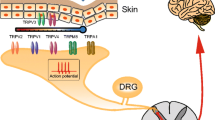
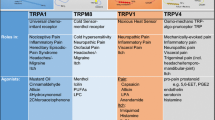
Abstract
Orofacial pain, including temporomandibular joint disorders pain, trigeminal neuralgia, dental pain, and debilitating headaches, affects millions of Americans each year with significant population health impact. Despite the existence of a large body of information on the subject, the molecular underpinnings of orofacial pain remain elusive. Two decades of research has identified that transient receptor potential (TRP) ion channels play a crucial role in pathological pain. A number of TRP ion channels are clearly expressed in the trigeminal sensory system and have critical functions in the transduction and pathogenesis of orofacial pain. Although there are many similarities, the orofacial sensory system shows some distinct peripheral and central pain processing and different sensitivities from the spinal sensory system. Relative to the extensive review on TRPs in spinally-mediated pain, the summary of TRPs in trigeminally-mediated pain has not been well-documented. This review focuses on the current experimental evidence involving TRP ion channels, particularly TRPV1, TRPA1, TRPV4, and TRPM8 in orofacial pain, and discusses their possible cellular and molecular mechanisms.

Similar content being viewed by others
Data Availability
Not applicable to this study as no dataset or material was generated during the current study.
References
Macfarlane TV, Blinkhorn AS, Davies RM, Ryan P, Worthington HV, Macfarlane GJ (2002) Orofacial pain: just another chronic pain? Results from a population-based survey. Pain 99(3):453–458. https://doi.org/10.1016/s0304-3959(02)00181-1
Chichorro JG, Porreca F, Sessle B (2017) Mechanisms of craniofacial pain. Cephalalgia 37(7):613–626. https://doi.org/10.1177/0333102417704187
Benoliel R, Sharav Y (2010) Chronic orofacial pain. Curr Pain Headache Rep 14(1):33–40. https://doi.org/10.1007/s11916-009-0085-y
González-Ramírez R, Chen Y, Liedtke WB, Morales-Lázaro SL (2017) TRP channels and pain. In: Emir TLR (ed) Neurobiology of TRP Channels. CRC Press/Taylor & Francis© 2018 by Taylor & Francis Group, LLC., Boca Raton (FL), pp. 125–147. https://doi.org/10.4324/9781315152837-8
Moore C, Gupta R, Jordt SE, Chen Y, Liedtke WB (2018) Regulation of pain and itch by TRP channels. Neurosci Bull 34(1):120–142. https://doi.org/10.1007/s12264-017-0200-8
Bereiter DA, Hargreaves KM, Hu JW (2008) 5.32 - trigeminal mechanisms of nociception: peripheral and brainstem organization. In: Masland RH, Albright TD, Albright TD et al (eds) The senses: A comprehensive reference. Academic Press, New York, pp. 435–460. https://doi.org/10.1016/B978-012370880-9.00174-2
Hargreaves KM (2011) Orofacial pain. Pain 152(3 Suppl):S25–S32. https://doi.org/10.1016/j.pain.2010.12.024
Rodriguez E, Sakurai K, Xu J, Chen Y, Toda K, Zhao S, Han BX, Ryu D et al (2017) A craniofacial-specific monosynaptic circuit enables heightened affective pain. Nat Neurosci 20(12):1734–1743. https://doi.org/10.1038/s41593-017-0012-1
Bongenhielm U, Boissonade FM, Westermark A, Robinson PP, Fried K (1999) Sympathetic nerve sprouting fails to occur in the trigeminal ganglion after peripheral nerve injury in the rat. Pain 82(3):283–288. https://doi.org/10.1016/s0304-3959(99)00064-0
Lopes DM, Denk F, McMahon SB (2017) The molecular fingerprint of dorsal root and trigeminal ganglion neurons. Front Mol Neurosci 10:304. https://doi.org/10.3389/fnmol.2017.00304
Vandewauw I, Owsianik G, Voets T (2013) Systematic and quantitative mRNA expression analysis of TRP channel genes at the single trigeminal and dorsal root ganglion level in mouse. BMC Neurosci 14:21. https://doi.org/10.1186/1471-2202-14-21
Benemei S, Dussor G (2019) TRP channels and migraine: recent developments and new therapeutic opportunities. Pharmaceuticals (Basel) 12(2). https://doi.org/10.3390/ph12020054
Dussor G, Yan J, Xie JY, Ossipov MH, Dodick DW, Porreca F (2014) Targeting TRP channels for novel migraine therapeutics. ACS Chem Neurosci 5(11):1085–1096. https://doi.org/10.1021/cn500083e
Benemei S, De Cesaris F, Fusi C, Rossi E, Lupi C, Geppetti P (2013) TRPA1 and other TRP channels in migraine. J Headache Pain 14(1):71. https://doi.org/10.1186/1129-2377-14-71
