Abstract
Lafora disease (LD; OMIM#274780) is a fatal rare neurodegenerative disorder characterized by generalized epileptic seizures and the presence of polyglucosan inclusions (PGs), called Lafora bodies (LBs), typically in the brain. LD is caused by mutations in two genes EPM2A or EPM2B, which encode respectively laforin, a glucan phosphatase, and malin, an E3-ubiquitin ligase. Much remains unknown about the molecular bases of LD and, unfortunately, appropriate treatment is still missing; therefore patients die within 10 years from the onset of the disease. Recently, we have identified neuroinflammation as one of the initial determinants in LD. In this work, we have investigated anti-inflammatory treatments as potential therapies in LD. With this aim, we have performed a preclinical study in an Epm2b−/− mouse model with propranolol, a β-adrenergic antagonist, and epigallocatechin gallate (EGCG), an antioxidant from green tea extract, both of which displaying additional anti-inflammatory properties. In vivo motor and cognitive behavioral tests and ex vivo histopathological brain analyses were used as parameters to assess the therapeutic potential of propranolol and EGCG. After 2 months of treatment, we observed an improvement not only in attention defects but also in neuronal disorganization, astrogliosis, and microgliosis present in the hippocampus of Epm2b−/− mice. In general, propranolol intervention was more effective than EGCG in preventing the appearance of astrocyte and microglia reactivity. In summary, our results confirm the potential therapeutic effectiveness of the modulators of inflammation as novel treatments in Lafora disease.






Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Turnbull J, Tiberia E, Striano P, Genton P, Carpenter S, Ackerley CA et al (2016) Lafora disease. Epileptic Disord 18(S2):38–62
Sakai M, Austin J, Witmer F, Trueb L (1970) Studies in myoclonus epilepsy (Lafora body form). II. Polyglucosans in the systemic deposits of myoclonus epilepsy and in corpora amylacea. Neurology 20(2):160–176
Lafora GR, Glueck B (1911) Beitrag zur histogpathologie der myoklonischen epilepsie. Gesamte Neurol Psychiatr 6:1–14
Minassian BA, Lee Jeffrey R, Herbrick JA, Huizenga J, Soder S, Mungall AJ et al (1998) Mutations in a gene encoding a novel protein tyrosine phosphatase cause progressive myoclonus epilepsy. Nat Genet 20(2):171–174
Serratosa JM, Gómez-Garre P, Gallardo ME, Anta B (1999) Beltrán-Valero De Bernabé D, Lindhout D, et al. A novel protein tyrosine phosphatase gene is mutated in progressive myoclonus epilepsy of the Lafora type (EPM2). Hum Mol Genet 8(2):345–352
Chan EM, Young EJ, Ianzano L, Munteanu I, Zhao X, Christopoulos CC, Avanzini G, Elia M et al (2003) Mutations in NHLRC1 cause progressive myoclonus epilepsy. Nat Genet 35(2):125–127
Gentry MS, Worby CA, Dixon JE (2005) Insights into Lafora disease: Malin is an E3 ubiquitin ligase that ubiquitinates and promotes the degradation of laforin. Proc Natl Acad Sci U S A 102(24):8501–8506
Lohi H, Ianzano L, Zhao XC, Chan EM, Turnbull J, Scherer SW, Ackerley CA, Minassian BA (2005) Novel glycogen synthase kinase 3 and ubiquitination pathways in progressive myoclonus epilepsy. Hum Mol Genet 14(18):2727–2736
Solaz-Fuster MC, Gimeno-Alcañiz JV, Ros S, Fernandez-Sanchez ME, Garcia-Fojeda B, Garcia OC, Vilchez D, Dominguez J et al (2008) Regulation of glycogen synthesis by the laforin - Malin complex is modulated by the AMP-activated protein kinase pathway. Hum Mol Genet 17(5):667–678
García-Gimeno M, Knecht E, Sanz P (2018) Lafora disease: a ubiquitination-related pathology. Cells 7(8):87
Nitschke F, Ahonen SJ, Nitschke S, Mitra S, Minassian BA (2018) Lafora disease — from pathogenesis to treatment strategies. Nat Rev Neurol 14(10):606–617
