Abstract
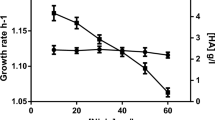
This study addresses the influence of upstream region sequence on the strength of has operon promoter in highly encapsulated S. equi subsp. zooepidemicus (SEZ). For this purpose, seven different strains were constructed. Each strain carries a point mutation in one of the following positions upstream of the has promoter: −43, −44, −49, and −50 bp. To facilitate measuring of the recombinant promoter relative strength, ß-glucuronidase gene was used as a reporter gene. Three mutations located in positions −49 and −50: AT, GT, and AG, positively impacted has promoter strength when compared to the wild type sequence GG. Conversely, two other mutations: TG and TT, exhibited a slight inhibitory effect. Further, three different strains carrying chromosomal mutations in the has promoter region were constructed. In two cases, the has operon is under the control of a stronger promoter and in the third strain the has operon is controlled by a weaker promoter. The laboratory fermenter scale cultivations confirmed the increase of hyaluronan yields for SEZPhasAG and SEZPhas2G, resulting 116 and 105 %, respectively. As expected, the yield of the hyaluronic acid of SEZPhas2B strain fell to 41 %.


Similar content being viewed by others
Abbreviations
- SEZ:
-
Streptococcus equi subsp. zooepidemicus
- HA:
-
Hyaluronic acid
- GlcUA:
-
Glucuronic acid
- GlcNAc:
-
N-acetylglucosamine
- GAS:
-
Group A streptococci
- TT:
-
Transcriptional terminator
References
Alberti, S., Ashbaugh, C. D., & Wessels, M. R. (1998). Structure of the has operon promoter and regulation of hyaluronic acid capsule expression in group A Streptococcus. Molecular Microbiology, 28, 343–353.
Ausubel, F., Brent, M. R., Kingston, R. E., Moore, D. D., Siedman, J. G., Smith, J. A., et al. (1987). Current protocols in molecular biology. New York: Wiley.
Blank, L. M., Hugenholtz, P., & Nielsen, L. K. (2008). Evolution of the hyaluronic acid synthesis (has) operon in Streptococcus zooepidemicus and other pathogenic streptococci. Journal of Molecular Evolution, 67, 13–22.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Chen, W. Y., Marcellin, E., Hung, J., & Nielsen, L. K. (2009). Hyaluronan molecular weight is controlled by UDP-N-acetylglucosamine concentration in Streptococcus zooepidemicus. Journal of Biological Chemistry, 284(27), 18007–18014.
Chien, L. J., & Lee, C. K. (2007). Enhanced hyaluronic acid production in Bacillus subtilis by coexpressing bacterial hemoglobin. Biotechnology Progress, 23(5), 1017–1022.
Chong, B. F., Blank, L. M., McLaughlin, R., & Nielsen, L. K. (2005). Microbial hyaluronic acid production. Applied Microbiology and Biotechnology, 66, 341–351.
Crater, D. L., & van de Rijn, I. (1995). Hyaluronic acid synthesis operon (has) expression in group A streptococci. Journal of Biological Chemistry, 270, 18452–18458.
DeAngelis, P. L., Jing, W., Graves, M. V., Burbank, D. E., & Van Etten, J. L. (1997). Hyaluronan synthase of Chlorella virus PBCV-1. Science, 278, 1800–1803.
DeAngelis, P. L., Papaconstantinou, J., & Weigel, P. H. (1993). Molecular cloning, identification, and sequence of the hyaluronan synthase gene from group A Streptococcus pyogenes. Journal of Biological Chemistry, 268, 19181–19184.
Dougherty, B. A., & van de Rijn, I. (1993). Molecular characterization of hasB from an operon required for hyaluronic acid synthesis in group A streptococci. Journal of Biological Chemistry, 10, 7118–7124.
Dougherty, B. A., & van de Rijn, I. (1994). Molecular characterization of hasA from an operon required for hyaluronic acid synthesis in group A streptococci. Journal of Biological Chemistry, 269, 169–175.
Grafe, S., Ellinger, T., & Malke, H. (1996). Structural dissection and functional analysis of the complex promoter of the streptokinase gene from Streptococcus equisimilis H46A. Medical Microbiology and Immunology, 185, 11–17.