Aroucha JM, Ximenes RC, Vasconcelos FM, Nery MW, Sougey EB (2014) Temporomandibular disorders and eating disorders: a literature review. Trends Psychiatry Psychother 36(1):11–15. https://doi.org/10.1590/2237-6089-2013-0006
Eaves ER, Nichter M, Ritenbaugh C, Sutherland E, Dworkin SF (2015) Works of illness and the challenges of social risk and the specter of pain in the lived experience of TMD. Med Anthropol Q 29(2):157–177. https://doi.org/10.1111/maq.12146
Ioi H, Kido MA, Zhang JQ, Yamaza T, Nakata S, Nakasima A, Tanaka T (2006) Capsaicin receptor expression in the rat temporomandibular joint. Cell Tissue Res 325(1):47–54. https://doi.org/10.1007/s00441-006-0183-7
Lund JP, Sadeghi S, Athanassiadis T, Caram Salas N, Auclair F, Thivierge B, Arsenault I, Rompré P et al (2010) Assessment of the potential role of muscle spindle mechanoreceptor afferents in chronic muscle pain in the rat masseter muscle. PLoS One 5(6):e11131. https://doi.org/10.1371/journal.pone.0011131
Ro JY, Lee JS, Zhang Y (2009) Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. Pain 144(3):270–277. https://doi.org/10.1016/j.pain.2009.04.021
Benbow T, Ranjbar Ekbatan M, Cairns BE (2020) α(1) adrenergic receptor activation has a dynamic effect on masticatory muscle afferent fibers. Neuropharmacology 175:108197. https://doi.org/10.1016/j.neuropharm.2020.108197
Sato J, Segami N, Yoshitake Y, Kaneyama K, Abe A, Yoshimura H, Fujimura K (2005) Expression of capsaicin receptor TRPV-1 in synovial tissues of patients with symptomatic internal derangement of the temporomandibular joint and joint pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 100(6):674–681. https://doi.org/10.1016/j.tripleo.2005.03.008
Wu YW, Hao T, Kou XX, Gan YH, Ma XC (2015) Synovial TRPV1 is upregulated by 17-β-estradiol and involved in allodynia of inflamed temporomandibular joints in female rats. Arch Oral Biol 60(9):1310–1318. https://doi.org/10.1016/j.archoralbio.2015.05.011
Wu YW, Bi YP, Kou XX, Xu W, Ma LQ, Wang KW, Gan YH, Ma XC (2010) 17-Beta-estradiol enhanced allodynia of inflammatory temporomandibular joint through upregulation of hippocampal TRPV1 in ovariectomized rats. J Neurosci 30(26):8710–8719. https://doi.org/10.1523/jneurosci.6323-09.2010
Simonic-Kocijan S, Zhao X, Liu W, Wu Y, Uhac I, Wang K (2013) TRPV1 channel-mediated bilateral allodynia induced by unilateral masseter muscle inflammation in rats. Mol Pain 9:68. https://doi.org/10.1186/1744-8069-9-68
Chung MK, Park J, Asgar J, Ro JY (2016) Transcriptome analysis of trigeminal ganglia following masseter muscle inflammation in rats. Mol Pain 12:174480691666852. https://doi.org/10.1177/1744806916668526
Chen Y, Williams SH, McNulty AL, Hong JH, Lee SH, Rothfusz NE, Parekh PK, Moore C et al (2013) Temporomandibular joint pain: a critical role for Trpv4 in the trigeminal ganglion. Pain 154(8):1295–1304. https://doi.org/10.1016/j.pain.2013.04.004
Wang K, Arendt-Nielsen L, Svensson P (2002) Capsaicin-induced muscle pain alters the excitability of the human jaw-stretch reflex. J Dent Res 81(9):650–654. https://doi.org/10.1177/154405910208100915
Wang S, Brigoli B, Lim J, Karley A, Chung MK (2018) Roles of TRPV1 and TRPA1 in spontaneous pain from inflamed masseter muscle. Neuroscience 384:290–299. https://doi.org/10.1016/j.neuroscience.2018.05.048
Wang S, Lim J, Joseph J, Wang S, Wei F, Ro JY, Chung MK (2017) Spontaneous and bite-evoked muscle pain are mediated by a common nociceptive pathway with differential contribution by TRPV1. J Pain 18(11):1333–1345. https://doi.org/10.1016/j.jpain.2017.06.005
Denadai-Souza A, Martin L, de Paula MA, de Avellar MC, Muscará MN, Vergnolle N, Cenac N (2012) Role of transient receptor potential vanilloid 4 in rat joint inflammation. Arthritis Rheum 64(6):1848–1858. https://doi.org/10.1002/art.34345
Lee SM, Cho YS, Kim TH, Jin MU, Ahn DK, Noguchi K, Bae YC (2012) An ultrastructural evidence for the expression of transient receptor potential ankyrin 1 (TRPA1) in astrocytes in the rat trigeminal caudal nucleus. J Chem Neuroanat 45(1–2):45–49. https://doi.org/10.1016/j.jchemneu.2012.07.003
