Abstract
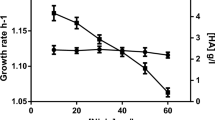
Streptococcus equi subsp. zooepidemicus is known to produce a hyaluronic acid capsule to resist the host immune defense. As the structure of the polysaccharide is identical to the one produced by humans, the bacteria S. equisubsp. zooepidemicusis used in biotechnological production of hyaluronic acid. In our laboratory we prepared mutated strains that are β-glucuronidase deficient. Comparing the wild-type strain, which is positive in β-glucuronidase activity, with the mutated strains named clone1 and clone2 in laboratory conditions, we observed that β-glucuronidase influences the production of hyaluronic acid considerably and the molecular weight of hyaluronan slightly. The production of hyaluronic acid by the mutated strains is higher by approximately 20% and the molecular weight is larger by about 2%. The significant increase in the production of hyaluronic acid and the slight increase in the molecular weight are probably caused by an absence of free β-glucuronic acid, due to its removal from the non-reducing termini of the polysaccharide by β-glucuronidase. The presence of free β-glucuronic acid would likely induce the expression of the β-glucuronic-acid-utilizing operon, which in turn would reflect into a misuse of energy in the glucose-rich media.






Similar content being viewed by others
References
Alho AM, Underhill CB (1989) The hyaluronate receptor is preferentially expressed on proliferating epithelial cells. J Cell Biol 108:1557–1565
Baudouy JR, Portalier R, Stoeber F (1981) Regulation of hexuronate system genes in Escherichia coli k-12: multiple regulation of the uxu operon by exuR and uxuR gene products. J Bacteriol 145:211–220
Bettelheim FA, Popdimirova N (1992) Hyaluronic acid—syneretic glycosaminoglycan. Curr Eye Res 11:411–419
Chanter N, Collin NC, Mumford JA (1994) Resistance of Streptococcus equi to equine polymorphonuclear leucocytes. Equine Inf Dis 7:201–206
DeAngelis LP, Padgett-McCue AJ (2000) Identification and molecular cloning of a chondroitin synthase from Pasteurella multocida type F. J Biol Chem 275:24124–24129
DeAngelis PL (2002) Microbial glycosaminoglycan glycosyltransferases. Glycobiology 12:9R–16R
Goldberg RL, Toole BP (1987) Hyaluronate inhibition of cell proliferation. Arthritis Rheum 30:769–778
Heinegard D, Sommarin Y (1987) Proteoglycans: an overview. Methods Enzymol 144D:305–319
Henrich CJ, Hawkes SP (1989) Molecular weight dependence of hyaluronic acid produced during oncogenic transformation. Cancer Biochem Biophys 10:257–267
Kass EH, Seastone CV (1944) The role of the mucoid polysaccharide (hyaluronic acid) in the virulence of group A hemolytic streptococci. J Exp Med 79:319–330
Kitchen JR, Cysyk RL (1995) Synthesis and release of hyaluronic acid by Swiss 3T3 fibroblasts. Biochemical J 309:649–656
Kujawa MJ, Carrino DA, Caplan AI (1986) Substrate-bonded hyaluronic acid exhibits a size-dependent stimulation of chondrogenic differentiation of stage 24 limb mesenchymal cells in culture. Dev Biol 114:519–528
