Environ Manag 210:104–113. https://doi.org/10.1016/j.jenvman.2018.01.015
Dagher D, de la Providencia I, Pitre F, St-Arnaud M, Hijri M (2019) Plant identity shaped rhizospheric microbial communities more strongly than bacterial bioaugmentation in petroleum hydrocarbon-polluted sediments. Front Microbiol 10:2144. https://doi.org/10.3389/fmicb.2019.02144
Asemoloye MD, Jonathan SG, Ahmad R (2019) Synergistic plant-microbes interactions in the rhizosphere: a potential headway for the remediation of hydrocarbon polluted soils. Int J Phytoremed 21:71–83. https://doi.org/10.1080/15226514.2018.1474437
Turkovskaya O, Muratova A (2019) Plant–bacterial degradation of polyaromatic hydrocarbons in the rhizosphere. Trends Biotechnol. https://doi.org/10.1016/j.tibtech.2019.04.010
Agarwal L, Prakash A, Purohit HJ (2019) Expression of autotrophic genes under CO2 environment and genome mining of desert bacterium Cupriavidus sp. HPC(L). Bioresour Technol Rep. https://doi.org/10.1016/j.biteb.2019.100258
Agarwal L, Qureshi A, Kalia VC, Kapley A, Purohit HJ, Singh RN (2014) Arid ecosystem: Future option for carbon sinks using microbial community intelligence. Curr Sci 106:1357–1363
Tikariha H, Purohit HJ (2019) Assembling a genome for novel nitrogen-fixing bacteria with capabilities for utilization of aromatic hydrocarbons. Genomics. https://doi.org/10.1016/j.ygeno.2018.12.005
Yu W, Chen H, Song J, Zhao C, Du X, Guo C, Song Q (2019) Microbial community from arid desert oilfield in response to accurate bio-stimulation remediation. Petrol Sci Technol 37:337–345. https://doi.org/10.1080/10916466.2018.1547752
Keller M, Zengler K (2004) Tapping into microbial diversity. Nat Rev Microbiol 2:141. https://doi.org/10.1038/nrmicro819
Lim SW, Tran TM, Abate AR (2015) PCR-activated cell sorting for cultivation-free enrichment and sequencing of rare microbes. PLoS ONE 10:e0113549. https://doi.org/10.1371/journal.pone.0113549
Kapley A, Purohit HJ (2009) Diagnosis of treatment efficiency in industrial wastewater treatment plants: a case study at a refinery ETP. Environ Sci Technol 43:3789–3795. https://doi.org/10.1021/es803296r
Tikariha H, Khardenavis AA, Purohit HJ (2018) Dissolved oxygen mediated quorum sensing phenomenon in the bacterial community to combat oxidative stress. Arch Microbiol 200:1371–1379. https://doi.org/10.1007/s00203-018-1551-x
Patel S, Homaei A, Patil S, Daverey A (2019) Microbial biosurfactants for oil spill remediation: pitfalls and potentials. Appl Microbiol Biotechnol 103:27–37. https://doi.org/10.1007/s00253-018-9434-2
Kapley A, Purohit HJ, Chhatre S, Shanker R, Chakrabarti T, Khanna P (1999) Osmotolerance and hydrocarbon degradation by genetically engineered bacterial consortium. Bioresour Technol 67:241–245. https://doi.org/10.1016/S0960-8524(98)00121-7
Kumari S, Regar RK, Manickam N (2018) Improved polycyclic aromatic hydrocarbon degradation in a crude oil by individual and a consortium of bacteria. Bioresour Technol 254:174–179. https://doi.org/10.1016/j.biortech.2018.01.075
Jha V, Tikariha H, Dafale N, Purohit HJ (2018) Exploring the rearrangement of sensory intelligence in Proteobacteria: insight of Pho regulon. World J Microbiol Biotechnol 34:172. https://doi.org/10.1007/s11274-018-2551-3
Puranik S, Shaligram S, Paliwal V, Raje DV, Kapley A, Purohit HJ (2012) Demonstration of sequential adaptation strategy for developing salt tolerance in bacteria for wastewater treatment: a study using Escherichia coli as model. Bioresour Technol 121C:282–289. https://doi.org/10.1016/j.biortech.2012.06.037
Puranik S, Purohit HJ (2014) Dependency of cellular decision making in physiology and influence of preceding growth conditions. Appl Biochem Biotechnol 174:1982–1998. https://doi.org/10.1007/s12010-014-1167-9
Puranik S, Purohit HJ (2015) Dynamic interactive events in gene regulation using E. coli dehydrogenase as a model. Funct Integr Genomics 15:175–188. https://doi.org/10.1007/s10142-014-0418-8