Asgar J, Zhang Y, Saloman JL, Wang S, Chung MK, Ro JY (2015) The role of TRPA1 in muscle pain and mechanical hypersensitivity under inflammatory conditions in rats. Neuroscience 310:206–215. https://doi.org/10.1016/j.neuroscience.2015.09.042
Kogawa EM, Calderon PS, Lauris JR, Araujo CR, Conti PC (2006) Evaluation of maximal bite force in temporomandibular disorders patients. J Oral Rehabil 33(8):559–565. https://doi.org/10.1111/j.1365-2842.2006.01619.x
Castrillon EE, Cairns BE, Ernberg M, Wang K, Sessle BJ, Arendt-Nielsen L, Svensson P (2008) Effect of peripheral NMDA receptor blockade with ketamine on chronic myofascial pain in temporomandibular disorder patients: a randomized, double-blinded, placebo-controlled trial. J Orofac Pain 22(2):122–130
Pereira LJ, Steenks MH, de Wijer A, Speksnijder CM, van der Bilt A (2009) Masticatory function in subacute TMD patients before and after treatment. J Oral Rehabil 36(6):391–402. https://doi.org/10.1111/j.1365-2842.2008.01920.x
Guo W, Zou S, Mohammad Z, Wang S, Yang J, Li H, Dubner R, Wei F et al (2019) Voluntary biting behavior as a functional measure of orofacial pain in mice. Physiol Behav 204:129–139. https://doi.org/10.1016/j.physbeh.2019.02.024
Akopian AN (2011) Regulation of nociceptive transmission at the periphery via TRPA1-TRPV1 interactions. Curr Pharm Biotechnol 12(1):89–94. https://doi.org/10.2174/138920111793937952
Jones MR, Urits I, Ehrhardt KP, Cefalu JN, Kendrick JB, Park DJ, Cornett EM, Kaye AD et al (2019) A comprehensive review of trigeminal neuralgia. Curr Pain Headache Rep 23(10):74. https://doi.org/10.1007/s11916-019-0810-0
International classification of orofacial pain, 1st edition (ICOP) (2020) Cephalalgia 40(2):129–221. https://doi.org/10.1177/0333102419893823
Rossi HL, Jenkins AC, Kaufman J, Bhattacharyya I, Caudle RM, Neubert JK (2012) Characterization of bilateral trigeminal constriction injury using an operant facial pain assay. Neuroscience 224:294–306. https://doi.org/10.1016/j.neuroscience.2012.08.015
Demartini C, Greco R, Zanaboni AM, Francesconi O, Nativi C, Tassorelli C, Deseure K (2018) Antagonism of transient receptor potential ankyrin type-1 channels as a potential target for the treatment of trigeminal neuropathic pain: study in an animal model. Int J Mol Sci 19(11). https://doi.org/10.3390/ijms19113320
Wu C, Xie N, Lian Y, Xu H, Chen C, Zheng Y, Chen Y, Zhang H (2016) Central antinociceptive activity of peripherally applied botulinum toxin type A in lab rat model of trigeminal neuralgia. Springerplus 5:431. https://doi.org/10.1186/s40064-016-2071-2
Urano H, Ara T, Fujinami Y, Hiraoka BY (2012) Aberrant TRPV1 expression in heat hyperalgesia associated with trigeminal neuropathic pain. Int J Med Sci 9(8):690–697. https://doi.org/10.7150/ijms.4706
Tsuboi Y, Honda K, Bae YC, Shinoda M, Kondo M, Katagiri A, Echizenya S, Kamakura S et al (2015) Morphological and functional changes in regenerated primary afferent fibres following mental and inferior alveolar nerve transection. Eur J Pain 19(9):1258–1266. https://doi.org/10.1002/ejp.650
Zakir HM, Mostafeezur RM, Suzuki A, Hitomi S, Suzuki I, Maeda T, Seo K, Yamada Y et al (2012) Expression of TRPV1 channels after nerve injury provides an essential delivery tool for neuropathic pain attenuation. PLoS One 7(9):e44023. https://doi.org/10.1371/journal.pone.0044023
Kim HY, Park CK, Cho IH, Jung SJ, Kim JS, Oh SB (2008) Differential Changes in TRPV1 expression after trigeminal sensory nerve injury. J Pain 9(3):280–288. https://doi.org/10.1016/j.jpain.2007.11.013
Kim YS, Chu Y, Han L, Li M, Li Z, LaVinka PC, Sun S, Tang Z et al (2014) Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron 81(4):873–887. https://doi.org/10.1016/j.neuron.2013.12.011
Zakir HM, Masuda Y, Kitagawa J (2020) A novel approach for detection of functional expression of TRPV1 channels on regenerated neurons following nerve injury. J Oral Sci 62(2):136–139. https://doi.org/10.2334/josnusd.19-0356
Cruz LS, Kopruszinski CM, Chichorro JG (2014) Intraganglionar resiniferatoxin prevents orofacial inflammatory and neuropathic hyperalgesia. Behav Pharmacol 25(2):112–118. https://doi.org/10.1097/fbp.0000000000000024
Wang S, Bian C, Yang J, Arora V, Gao Y, Wei F, Chung MK (2020) Ablation of TRPV1+ afferent terminals by capsaicin mediates long-lasting analgesia for trigeminal neuropathic pain. eNeuro 7(3):ENEURO.0118–ENEU20.2020. https://doi.org/10.1523/eneuro.0118-20.2020