Kumar S, Kumar P, Ponting JM, Sattar A, Rooney P, Pye D, Hunter RD (1992) Biotinylated hyaluronan: a versatile and highly sensitive probe capable of detecting nanogram level of hyaluronan binding proteins (hyaladherins) on electroblots by a novel affinity detection procedure. In: Maragoudakis, ME (ed) Angiogenesis in health and disease, NATO ASI Series A, vol 227. Plenum, New York, pp 253–263
Laurent TC, Fraser JR (1992) Hyaluronan. Faseb J 6:2397–2404
McCarty MF (1996) Glucosamine for wound healing. Med Hypotheses 47:273–275
Moses AE, Wessels MR, Zalcman K, Alberti S, Natanson-Yaron S, Menes T, Hanski E (1997) Relative contribution of hyaluronic acid capsule and M protein to virulence in a mucoid strain of the group A Streptococcus. Inf Immun 65:64–71
Pham PL, Dupont I, Roy D, Lapointe G, Cerning J (2000) Production of exopolysaccharide by Lactobacillus rhamnosum R and analysis of its enzymatic degradation during prolonged fermentation. Appl Environ Microbiol 66:2302–2310
Poole AR (1986) Proteoglycans in health and disease: structures and functions. Biochem J 236:1–14
Roberts IS (1996) The biochemistry and genetics of capsular polysaccharide production in bacteria. Annu Rev Microbiol 50:285–315
Shulami S, Gat O, Sonenshein AL, Shoham Y (1999) The glucuronic acid utilization gene cluster from Bacillus stearothermophilus T-6. J Bacteriol 181:3695–3704
Toole BP (1990) Hyaluronan and its binding proteins, the hyaladherins. Curr Opin Cell Biol 2:839–844
Turley EA, Bowman P, Kytryk MA (1985) Effects of hyaluronate and hyaluronate binding proteins on cell motile and contact behaviour. J Cell Sci 78:133–145
Van Brunt J (1986) More to hyaluronic acid than meets the eye. Biotechnology 4:780–782
Weindl G, Schaller M, Schäfer-Korting M, Korting HC (2004) Hyaluronic acid in the treatment and prevention of skin diseases: molecular biological, pharmaceutical and clinical aspects. Skin Pharmacol Physiol 17:207–213
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A
First day: clone1 | ||||
Cultivation time (h) | MW (Da) | Concentration (mg/ml) | Cell number | pH |
6 | 7.31 | |||
8 | 1.969×106 | 0.1152 | 2.28×108 | 6.48 |
1.963×106 | 0.1085 | |||
10 | 2.323×106 | 0.1699 | 3.74×108 | 5.1 |
2.384×106 | 0.1594 | |||
12 | 4.53×108 | 4.95 | ||
2.274×106 | 0.2357 | |||
14 | 2.240×106 | 0.2964 | 4.93×108 | 4.95 |
2.110×106 | 0.3029 | |||
16 | 2.305×106 | 0.3255 | 5.28×108 | 5.15 |
2.213×106 | 0.3423 | |||
18 | 2.239×106 | 0.3696 | 5.26×108 | 5.18 |
20 | 2.257×106 | 0.4184 | 5.49×108 | 5.25 |
2.221×106 | 0.4247 | |||
Appendix B
First day: clone2 | ||||
Cultivation time (h) | MW (Da) | Concentration (mg/ml) | Cell number | pH |
6 | 7.3 | |||
8 | 2.493×106 | 0.0889 | 2.47×108 | 6.25 |
2.318×106 | 0.0924 | |||
10 | 2.367×106 | 0.1716 | 3.86×108 | 4.9 |
2.374×106 | 0.1679 | |||
12 | 4.63×108 | 4.9 | ||
2.394×106 | 0.2184 | |||
14 | 2.264×106 | 0.2895 | 5.03×108 | 4.95 |
2.343×106 | 0.2756 | |||
16 | 2.372×106 | 0.3033 | 5.29×108 | 5.05 |
2.268×106 | 0.3151 | |||
18 | 2.289×106 | 0.3499 | 5.37×108 | 5.24 |
2.190×106 | 0.3641 | |||
20 | 2.239×106 | 0.4111 | 5.44×108 | 5.24 |
2.244×106 | 0.4065 | |||
Appendix C
First day: wild type | ||||
Cultivation time (h) | MW (Da) | Concentration (mg/ml) | Cell number | pH |