Ganesh S, Delgado-Escueta AV, Sakamoto T, Avila MR, Machado-Salas J, Hoshii Y, Akagi T, Gomi H et al (2002) Targeted disruption of the Epm2a gene causes formation of Lafora inclusion bodies, neurodegeneration, ataxia, myoclonus epilepsy and impaired behavioral response in mice. Hum Mol Genet 11(11):1251–1262
DePaoli-Roach AA, Tagliabracci VS, Segvich DM, Meyer CM, Irimia JM, Roach PJ (2010) Genetic depletion of the malin E3 ubiquitin ligase in mice leads to Lafora bodies and the accumulation of insoluble laforin. J Biol Chem 285(33):25372–25381
Turnbull J, Wang P, Girard JM, Ruggieri A, Wang TJ, Draginov AG, Kameka AP, Pencea N et al (2010) Glycogen hyperphosphorylation underlies Lafora body formation. Ann Neurol 68(6):925–933
Criado O, Aguado C, Gayarre J, Duran-Trio L, Garcia-Cabrero AM, Vernia S, San Millán B, Heredia M et al (2012) Lafora bodies and neurological defects in malin-deficient mice correlate with impaired autophagy. Hum Mol Genet 21(7):1521–1533
García-Cabrero AM, Marinas A, Guerrero R, Rodríguez De Córdoba S, Serratosa JM, Sánchez MP (2012) Laforin and malin deletions in mice produce similar neurologic impairments. J Neuropathol Exp Neurol 71(5):413–421
Valles-Ortega J, Duran J, Garcia-Rocha M, Bosch C, Saez I, Pujadas L, Serafin A, Cañas X et al (2011) Neurodegeneration and functional impairments associated with glycogen synthase accumulation in a mouse model of Lafora disease. EMBO Mol Med 3:667–681
Aguado C, Sarkar S, Korolchuk VI, Criado O, Vernia S, Boya P, Sanz P, de Córdoba SR et al (2010) Laforin, the most common protein mutated in Lafora disease, regulates autophagy. Hum Mol Genet 19(14):2867–2876
Vernia S, Rubio T, Heredia M, Rodríguez de Córdoba S, Sanz P (2009) Increased endoplasmic reticulum stress and decreased proteasomal function in Lafora disease models lacking the phosphatase laforin. PLoS One 4(6):e5907
Vernia S, Solaz-Fuster MC, Gimeno-Alcañiz JV, Rubio T, García-Haro L, Foretz M, de Córdoba SR, Sanz P (2009) AMP-activated protein kinase phosphorylates R5/PTG, the glycogen targeting subunit of the R5/PTG-protein phosphatase 1 holoenzyme, and accelerates its down-regulation by the laforin-malin complex. J Biol Chem 284(13):8247–8255
Upadhyay M, Agarwal S, Bhadauriya P, Ganesh S (2017) Loss of laforin or malin results in increased Drp1 level and concomitant mitochondrial fragmentation in Lafora disease mouse models. Neurobiol Dis 100:39–51
Lahuerta M, Aguado C, Sánchez-Martín P, Sanz PKE (2018) Degradation of altered mitochondria by autophagy is impaired in Lafora disease. FEBS J 285(11):2071–2090
Romá-Mateo C, Aguado C, García-Giménez JL, Knecht E, Sanz P, Pallardó FV (2015) Oxidative stress, a new hallmark in the pathophysiology of Lafora progressive myoclonus epilepsy. Free Radic Biol Med 88:30–41
Berthier A, Payá M, García-Cabrero AM, Ballester MI, Heredia M, Serratosa JM, Sánchez MP, Sanz P (2016) Pharmacological interventions to ameliorate neuropathological symptoms in a mouse model of Lafora disease. Mol Neurobiol 53(2):1296–1309
Augé E, Pelegrí C, Manich G, Cabezón I, Guinovart JJ, Duran J, Vilaplana J (2018) Astrocytes and neurons produce distinct types of polyglucosan bodies in Lafora disease. Glia 66(10):2094–2107
Rubio-Villena C, Viana R, Bonet J, Garcia-Gimeno MA, Casado M, Heredia M, Sanz P (2018) Astrocytes: new players in progressive myoclonus epilepsy of Lafora type. Hum Mol Genet 27(7):1290–1300
Lahuerta M, Gonzalez D, Aguado C, Fathinajafabadi A, García-Giménez JL, Moreno-Estellés M, Romá-Mateo C, Knecht E et al (2020) Reactive glia-derived neuroinflammation: a novel hallmark in Lafora progressive myoclonus epilepsy that progresses with age. Mol Neurobiol 57(3):1607–1621
Ransohoff RM (2016) How neuroinflammation contributes to neurodegeneration. Science (80- ) 353(6301):777–783