Mou J, Paillard F, Turnbull B, Trudeau J, Stoker M, Katz NP (2013) Efficacy of Qutenza® (capsaicin) 8% patch for neuropathic pain: a meta-analysis of the Qutenza Clinical Trials Database. Pain 154(9):1632–1639. https://doi.org/10.1016/j.pain.2013.04.044
Treede RD, Wagner T, Kern KU, Husstedt IW, Arendt G, Birklein F, Cegla T, Freynhagen R et al (2013) Mechanism- and experience-based strategies to optimize treatment response to the capsaicin 8% cutaneous patch in patients with localized neuropathic pain. Curr Med Res Opin 29(5):527–538. https://doi.org/10.1185/03007995.2013.781019
Sugawara S, Shinoda M, Hayashi Y, Saito H, Asano S, Kubo A, Shibuta I, Furukawa A et al (2019) Increase in IGF-1 expression in the injured infraorbital nerve and possible implications for orofacial neuropathic pain. Int J Mol Sci 20(24). https://doi.org/10.3390/ijms20246360
Trevisan G, Benemei S, Materazzi S, De Logu F, De Siena G, Fusi C, Fortes Rossato M, Coppi E et al (2016) TRPA1 mediates trigeminal neuropathic pain in mice downstream of monocytes/macrophages and oxidative stress. Brain 139(Pt 5):1361–1377. https://doi.org/10.1093/brain/aww038
Zhang Y, Su Q, Lian Y, Chen Y (2019) Botulinum toxin type A reduces the expression of transient receptor potential melastatin 3 and transient receptor potential vanilloid type 4 in the trigeminal subnucleus caudalis of a rat model of trigeminal neuralgia. Neuroreport 30(10):735–740. https://doi.org/10.1097/wnr.0000000000001268
Zuo X, Ling JX, Xu GY, Gu JG (2013) Operant behavioral responses to orofacial cold stimuli in rats with chronic constrictive trigeminal nerve injury: Effects of menthol and capsazepine. Mol Pain 9:28. https://doi.org/10.1186/1744-8069-9-28
Neumann S, Doubell TP, Leslie T, Woolf CJ (1996) Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature 384(6607):360–364. https://doi.org/10.1038/384360a0
Schäfers M, Geis C, Svensson CI, Luo ZD, Sommer C (2003) Selective increase of tumour necrosis factor-alpha in injured and spared myelinated primary afferents after chronic constrictive injury of rat sciatic nerve. Eur J Neurosci 17(4):791–804. https://doi.org/10.1046/j.1460-9568.2003.02504.x
Binshtok AM, Bean BP, Woolf CJ (2007) Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature 449(7162):607–610. https://doi.org/10.1038/nature06191
Mishra SK, Hoon MA (2010) Ablation of TrpV1 neurons reveals their selective role in thermal pain sensation. Mol Cell Neurosci 43(1):157–163. https://doi.org/10.1016/j.mcn.2009.10.006
Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP (2006) TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50(2):277–289. https://doi.org/10.1016/j.neuron.2006.03.042
Morra ME, Elgebaly A, Elmaraezy A, Khalil AM, Altibi AM, Vu TL, Mostafa MR, Huy NT et al (2016) Therapeutic efficacy and safety of botulinum toxin a therapy in trigeminal neuralgia: a systematic review and meta-analysis of randomized controlled trials. J Headache Pain 17(1):63. https://doi.org/10.1186/s10194-016-0651-8
Rubis A, Juodzbalys G (2020) The use of botulinum toxin a in the management of trigeminal neuralgia: a systematic literature review. J Oral Maxillofac Res 11(2):e2. https://doi.org/10.5037/jomr.2020.11202
Shimizu T, Shibata M, Toriumi H, Iwashita T, Funakubo M, Sato H, Kuroi T, Ebine T et al (2012) Reduction of TRPV1 expression in the trigeminal system by botulinum neurotoxin type-A. Neurobiol Dis 48(3):367–378. https://doi.org/10.1016/j.nbd.2012.07.010
Gill CH, Randall A, Bates SA, Hill K, Owen D, Larkman PM, Cairns W, Yusaf SP et al (2004) Characterization of the human HCN1 channel and its inhibition by capsazepine. Br J Pharmacol 143(3):411–421. https://doi.org/10.1038/sj.bjp.0705945
Orio P, Madrid R, de la Peña E, Parra A, Meseguer V, Bayliss DA, Belmonte C, Viana F (2009) Characteristics and physiological role of hyperpolarization activated currents in mouse cold thermoreceptors. J Physiol 587(Pt 9):1961–1976. https://doi.org/10.1113/jphysiol.2008.165738
Chung G, Jung SJ, Oh SB (2013) Cellular and molecular mechanisms of dental nociception. J Dent Res 92(11):948–955. https://doi.org/10.1177/0022034513501877