6 | 7.48 | |||
8 | 2.570×106 | 0.0396 | 1.21×108 | 6.98 |
2.611×106 | 0.0373 | |||
10 | 2.441×106 | 0.1173 | 2.85×108 | 5.4 |
2.460×106 | 0.1147 | |||
12 | 2.253×106 | 0.1960 | 4.00×108 | 4.75 |
2.136×106 | 0.2092 | |||
14 | 2.209×106 | 0.2505 | 4.41×108 | 4.84 |
2.341×106 | 0.2329 | |||
16 | 2.386×106 | 0.2662 | 4.82×108 | 4.9 |
2.242×106 | 0.2805 | |||
18 | 2.220×106 | 0.3208 | 4.69×108 | 4.88 |
2.361×106 | 0.3020 | |||
20 | 2.282×106 | 0.3387 | 4.84×108 | 4.86 |
2.275×106 | 0.3392 | |||
Appendix D
Second day: clone1 | ||||
Cultivation time (h) | MW (Da) | Concentration (mg/ml) | Cell number | pH |
6 | 5.36 | |||
8 | 2.461×106 | 0.1740 | 3.97×108 | 4.98 |
2.457×106 | 0.1708 | |||
10 | 2.284×106 | 0.2453 | 4.69×108 | 4.97 |
2.293×106 | 0.2461 | |||
12 | 2.256×106 | 0.2901 | 5.06×108 | 5.08 |
2.384×106 | 0.2728 | |||
14 | 2.298×106 | 0.3203 | 5.28×108 | 5.28 |
2.226×106 | 0.3269 | |||
16 | 2.193×106 | 0.3869 | 5.35×108 | 5.41 |
2.284×106 | 0.3675 | |||
18 | 2.264×106 | 0.4048 | 5.47×108 | 5.3 |
2.178×106 | 0.4281 | |||
20 | 2.204×106 | 0.4452 | 5.44×108 | 5.78 |
2.184×106 | 0.4501 | |||
Appendix E
Second day: clone2 | ||||
Cultivation time (h) | MW (Da) | Concentration (mg/ml) | Cell number | pH |
6 | 5.36 | |||
8 | 2.497×106 | 0.1735 | 3.98×108 | 4.9 |
2.347×106 | 0.1837 | |||
10 | 2.357×106 | 0.2402 | 4.71×108 | 4.96 |
2.337×106 | 0.2397 | |||
12 | 2.209×106 | 0.3036 | 5.14×108 | 5.05 |
2.260×106 | 0.2939 | |||
14 | 2.260×106 | 0.3359 | 5.29×108 | 5.26 |
2.265×106 | 0.3342 | |||
16 | 2.271×106 | 0.3722 | 5.51×108 | 5.47 |
2.295×106 | 0.3665 | |||
18 | 2.131×106 | 0.4354 | 5.60×108 | 5.33 |
2.176×106 | 0.4304 | |||
20 | 2.231×106 | 0.4678 | 5.54×108 | 5.7 |
2.178×106 | 0.4724 | |||
Appendix F
Second day: wild type | ||||
Cultivation time (h) | MW (Da) | Concentration (mg/ml) | Cell number | pH |
6 | 5.28 | |||
8 | 2.483×106 | 0.1455 | 3.95×108 | 4.84 |
2.380×106 | 0.1447 | |||
10 | 2.264×106 | 0.2124 | 4.53×108 | 4.8 |
2.222×106 | 0.2150 | |||
12 | 2.188×106 | 0.2567 | 4.78×108 | 4.83 |
2.239×106 | 0.2528 | |||
14 | 2.159×106 | 0.2814 | 4.89×108 | 4.87 |
2.179×106 | 0.2781 | |||
16 | 2.006×106 | 0.3315 | 5.11×108 | 5.07 |
2.170×106 | 0.3119 | |||
18 | 2.104×106 | 0.3390 | 5.13×108 | 5.05 |
2.170×106 | 0.3239 | |||
20 | 2.133×106 | 0.3576 | 5.13×108 | 5.27 |
2.025×106 | 0.3692 | |||
Appendix G
Third day: clone1 | ||||
Cultivation time (h) | MW (Da) | Concentration (mg/ml) | Cell number | pH |
6 | 6.06 | |||
8 | 2.378×106 | 0.1714 | 3.77×108 | 5.00 |
2.391×106 | 0.1696 | |||
10 | 2.294×106 | 0.2393 | 4.50×108 | 5.00 |
2.224×106 | 0.2384 | |||
12 | 2.154×106 | 0.3148 | 4.97×108 | 5.05 |
2.287×106 | 0.3044 | |||
14 | 2.285×106 | 0.3230 | 5.20×108 | 5.15 |
2.299×106 | 0.3247 | |||
16 | 2.293×106 | 0.3644 | 5.27×108 | 5.34 |
2.261×106 | 0.3772 | |||
18 | 2.199×106 | 0.4445 | 5.45×108 | 5.45 |
2.382×106 | 0.4074 | |||
20 | 2.354×106 | 0.4520 | 5.54×108 | 5.54 |
2.200×106 | 0.4677 | |||
Appendix H
Third day: clone2 | ||||
Cultivation time (h) | MW (Da) | Concentration (mg/ml) | Cell number | pH |
6 | 5.58 | |||
8 | 2.289×106 | 0.1769 | 3.90×108 | 4.94 |