Muñoz-Ballester C, Santana N, Perez-Jimenez E, Viana R, Artigas F, Sanz P (2019) In vivo glutamate clearance defects in a mouse model of Lafora disease. Exp Neurol 320:112959
Muñoz-Ballester C, Berthier A, Viana R, Sanz P (2016) Homeostasis of the astrocytic glutamate transporter GLT-1 is altered in mouse models of Lafora disease. Biochim Biophys Acta 1862(6):1074–1083
Ortolano S, Vieitez I, Agis-Balboa RC, Spuch C (2014) Loss of GABAergic cortical neurons underlies the neuropathology of Lafora disease. Mol Brain 7:7
García-Cabrero AM, Sánchez-Elexpuru G, Serratosa JM, Sánchez MP (2014) Enhanced sensitivity of laforin- and malin-deficient mice to the convulsant agent pentylenetetrazole. Front Neurosci 8:291
Duran J, Gruart A, García-Rocha M, Delgado-García JM, Guinovart JJ (2014) Glycogen accumulation underlies neurodegeneration and autophagy impairment in lafora disease. Hum Mol Genet 23(12):3147–3156
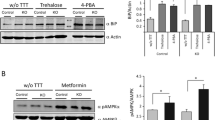
Sánchez-Elexpuru G, Serratosa JM, Sanz P, Sánchez MP (2017) 4-Phenylbutyric acid and metformin decrease sensitivity to pentylenetetrazol-induced seizures in a malin knockout model of Lafora disease. Neuroreport 28(5):268–271
Bisulli F, Muccioli L, D’Orsi G, Canafoglia L, Freri E, Licchetta L et al (2019) Treatment with metformin in twelve patients with Lafora disease. Orphanet J Rare Dis 14:149
Brewer MK, Uittenbogaard A, Austin GL, Segvich DM, DePaoli-Roach A, Roach PJ et al (2019) Targeting pathogenic Lafora bodies in Lafora disease using an antibody-enzyme fusion. Cell Metab. 30(4):689–705.e6
Delpech JC, Madore C, Nadjar A, Joffre C, Wohleb ES, Layé S (2015) Microglia in neuronal plasticity: influence of stress. Neuropharmacology 96:19–28
Kota DJ, Prabhakara KS, van Brummen AJ, Bedi S, Xue H, DiCarlo B, Cox CS Jr, Olson SD (2016) Propranolol and medenchymal stromal cells combine to treat traumatic brain injury. Stem Cells Transl Med 5:33–44
Lin SY, Wang YY, Chang CY, Wu CC, Chen WY, Kuan YH, Liao SL, Chen CJ (2020) Effects of β-Adrenergic blockade on metabolic and inflammatory responses in a rat model of ischemic stroke. Cells 9(6):1373
Woiciechowsky C, Schöning B, Stoltenburg-Didinger G, Stockhammer F, Volk HD (2004) Brain-IL-1β triggers astrogliosis through induction of IL-6: inhibition by propranolol and IL-10. Med Sci Monit 10(9):325–330
Dobarro M, Gerenu G, Ramírez MJ (2013) Propranolol reduces cognitive deficits, amyloid and tau pathology in Alzheimer’s transgenic mice. Int J Neuropsychopharmacol 16(10):2245–2257
Herges K, Millward JM, Hentschel N, Infante-Duarte C, Aktas O, Zipp F (2011) Neuroprotective effect of combination therapy of Glatiramer acetate and epigallocatechin-3-gallate in neuroinflammation. PLoS One 6(10):e25456
Cascella M, Bimonte S, Muzio MR, Schiavone V, Cuomo A (2017) The efficacy of Epigallocatechin-3-gallate (green tea) in the treatment of Alzheimer’s disease: an overview of pre-clinical studies and translational perspectives in clinical practice. Infect Agent Cancer 12:36
Wang J, Li P, Qin T, Sun D, Zhao X, Zhang B (2020) Protective effect of epigallocatechin-3-gallate against neuroinflammation and anxiety-like behavior in a rat model of myocardial infarction. Brain Behav 10(6):1–9
Fischer W (2002) Anticonvulsant profile and mechanism of action of propanolol and its two enantiomers. Seizure 11(5):285–302
Lalonde R, Strazielle C (2011) Brain regions and genes affecting limb-clasping responses. Brain Res Rev 67(1–2):252–259
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85(3):367–370
Luong TN, Carlisle HJ, Southwell A, Patterson PH (2011) Assessment of motor balance and coordination in mice using the balance beam. J Vis Exp 49:2376
Matsuura K, Kabuto H, Makino H, Ogawa N (1997) Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J Neurosci Methods 73(1):45–48