Ichikawa H, Sugimoto T (2001) VR1-immunoreactive primary sensory neurons in the rat trigeminal ganglion. Brain Res 890(1):184–188. https://doi.org/10.1016/s0006-8993(00)03253-4
Chung MK, Lee J, Duraes G, Ro JY (2011) Lipopolysaccharide-induced pulpitis up-regulates TRPV1 in trigeminal ganglia. J Dent Res 90(9):1103–1107. https://doi.org/10.1177/0022034511413284
Stenholm E, Bongenhielm U, Ahlquist M, Fried K (2002) VRl- and VRL-l-like immunoreactivity in normal and injured trigeminal dental primary sensory neurons of the rat. Acta Odontol Scand 60(2):72–79. https://doi.org/10.1080/000163502753509455
Park CK, Kim MS, Fang Z, Li HY, Jung SJ, Choi SY, Lee SJ, Park K et al (2006) Functional expression of thermo-transient receptor potential channels in dental primary afferent neurons: implication for tooth pain. J Biol Chem 281(25):17304–17311. https://doi.org/10.1074/jbc.M511072200
Kim HY, Chung G, Jo HJ, Kim YS, Bae YC, Jung SJ, Kim JS, Oh SB (2011) Characterization of dental nociceptive neurons. J Dent Res 90(6):771–776. https://doi.org/10.1177/0022034511399906
Okumura R, Shima K, Muramatsu T, Nakagawa K, Shimono M, Suzuki T, Magloire H, Shibukawa Y (2005) The odontoblast as a sensory receptor cell? The expression of TRPV1 (VR-1) channels. Arch Histol Cytol 68(4):251–257. https://doi.org/10.1679/aohc.68.251
Tsumura M, Sobhan U, Muramatsu T, Sato M, Ichikawa H, Sahara Y, Tazaki M, Shibukawa Y (2012) TRPV1-mediated calcium signal couples with cannabinoid receptors and sodium-calcium exchangers in rat odontoblasts. Cell Calcium 52(2):124–136. https://doi.org/10.1016/j.ceca.2012.05.002
Morgan CR, Rodd HD, Clayton N, Davis JB, Boissonade FM (2005) Vanilloid receptor 1 expression in human tooth pulp in relation to caries and pain. J Orofac Pain 19(3):248–260
Ohkura M, Ohkura N, Yoshiba N, Yoshiba K, Ida-Yonemochi H, Ohshima H, Saito I, Okiji T (2018) Orthodontic force application upregulated pain-associated prostaglandin-I(2)/PGI(2)-receptor/TRPV1 pathway-related gene expression in rat molars. Odontology 106(1):2–10. https://doi.org/10.1007/s10266-017-0309-2
Wang S, Kim M, Ali Z, Ong K, Pae EK, Chung MK (2019) TRPV1 and TRPV1-expressing nociceptors mediate orofacial pain behaviors in a mouse model of orthodontic tooth movement. Front Physiol 10:1207. https://doi.org/10.3389/fphys.2019.01207
Watase T, Shimizu K, Komiya H, Ohara K, Iwata K, Ogiso B (2018) Involvement of transient receptor potential vanilloid 1 channel expression in orofacial cutaneous hypersensitivity following tooth pulp inflammation. J Oral Sci 60(1):8–13. https://doi.org/10.2334/josnusd.16-0854
Ito M, Ono K, Hitomi S, Nodai T, Sago T, Yamaguchi K, Harano N, Gunnjigake K et al (2017) Prostanoid-dependent spontaneous pain and PAR(2)-dependent mechanical allodynia following oral mucosal trauma: involvement of TRPV1, TRPA1 and TRPV4. Mol Pain 13:1744806917704138. https://doi.org/10.1177/1744806917704138
Ichikawa H, Sugimoto T (2000) Vanilloid receptor 1-like receptor-immunoreactive primary sensory neurons in the rat trigeminal nervous system. Neuroscience 101(3):719–725. https://doi.org/10.1016/s0306-4522(00)00427-9
Gibbs JL, Melnyk JL, Basbaum AI (2011) Differential TRPV1 and TRPV2 channel expression in dental pulp. J Dent Res 90(6):765–770. https://doi.org/10.1177/0022034511402206
Sato M, Sobhan U, Tsumura M, Kuroda H, Soya M, Masamura A, Nishiyama A, Katakura A et al (2013) Hypotonic-induced stretching of plasma membrane activates transient receptor potential vanilloid channels and sodium-calcium exchangers in mouse odontoblasts. J Endod 39(6):779–787. https://doi.org/10.1016/j.joen.2013.01.012
Wen W, Que K, Zang C, Wen J, Sun G, Zhao Z, Li Y (2017) Expression and distribution of three transient receptor potential vanilloid(TRPV) channel proteins in human odontoblast-like cells. J Mol Histol 48(5–6):367–377. https://doi.org/10.1007/s10735-017-9735-2
Bakri MM, Yahya F, Munawar KMM, Kitagawa J, Hossain MZ (2018) Transient receptor potential vanilloid 4 (TRPV4) expression on the nerve fibers of human dental pulp is upregulated under inflammatory condition. Arch Oral Biol 89:94–98. https://doi.org/10.1016/j.archoralbio.2018.02.011