2.336×106 | 0.1710 | |||
10 | 2.244×106 | 0.2407 | 4.62×108 | 5.00 |
2.222×106 | 0.2420 | |||
12 | 2.354×106 | 0.2662 | 4.98×108 | 5.10 |
2.392×106 | 0.2712 | |||
14 | 2.144×106 | 0.3344 | 5.15×108 | 5.23 |
2.185×106 | 0.3292 | |||
16 | 2.188×106 | 0.3572 | 5.26×108 | 5.45 |
2.168×106 | 0.3548 | |||
18 | 2.254×106 | 0.4158 | 5.35×108 | 5.55 |
2.248×106 | 0.4147 | |||
20 | 2.413×106 | 0.4262 | 5.43×108 | 5.68 |
2.351×106 | 0.4264 | |||
Appendix I
Third day: wild type | ||||
Cultivation time (h) | MW (Da) | Concentration (mg/ml) | Cell number | pH |
6 | 6.46 | |||
8 | 2.425×106 | 0.1457 | 3.32×108 | 5.09 |
2.456×106 | 0.1446 | |||
10 | 2.329×106 | 0.2064 | 4.14×108 | 4.85 |
12 | 2.256×106 | 0.2421 | 4.45×108 | 4.95 |
2.250×106 | 0.2438 | |||
14 | 2.285×106 | 0.2562 | 4.47×108 | 4.87 |
2.186×106 | 0.2780 | |||
16 | 2.190×106 | 0.3043 | 4.52×108 | 4.95 |
2.227×106 | 0.2865 | |||
18 | 2.268×106 | 0.3219 | 4.62×108 | 4.96 |
2.193×106 | 0.3205 | |||
20 | 2.233×106 | 0.3411 | 4.72×108 | 5.15 |
2.245×106 | 0.3229 | |||
Appendix J
Fourth day: clone1 | ||||
Cultivation time (h) | MW (Da) | Concentration (mg/ml) | Cell number | pH |
6 | 6.85 | |||
8 | 2.168×106 | 0.1532 | 3.19×108 | 5.16 |
10 | 2.145×106 | 0.2205 | 4.26×108 | 4.96 |
12 | 2.143×106 | 0.2785 | 4.74×108 | 4.93 |
2.190×106 | 0.2705 | |||
14 | 2.210×106 | 0.2835 | 5.03×108 | 5.17 |
2.197×106 | 0.2908 | |||
16 | 2.297×106 | 0.3370 | 5.19×108 | 5.25 |
2.293×106 | 0.3389 | |||
18 | 2.215×106 | 0.4039 | 5.32×108 | 5.4 |
2.250×106 | 0.3885 | |||
20 | 2.243×106 | 0.4236 | 5.38×108 | 5.94 |
Appendix K
Fourth day: clone2 | ||||
Cultivation time (h) | MW (Da) | Concentration (mg/ml) | Cell number | pH |
6 | 6.31 | |||
8 | 2.260×106 | 0.1873 | 3.96×108 | 4.85 |
2.276×106 | 0.1824 | |||
10 | 2.354×106 | 0.2402 | 4.84×108 | 5.00 |
2.241×106 | 0.2569 | |||
12 | 2.354×106 | 0.3103 | 5.23×108 | 4.86 |
2.243×106 | 0.3168 | |||
14 | 2.130×106 | 0.3604 | 5.46×108 | 5.16 |
2.239×106 | 0.3559 | |||
16 | 2.176×106 | 0.3945 | 5.63×108 | 5.20 |
2.108×106 | 0.4123 | |||
18 | 2.354×106 | 0.4386 | 5.75×108 | 5.40 |
2.240×106 | 0.4469 | |||
20 | 2.223×106 | 0.4834 | 5.80×108 | 5.97 |
2.221×106 | 0.4804 | |||
Appendix L
Fourth day: wild type | ||||
Cultivation time (h) | MW (Da) | Concentration (mg/ml) | Cell number | pH |
6 | 5.94 | |||
8 | 2.178×106 | 0.1870 | 3.91×108 | 4.85 |
2.279×106 | 0.1831 | |||
10 | 2.253×106 | 0.2574 | 4.63×108 | 4.90 |
2.159×106 | 0.2639 | |||
12 | 2.295×106 | 0.2747 | 4.82×108 | 4.85 |
14 | 2.121×106 | 0.3252 | 4.94×108 | 4.90 |
2.118×106 | 0.3245 | |||
16 | 2.064×106 | 0.3520 | 5.00×108 | 4.93 |
2.156×106 | 0.3370 | |||
18 | 2.258×106 | 0.3406 | 4.99×108 | 5.04 |
2.258×106 | 0.3383 | |||
20 | 2.247×106 | 0.3422 | 5.08×108 | 5.42 |
2.196×106 | 0.3462 | |||
Rights and permissions
About this article
Cite this article
Krahulec, J., Krahulcová, J. Increase in hyaluronic acid production by Streptococcus equi subsp. zooepidemicus strain deficient in β-glucuronidase in laboratory conditions. Appl Microbiol Biotechnol 71, 415–422 (2006). https://doi.org/10.1007/s00253-005-0173-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-0173-9