Graves, M. V., Burbank, D. E., Roth, R., Heuser, J., DeAngelis, P. L., & Van Etten, J. L. (1999). Hyaluronan synthesis in virus PBCV-1 infected Chlorella-like green algae. Virology, 257, 15–23.
Heldermon, C. D., Tlapak-Simmons, V. L., Baggenstoss, B. A., & Weigel, P. H. (2001). Site-direct mutation of conserved cysteine residues does not inactivate the Streptococcus pyogenes hyaluronan synthase. Glycobiology, 11, 1017–1024.
Husman, L. K., Yung, D. L., Hollingshead, S. K., & Scott, J. R. (1997). Role of putative virulence of Streptococcus pyogenes in mouse models of long-term throat colonization and pneumonia. Infection and Immunity, 65, 1422–1430.
Johnson, D. R., Stevens, D. L., & Kaplan, E. L. (1992). Epidemiologic analysis of group A streptococcal serotypes associated with severe systemic infections, rheumatic fever, or uncomplicated pharyngitis. Journal of Infectious Diseases, 166, 374–382.
Kendall, F., Heidelberger, M., & Dawson, M. (1937). A serologically inactive polysaccharide elaborated by mucoid strains of group A hemolytic Streptococcus. Journal of Biological Chemistry, 118, 61–69.
Kovacic, R. T. (1987). The 0°C closed complexes between Escherichia coli RNA polymerase and two promoters, T7–A3 and lacUV. Biological Chemistry, 262, 13654–13661.
Krahulec, J., & Krahulcová, J. (2006). Increase in hyaluronic acid production by Streptococcus equi subspecies zooepidemicus strain deficient in β-glucuronidase in laboratory conditions. Applied Microbiology and Biotechnology, 71(4), 415–422.
Krahulec, J., & Krahulcová, J. (2007). Characterization of the new β-glucuronidase from Streptococcus equi subsp. zooepidemicus. Applied Microbiology and Biotechnology, 74(5), 1016–1022.
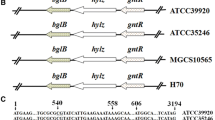
Krahulec, J., Tlustá, M., Stuchlík, S., & Turňa, J. (2011). Structure of the has operon promoter and the effect of mutations on the has promoter strength in Streptococcus equi subsp. zooepidemicus. Molecular Biotechnology, 49(2), 166–175.
Kumari, K., Baggenstoss, B. A., Parker, A. L., & Weigel, P. H. (2006). Mutation of two intramembrane polar residues conserved within the hyaluronan synthase family alters hyaluronan product size. Journal of Biological Chemistry, 281, 11755–11760.
Kumari, K., Tlapak-Simmons, V. L., Baggenstoss, B. A., & Weigel, P. H. (2002). The streptococcal hyaluronan synthases are inhibited by sulfhydryl-modifying reagents, but conserved cysteine residues are not essential for enzyme function. Journal of Biological Chemistry, 277, 13943–13951.
Landstein, D., Graves, M. V., Burbank, D. E., DeAngelis, P. L., & Van Etten, J. L. (1998). Chlorella virus PBCV-1 encodes functional glutamine: Fructose-6-phosphate amidotransferase and UDP-glucose dehydrogenase enzymes. Virology, 250, 388–396.
Laurent, T. C., & Fraser, J. R. E. (1992). Hyaluronan. FASEB Journal, 6, 2397–2404.
Lee, J. Y., & Spicer, A. P. (2000). Hyaluronan: A multifunctional, megaDalton, stealth molecule. Current Opinion in Cell Biology, 12, 581–586.
Marcellin, E., Chen, W. Y., & Nielsen, K. L. (2010). Understanding effect on hyaluronic acid molecular weight produced by Streptococcus equi subsp. zooepidemicus. Metabolic Engineering, 12, 62–69.
McCure, P. M., & Wilson, W. D. (1989). Equine mastitis: A review of 28 cases. Equine Veterinary Journal, 21, 351–353.