Michot B, Lee CS, Gibbs JL (2018) TRPM8 and TRPA1 do not contribute to dental pulp sensitivity to cold. Sci Rep 8(1):13198. https://doi.org/10.1038/s41598-018-31487-2
Kim YS, Jung HK, Kwon TK, Kim CS, Cho JH, Ahn DK, Bae YC (2012) Expression of transient receptor potential ankyrin 1 in human dental pulp. J Endod 38(8):1087–1092. https://doi.org/10.1016/j.joen.2012.04.024
Tazawa K, Ikeda H, Kawashima N, Okiji T (2017) Transient receptor potential melastatin (TRPM) 8 is expressed in freshly isolated native human odontoblasts. Arch Oral Biol 75:55–61. https://doi.org/10.1016/j.archoralbio.2016.12.007
Yeon KY, Chung G, Shin MS, Jung SJ, Kim JS, Oh SB (2009) Adult rat odontoblasts lack noxious thermal sensitivity. J Dent Res 88(4):328–332. https://doi.org/10.1177/0022034509334100
Haas ET, Rowland K, Gautam M (2011) Tooth injury increases expression of the cold sensitive TRP channel TRPA1 in trigeminal neurons. Arch Oral Biol 56(12):1604–1609. https://doi.org/10.1016/j.archoralbio.2011.06.014
El Karim I, McCrudden MT, Linden GJ, Abdullah H, Curtis TM, McGahon M, About I, Irwin C et al (2015) TNF-α-induced p38MAPK activation regulates TRPA1 and TRPV4 activity in odontoblast-like cells. Am J Pathol 185(11):2994–3002. https://doi.org/10.1016/j.ajpath.2015.07.020
El Karim IA, McCrudden MT, McGahon MK, Curtis TM, Jeanneau C, Giraud T, Irwin CR, Linden GJ et al (2016) Biodentine reduces tumor necrosis factor alpha-induced TRPA1 expression in odontoblastlike cells. J Endod 42(4):589–595. https://doi.org/10.1016/j.joen.2015.12.017
Morii A, Miyamura Y, Sago MI, Mizuhara M, Shikayama T, Naniwa M, Hitomi S, Ujihara I et al (2020) Orthodontic force-induced oxidative stress in the periodontal tissue and dental pulp elicits nociception via activation/sensitization of TRPA1 on nociceptive fibers. Free Radic Biol Med 147:175–186. https://doi.org/10.1016/j.freeradbiomed.2019.12.016
Kwon M, Baek SH, Park CK, Chung G, Oh SB (2014) Single-cell RT-PCR and immunocytochemical detection of mechanosensitive transient receptor potential channels in acutely isolated rat odontoblasts. Arch Oral Biol 59(12):1266–1271. https://doi.org/10.1016/j.archoralbio.2014.07.016
Won J, Vang H, Kim JH, Lee PR, Kang Y, Oh SB (2018) TRPM7 mediates mechanosensitivity in adult rat odontoblasts. J Dent Res 97(9):1039–1046. https://doi.org/10.1177/0022034518759947
Tsumura M, Sobhan U, Sato M, Shimada M, Nishiyama A, Kawaguchi A, Soya M, Kuroda H et al (2013) Functional expression of TRPM8 and TRPA1 channels in rat odontoblasts. PLoS One 8(12):e82233. https://doi.org/10.1371/journal.pone.0082233
El Karim IA, Linden GJ, Curtis TM, About I, McGahon MK, Irwin CR, Lundy FT (2011) Human odontoblasts express functional thermo-sensitive TRP channels: implications for dentin sensitivity. Pain 152(10):2211–2223. https://doi.org/10.1016/j.pain.2010.10.016
Alvarado LT, Perry GM, Hargreaves KM, Henry MA (2007) TRPM8 axonal expression is decreased in painful human teeth with irreversible pulpitis and cold hyperalgesia. J Endod 33(10):1167–1171. https://doi.org/10.1016/j.joen.2007.06.018
Kim YS, Kim YJ, Paik SK, Cho YS, Kwon TG, Ahn DK, Kim SK, Yoshida A et al (2009) Expression of metabotropic glutamate receptor mGluR5 in human dental pulp. J Endod 35(5):690–694. https://doi.org/10.1016/j.joen.2009.02.005
Renton T, Yiangou Y, Baecker PA, Ford AP, Anand P (2003) Capsaicin receptor VR1 and ATP purinoceptor P2X3 in painful and nonpainful human tooth pulp. J Orofac Pain 17(3):245–250
Solé-Magdalena A, Martínez-Alonso M, Coronado CA, Junquera LM, Cobo J, Vega JA (2018) Molecular basis of dental sensitivity: the odontoblasts are multisensory cells and express multifunctional ion channels. Ann Anat 215:20–29. https://doi.org/10.1016/j.aanat.2017.09.006
Nakano Y, Le MH, Abduweli D, Ho SP, Ryazanova LV, Hu Z, Ryazanov AG, Den Besten PK et al (2016) A critical role of TRPM7 as an ion channel protein in mediating the mineralization of the craniofacial hard tissues. Front Physiol 7:258. https://doi.org/10.3389/fphys.2016.00258
Won J, Kim JH, Oh SB (2018) Molecular expression of Mg(2+) regulator TRPM7 and CNNM4 in rat odontoblasts. Arch Oral Biol 96:182–188. https://doi.org/10.1016/j.archoralbio.2018.09.011
Markowitz K (2010) Pretty painful: why does tooth bleaching hurt? Med Hypotheses 74(5):835–840. https://doi.org/10.1016/j.mehy.2009.11.044