Purohit HJ, Khardenavis AA, Vaidya AN, Kalia VC (2017) Mining the microbial community for redefining the bioprocess in the future, Ch 19. In: Purohit HJ, Kalia VC, Khardenavis AA, Vaidya AN (eds) Optimization and applicability of bioprocess. Springer, New Delhi, pp 409–418. https://doi.org/10.1007/978-981-10-6863-8_19
Kalia VC (2013) Quorum sensing inhibitors: an overview. Biotechnol Adv 31:224–245. https://doi.org/10.1016/j.biotechadv.2012.10.004
Kalia VC, Kumar P (2015) Potential applications of quorum sensing inhibitors in diverse fields, Ch 29. In: Kalia VC (ed) Quorum sensing vs quorum quenching: a battle with no end in sight. Springer, New York, pp 359–370. https://doi.org/10.1007/978-81-322-1982-8_29. ISBN 978-81-322-1981-1
Kalia VC, Kumar P (2015) The battle: quorum-sensing inhibitors versus evolution of bacterial resistance, Ch 31. In: Kalia VC (ed) Quorum sensing vs quorum quenching: a battle with no end in sight. Springer, New York, pp 385–391. https://doi.org/10.1007/978-81-322-1982-8_31. ISBN 978-81-322-1981-1
Kalia VC, Patel SKS, Kang YC, Lee J-K (2019) Quorum sensing inhibitors as antipathogens: biotechnological applications. Biotechnol Adv 37:68–90. https://doi.org/10.1016/j.biotechadv.2018.11.006
Kalia VC, Wood TK, Kumar P (2014) Evolution of resistance to quorum-sensing inhibitors. Microb Ecol 68:13–23. https://doi.org/10.1007/s00248-013-0316-y
Kalia VC, Koul S, Ray S, Prakash S (2018) Targeting quorum sensing mediated Staphylococcus aureus biofilms, Ch 02. In: Kalia VC (ed) Biotechnological applications of quorum sensing inhibitors. Springer, Singapore, pp 23–32. https://doi.org/10.1007/978-981-10-9026-4_2
Saini AK, Kalia VC (2017) Potential challenges and alternative approaches in metabolic engineering of bioactive compounds in industrial setup, Ch 19. In: Kalia VC, Saini AK (eds) Metabolic engineering for bioactive compounds: strategies and processes. Springer, Singapore, pp 405–412. https://doi.org/10.1007/978-981-10-5511-9_19
Xia S, Song Z, Jeyakumar P et al (2019) A critical review on bioremediation technologies for Cr(VI)-contaminated soils and wastewater. Crit Rev Environ Sci Technol 49:1027–1078. https://doi.org/10.1080/10643389.2018.1564526
Cameselle C, Gouveia S, Urréjola S (2019) Benefits of phytoremediation amended with DC electric field. Application to soils contaminated with heavy metals. Chemosphere 229:481–488. https://doi.org/10.1016/j.chemosphere.2019.04.222
Purohit HJ, Tikariha H, Kalia VC (2018) Current scenario on application of computational tools in biological systems, Ch 01. In: Purohit HJ, Kalia VC, More R (eds) Soft computing for biological systems. Springer, Singapore, pp 1–12. https://doi.org/10.1007/978-981-10-7455-4_1
Ghosh S, Qureshi A, Purohit HJ (2017) Biofilm micro-environments: modeling approach, Ch 15. In: Purohit HJ, Kalia VC, Khardenavis AA, Vaidya AN (eds) Optimization and applicability of bioprocess. Springer, New Delhi, pp 305–324. https://doi.org/10.1007/978-981-10-6863-8_15
Purohit HJ, Tikariha H, Kalia VC (2018) Future perspectives of computational biology: demanding shifts in analytical thinking to unfold biological complexities, Ch 17. In: Purohit HJ, Kalia VC, More R (eds) Soft computing for biological systems. Springer, Singapore, pp 283–293. https://doi.org/10.1007/978-981-10-7455-4_17
Acknowledgements
This research was supported a Grant from the Intelligent Synthetic Biology Center of Global Frontier Project (2013M3A6A8073184) funded by the Ministry of Science, ICT and Future Planning, Republic of Korea (JKL). This research was supported by Brain Pool Grant (NRF-2019H1D3A2A01060226) by National Research Foundation of Korea to work at Konkuk University (VCK).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, JK., Kalia, V.C. Mapping Microbial Capacities for Bioremediation: Genes to Genomics. Indian J Microbiol 60, 45–53 (2020). https://doi.org/10.1007/s12088-019-00842-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-019-00842-w