McIver, K. S., Heath, A. S., Green, B. D., & Scott, J. R. (1995). Specific binding of the activator Mga to promoter sequences of the emm and scpA genes in the group A streptococcus. Journal of Bacteriology, 177, 6619–6624.
Meyer, K. (1934). The polysaccharide of the vitreous humor. Journal of Biological Chemistry, 107, 629–634.
Morrison, D. A., & Jaurin, R. (1990). Streptococcus pneumoniae possesses canonical Escherichia coli (sigma 70) promoters. Molecular Microbiology, 4, 1143–1152.
Moses, A. E., Wessels, M. R., Zalcman, K., Alberti, S., Natanson-Yaron, S., Menes, T., et al. (1997). Relative contributions of hyaluronic acid capsule and M protein to virulence in a mucoid strain of group A Streptococcus. Infection and Immunity, 65, 64–71.
Nimrod, A. (1998). Method of producing high molecular weight sodium hyaluronate by fermentation of streptococcus. US Patent, 4(780), 414.
O’Regan, M., Martini, I., Crescenzi, F., DeLuka, C., et al. (1994). Molecular mechanisms and genetics of hyaluronan biosynthesis. International Journal of Biological Macromolecules, 16, 283–286.
Schickor, P., Metzger, W., Werel, W., Lederer, H., & Heumann, H. (1990). Topography of intermediates in transcription initiation in E. coli. EMBO Journal, 9, 2215–2220.
Schrager, H. M., Rheinwald, J. G., & Wessels, M. R. (1996). Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. Journal of Clinical Investigation, 98, 1954–1958.
Stocks, S. M. & Brown, S. (2007). Production of low molecular weight hyaluronic acid. Patent WO2007/093179.
Weigel, P. H. (2002). Functional characteristics and catalytic mechanisms of the bacterial hyaluronan synthases. IUBMB Life, 54, 201–211.
Weigel, P. H., & DeAngelis, P. L. (2007). Hyaluronan synthases: A decade-plus of novel glycosyltransferases. Journal of Biological Chemistry, 282(51), 36777–36781.
Weigel, P. H., Hascall, V. C., & Tammi, M. (1997). Hyaluronan synthases. Journal of Biological Chemistry, 272, 13997–14000.
Weissman, B., & Meyer, K. (1954). The structure of hyalobiuronic acid and of hyaluronic acid from umbilical cord. Journal of the American Chemical Society, 76, 1753–1757.
Wessels, M. R., & Bronze, M. S. (1994). Critical role of the group A streptococcal capsule in pharyngeal colonization and infection in mice. Proceedings of the National Academy of Sciences USA, 91, 12238–12242.
Wessels, M. R., Moses, A. E., Goldberg, J. B., & DiCesare, T. J. (1991). Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proceedings of the National Academy of Sciences USA, 88, 8317–8321.
Wibawan, I. W., Pasaribu, F. H., Utama, I. H., Abdulmawjood, A., & Lammler, C. (1999). The role of hyaluronic acid capsular material of Streptococcus equi subsp. zooepidemicus in mediating adherence to HeLa cells and in resisting phagocytosis. Research in Veterinary Science, 67, 131–135.
Widner, B., Behr, R., Von Dollen, S., Tang, M., Heu, T., Sloma, A., et al. (2005). Hyaluronic acid production in Bacillus subtilis. Applied and Environment Microbiology, 71(7), 3747–3752.
Widner, B., Thomas, M., Sternberg, D., Lammon, D., Behr, R., & Sloma, A. (2000). Development of marker-free strains of Bacillus subtilis capable of secreting high levels of industrial enzymes. Journal of Industrial Microbiology and Biotechnology, 25(2), 04–212.
Yamada, T., & Kawasaki, T. (2005). Microbial synthesis of hyaluronan and chitin: New approaches. Journal of Bioscience and Bioengineering, 99(6), 521–528.
Acknowledgments
This study was supported by Contipro Biotech s r.o.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tlustá, M., Krahulec, J., Pepeliaev, S. et al. Production of Hyaluronic Acid by Mutant Strains of Group C Streptococcus . Mol Biotechnol 54, 747–755 (2013). https://doi.org/10.1007/s12033-012-9622-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-012-9622-8