Gunthorpe MJ, Chizh BA (2009) Clinical development of TRPV1 antagonists: targeting a pivotal point in the pain pathway. Drug Discov Today 14(1–2):56–67. https://doi.org/10.1016/j.drudis.2008.11.005
Mickle AD, Shepherd AJ, Mohapatra DP (2016) Nociceptive TRP channels: sensory detectors and transducers in multiple pain pathologies. Pharmaceuticals (Basel) 9(4). https://doi.org/10.3390/ph9040072
Quiding H, Jonzon B, Svensson O, Webster L, Reimfelt A, Karin A, Karlsten R, Segerdahl M (2013) TRPV1 antagonistic analgesic effect: a randomized study of AZD1386 in pain after third molar extraction. Pain 154(6):808–812. https://doi.org/10.1016/j.pain.2013.02.004
Gavva NR, Bannon AW, Surapaneni S, Hovland DN Jr, Lehto SG, Gore A, Juan T, Deng H et al (2007) The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci 27(13):3366–3374. https://doi.org/10.1523/jneurosci.4833-06.2007
Wang H, Siemens J (2015) TRP ion channels in thermosensation, thermoregulation and metabolism. Temperature (Austin) 2(2):178–187. https://doi.org/10.1080/23328940.2015.1040604
Moran MM, Szallasi A (2018) Targeting nociceptive transient receptor potential channels to treat chronic pain: current state of the field. Br J Pharmacol 175(12):2185–2203. https://doi.org/10.1111/bph.14044
Gomtsyan A, McDonald HA, Schmidt RG, Daanen JF, Voight EA, Segreti JA, Puttfarcken PS, Reilly RM et al (2015) TRPV1 ligands with hyperthermic, hypothermic and no temperature effects in rats. Temperature (Austin) 2(2):297–301. https://doi.org/10.1080/23328940.2015.1046013
Voight EA, Gomtsyan AR, Daanen JF, Perner RJ, Schmidt RG, Bayburt EK, DiDomenico S, McDonald HA et al (2014) Discovery of (R)-1-(7-chloro-2,2-bis(fluoromethyl)chroman-4-yl)-3-(3-methylisoquinolin-5-yl)urea (A-1165442): a temperature-neutral transient receptor potential vanilloid-1 (TRPV1) antagonist with analgesic efficacy. J Med Chem 57(17):7412–7424. https://doi.org/10.1021/jm500916t
Lehto SG, Tamir R, Deng H, Klionsky L, Kuang R, Le A, Lee D, Louis JC et al (2008) Antihyperalgesic effects of (R,E)-N-(2-hydroxy-2,3-dihydro-1H-inden-4-yl)-3-(2-(piperidin-1-yl)-4-(trifluoromethyl)phenyl)-acrylamide (AMG8562), a novel transient receptor potential vanilloid type 1 modulator that does not cause hyperthermia in rats. J Pharmacol Exp Ther 326(1):218–229. https://doi.org/10.1124/jpet.107.132233
Chizh BA, O'Donnell MB, Napolitano A, Wang J, Brooke AC, Aylott MC, Bullman JN, Gray EJ et al (2007) The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain 132(1–2):132–141. https://doi.org/10.1016/j.pain.2007.06.006
Chung MK, Campbell JN (2016) Use of capsaicin to treat pain: mechanistic and therapeutic considerations. Pharmaceuticals (Basel) 9(4). https://doi.org/10.3390/ph9040066
Yong YL, Tan LT, Ming LC, Chan KG, Lee LH, Goh BH, Khan TM (2016) The effectiveness and safety of topical capsaicin in postherpetic neuralgia: a systematic review and meta-analysis. Front Pharmacol 7:538. https://doi.org/10.3389/fphar.2016.00538
Weng HJ, Patel KN, Jeske NA, Bierbower SM, Zou W, Tiwari V, Zheng Q, Tang Z et al (2015) Tmem100 is a regulator of TRPA1-TRPV1 complex and contributes to persistent pain. Neuron 85(4):833–846. https://doi.org/10.1016/j.neuron.2014.12.065
Chen Y, Kanju P, Fang Q, Lee SH, Parekh PK, Lee W, Moore C, Brenner D et al (2014) TRPV4 is necessary for trigeminal irritant pain and functions as a cellular formalin receptor. Pain 155(12):2662–2672. https://doi.org/10.1016/j.pain.2014.09.033
Vermeulen W, De Man JG, De Schepper HU, Bult H, Moreels TG, Pelckmans PA, De Winter BY (2013) Role of TRPV1 and TRPA1 in visceral hypersensitivity to colorectal distension during experimental colitis in rats. Eur J Pharmacol 698(1–3):404–412. https://doi.org/10.1016/j.ejphar.2012.10.014
Schwartz ES, Christianson JA, Chen X, La JH, Davis BM, Albers KM, Gebhart GF (2011) Synergistic role of TRPV1 and TRPA1 in pancreatic pain and inflammation. Gastroenterology 140(4):1283-1291.e1281-1282. https://doi.org/10.1053/j.gastro.2010.12.033
Kanju P, Chen Y, Lee W, Yeo M, Lee SH, Romac J, Shahid R, Fan P et al (2016) Small molecule dual-inhibitors of TRPV4 and TRPA1 for attenuation of inflammation and pain. Sci Rep 6:26894. https://doi.org/10.1038/srep26894