Korpi ER, Koikkalainen P, Vekovischeva OY, Mäkaela R, Kleinz R, Uusi-oukari M et al (1998) Cerebellar granule-cell-specific GABA A receptors attenuate benzodiazepine-induced ataxia : evidence from α 6-subunit-deficient mice. Eur J Neurosci 11:233–240
Sarnyai Z, Sibille EL, Pavlides C, Fenster RJ, McEwen BS, Tóth M (2000) Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin1A receptors. Proc Natl Acad Sci U S A 97(26):14731–14736
Vogel-Ciernia A, Wood M (2014) Examining object localtion and object recognition memory in mice. Curr Protoc Neurosci 69:8.31.1–8.31.17
Seese RR, Wang K, Yao YQ, Lynch G, Gall CM (2014) Spaced training rescues memory and ERK1/2 signaling in fragile X syndrome model mice. Proc Natl Acad Sci U S A 111(47):16907–16912
Can A, Dao DT, Terrillion CE, Piantadosi SC, Bhat S, Gould TD (2012) The tail suspension test. J Vis Exp 59:e3769
Calpe-López C, García-Pardo MP, Martínez-Caballero MA, Santos-Ortíz A, Aguilar MA (2020) Behavioral traits associated with resilience to the effects of repeated social defeat on cocaine-induced conditioned place preference in mice. Front Behav Neurosci 13(January):1–20
RStudio_Team (2020) RStudio: Integrated Development for R. RStudio. PBC, Boston
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Erlbaum, Hillsdale
Kraemer HC, Morgan GA, Leech NL, Gliner JA, Vaske JJ, Harmon RJ (2003) Measures of clinical significance. J Am Acad Child Adolesc Psychiatry 42(12):1524–1529
López-González I, Viana R, Sanz P, Ferrer I (2017) Inflammation in Lafora disease: evolution with disease progression in Laforin and Malin knock-out mouse models. Mol Neurobiol 54(5):3119–3130
Felsky D, Roostaei T, Nho K, Risacher SL, Bradshaw EM, Petyuk V et al (2019) Neuropathological correlates and genetic architecture of microglial activation in elderly human brain. Nat Commun 10(1):1–12
Dutta S, Sengupta P (2016) Men and mice: Relating their ages. Life Sci 152:244–248
Taneja K, Ganesh S (2020) Dendritic spine abnormalities correlate with behavioral and cognitive deficits in mouse models of Lafora disease. J Comp Neurol. https://doi.org/10.1002/cne.25006
Navarro-Cruz AR, Ramírez Y, Ayala R, Ochoa-Velasco C, Brambila E, Avila-Sosa R, Pérez-Fernández S et al (2017) Effect of chronic administration of resveratrol on cognitive performance during aging process in rats. Oxidative Med Cell Longev 2017:1–8
Gao Q, Song H, Wang XT, Liang Y, Xi YJ, Gao Y et al (2017) Molecular hydrogen increases resilience to stress in mice. Sci Rep 7(1):1–12
Hodges-Savola C, Rogers SD, Ghilardi JR, Timm DR, Mantyh PW (1996) β-Adrenergic receptors regulate astrogliosis and cell proliferation in the central nervous system in vivo. Glia 17(1):52–62
Funding
This work was supported by a grant from the Spanish Ministry of Science and Innovation SAF2017-83151-R and a grant from the National Institutes of Health P01 NS097197, which established the Lafora Epilepsy Cure Initiative (LECI), to P.S.
Author information
Authors and Affiliations
Contributions
MH and BM performed all experiments. BM analyzed and interpreted the data and was a major contributor in writing the manuscript. PS interpreted the data and wrote the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Ethics Approval and Consent to Participate
All animal experiments were approved by the animal committee of the Instituto de Biomedicina de Valencia-CSIC [Permit Number: INTRA12 (IBV-4)] and carried out in accordance with recommendations for the Care and Use of Laboratory Animals of the Consejo Superior de Investigaciones Cientificas (CSIC, Spain).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 3726 kb)
Rights and permissions
About this article
Cite this article
Mollá, B., Heredia, M. & Sanz, P. Modulators of Neuroinflammation Have a Beneficial Effect in a Lafora Disease Mouse Model. Mol Neurobiol 58, 2508–2522 (2021). https://doi.org/10.1007/s12035-021-02285-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02285-1