Häggman-Henrikson B, Liv P, Ilgunas A, Visscher CM, Lobbezoo F, Durham J, Lövgren A (2020) Increasing gender differences in the prevalence and chronification of orofacial pain in the population. Pain. Publish Ahead of Print. https://doi.org/10.1097/j.pain.0000000000001872
Halpern LR, Levine M, Dodson TB (2007) Sexual dimorphism and temporomandibular disorders (TMD). Oral Maxillofac Surg Clin North Am 19(2):267–277, viii. https://doi.org/10.1016/j.coms.2007.01.012
Cairns BE (2007) The influence of gender and sex steroids on craniofacial nociception. Headache 47(2):319–324. https://doi.org/10.1111/j.1526-4610.2006.00708.x
Mecklenburg J, Zou Y, Wangzhou A, Garcia D, Lai Z, Tumanov AV, Dussor G, Price TJ et al (2020) Transcriptomic sex differences in sensory neuronal populations of mice. Sci Rep 10(1):15278. https://doi.org/10.1038/s41598-020-72285-z
Caudle RM, Caudle SL, Jenkins AC, Ahn AH, Neubert JK (2017) Sex differences in mouse transient receptor potential cation channel, subfamily M, member 8 expressing trigeminal ganglion neurons. PLoS One 12(5):e0176753. https://doi.org/10.1371/journal.pone.0176753
Artero-Morales M, González-Rodríguez S, Ferrer-Montiel A (2018) TRP channels as potential targets for sex-related differences in migraine pain. Front Mol Biosci 5:73. https://doi.org/10.3389/fmolb.2018.00073
Zakrzewska JM, Wu J, Mon-Williams M, Phillips N, Pavitt SH (2017) Evaluating the impact of trigeminal neuralgia. Pain 158(6):1166–1174. https://doi.org/10.1097/j.pain.0000000000000853
Giannakopoulos NN, Keller L, Rammelsberg P, Kronmüller KT, Schmitter M (2010) Anxiety and depression in patients with chronic temporomandibular pain and in controls. J Dent 38(5):369–376. https://doi.org/10.1016/j.jdent.2010.01.003
Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, Bair E, Baraian C et al (2013) Psychological factors associated with development of TMD: the OPPERA prospective cohort study. J Pain 14(12 Suppl):T75–T90. https://doi.org/10.1016/j.jpain.2013.06.009
Smith JG, Elias LA, Yilmaz Z, Barker S, Shah K, Shah S, Renton T (2013) The psychosocial and affective burden of posttraumatic neuropathy following injuries to the trigeminal nerve. J Orofac Pain 27 (4):293-303. doi:https://doi.org/10.11607/jop.1056
Schmidt K, Schunke O, Forkmann K, Bingel U (2015) Enhanced short-term sensitization of facial compared with limb heat pain. J Pain 16(8):781–790. https://doi.org/10.1016/j.jpain.2015.05.003
Schmidt K, Forkmann K, Sinke C, Gratz M, Bitz A, Bingel U (2016) The differential effect of trigeminal vs. peripheral pain stimulation on visual processing and memory encoding is influenced by pain-related fear. Neuroimage 134:386–395. https://doi.org/10.1016/j.neuroimage.2016.03.026
Hunt SP, Mantyh PW (2001) The molecular dynamics of pain control. Nat Rev Neurosci 2(2):83–91. https://doi.org/10.1038/35053509
Craig AD (1995) Distribution of brainstem projections from spinal lamina I neurons in the cat and the monkey. J Comp Neurol 361(2):225–248. https://doi.org/10.1002/cne.903610204
Gauriau C, Bernard JF (2002) Pain pathways and parabrachial circuits in the rat. Exp Physiol 87(2):251–258. https://doi.org/10.1113/eph8702357
Acknowledgments
The authors acknowledge the support by the National Institutes of Health Grant DE027454 to YC.
Funding
This work was supported by the National Institutes of Health Grant DE027454 to YC.
Author information
Authors and Affiliations
Contributions
YHL and YC conceived the original idea and designed the outlines of the study. YHL, AS, QJZ, PW, and YC performed the literature review and wrote the draft of the manuscript. YHL and YC prepared the figures and tables for the manuscript. All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors of the manuscript declare that there are no conflicts of interest.
Consent to Participate
Not applicable to this study.
Consent for Publication
Not applicable to this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Luo, Y., Suttle, A., Zhang, Q. et al. Transient Receptor Potential (TRP) Ion Channels in Orofacial Pain. Mol Neurobiol 58, 2836–2850 (2021). https://doi.org/10.1007/s12035-021-02284-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02284-2