Abstract
Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) belong to the same peptide family and exert a variety of biological functions. Both PACAP and VIP have protective effects in several tissues. While PACAP is known to be a stronger retinoprotective peptide, VIP has very potent anti-inflammatory effects. The need for a non-invasive therapeutic approach has emerged and PACAP has been shown to be retinoprotective when administered in the form of eye drops as well. The cell penetrating peptide TAT is composed of 11 amino acids and tagging of TAT at the C-terminus of neuropeptides PACAP/VIP can enhance the traversing ability of the peptides through the biological barriers. We hypothesized that TAT-bound PACAP and VIP could be more effective in exerting retinoprotective effects when given in eye drops, by increasing the traversing efficacy and enhancing the activation of the PAC1 receptor. Rats were subjected to bilateral carotid artery occlusion (BCCAO), and retinas were processed for histological analysis 14 days later. The efficiency of the TAT-bound peptides to reach the retina was assessed as well as their cAMP increasing ability. Our present study provides evidence, for the first time, that topically administered PACAP and VIP derivatives (PACAP-TAT and VIP-TAT) attenuate ischemic retinal degeneration via the PAC1 receptor presumably due to a multifactorial protective mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
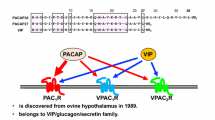
Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) belong to the same peptide family. PACAP exists in 27 and 38 amino acid forms, and the shorter peptide shows 67% homology with VIP. They also share their receptors: VPAC1 and VPAC2 receptors bind both VIP and PACAP. However, PACAP also has a specific PAC1 receptor, which only binds PACAP. PACAP has a widespread occurrence in the body and a broad array of functions (Reglodi and Tamas 2016). Among others, PACAP influences gastrointestinal, urinary and cardiovascular functions (Heppner et al. 2018; Parsons and May 2018; Reglodi et al. 2018a), plays a role in reproduction and pregnancy (Lajko et al. 2018; Reglodi et al. 2012; Ross et al. 2018), has diverse behavioral and cognitive functions (Farkas et al. 2017; Gupta et al. 2018; Han et al. 2014; King et al. 2017), plays roles during both early development and aging (Fulop et al. 2018; Reglodi et al. 2018b; Watanabe et al. 2007), as well as influences the functions of both endocrine and exocrine glands (Bardosi et al. 2016; Egri et al. 2016; Prevost et al. 2013; Sasaki et al. 2017). VIP has also been shown to have diverse actions in addition to the originally described vasodilatory effects (Gozes 2008; Hill et al. 2007; Moody and Gozes 2007; Vu et al. 2015). VIP was originally isolated as a vasoactive peptide in the airways, later confirmed in the gastrointestinal tract (Vu et al. 2015). VIP is involved, among others, in immunomodulatory pathways (Abad and Tan 2018; Carrión et al. 2016; Jimeno et al. 2014), in nervous system development and in the acquisition of certain neurological disorders (Maugeri et al. 2018a, b; Morell et al. 2012).
Both PACAP and VIP exert protective effects in several tissues (Brifault et al. 2016; Giladi et al. 2007; Reglodi et al. 2011; Shioda and Gozes 2011). VIP has stronger anti-inflammatory effects (Olson et al. 2015), while PACAP is a more potent antiapoptotic peptide (Reglodi et al. 2018c). In the eye, VIP and PACAP have various biological effects. Among others, PACAP has been described to participate in the iris sphincter functions (Yoshitomi et al. 2002), stimulates tear secretion (Nakamachi et al. 2016; Shioda et al. 2018) and modulates its composition (Gaal et al. 2008), influences corneal keratinization and wound repair (Ma et al. 2015; Nakamachi et al. 2016) and is involved in the sensory innervation of the ocular surface (Wang et al. 1996). PACAP and VIP also have protective effects on the corneal endothelium (Koh et al. 2017; Maugeri et al. 2018a, b). Both peptides and their receptors are distributed also in the retina, where they are involved in information processing of visual stimuli (Akrouh and Kerschensteiner 2015; Atlasz et al. 2016; Dragich et al. 2010; Pérez de Sevilla Müller et al. 2017; Webb et al. 2013) and have trophic functions (Endo et al. 2011; Fabian et al. 2012).
The retinoprotective effects of PACAP are well-documented and have been proven in different injury models, such as excitotoxic, ischemic, UV light-induced, traumatic, diabetic and oxygen-induced injuries (Atlasz et al. 2008, 2011, 2016; Kvarik et al. 2016; Shioda et al. 2016; Szabadfi et al. 2016; Vaczy et al. 2016). VIP, on the other hand, seems to be a less potent retinoprotective peptide. VIP has been shown to exert retinoprotective effects mainly in conditions involving inflammatory processes (Shi et al. 2016; Tunçel et al. 1996). However, in ischemic retinopathy, VIP was proven to be ten times less active than PACAP (Szabadfi et al. 2012). In most in vivo retinal disease models, PACAP and VIP have been administered as intravitreal injection in order to guarantee that the injected peptides reach the retina in high enough concentrations to exert protective effects. As PACAP exerts dramatic retinoprotective effects proven by dozens of studies, therapeutic use is implied and so the need for a non-invasive approach has emerged. One possible approach is to enhance cell penetration of these peptides. The cell penetrating peptide TAT (GRKKRRQRRRPQ) is derived from the HIV Tat protein (Schwarze et al. 1999). TAT has protein transduction domains (PTDs) with the ability to efficiently traverse cellular membranes. TAT can not only transfer different types of molecules (peptides, large molecular proteins, DNAs) into a variety of cell types, but can also bring the linked molecules across many biological barriers such as the blood-brain barrier (BBB), mucosal barrier and lung respiratory epithelium in vivo (Dietz and Bähr 2004). Our previous study reported that the tagging of TAT at the C-terminus of neuropeptides PACAP/VIP enhanced the traversing ability of the peptides through the biological barriers, such as BBB and blood–air barrier and blood–testis barrier (Yu et al. 2012a, b). Furthermore, we found that VIP-TAT has higher activity on the activation of PACAP preferring PAC1receptor than VIP (Yu et al. 2014). The structure analysis showed that TAT has a two-dimensional structure similar to that of PACAP(28–38), and PACAP(28–38) has been shown to facilitate the binding and the activation of PAC1-R (Vaudry et al. 2009).
PACAP in the form of eye drops has first been shown to exert local effects on the cornea. It has been shown to enhance corneal wound regeneration and nerve regrowth after injuries (Fukiage et al. 2007; Ma et al. 2015; Nakamachi et al. 2016; Shioda et al. 2018). Similarly, VIP has been shown to enhance corneal wound repair after alkali burn injury (Tuncel et al. 2016). Our recent studies have demonstrated that PACAP eye drops not only lead to topical effects, but PACAP is able to pass the ocular barriers and reach the retina, where it can exert retinoprotective effects (Werling et al. 2016, 2017). We hypothesize that the passage through ocular layers can be further enhanced by the binding of TAT peptide, which is known to increase passage of peptides through biological barriers. We have previously shown that intravitreally administered VIP is able to protect the retina against hypoperfusion-induced injury, but only in a dose ten times higher than that of PACAP (Szabadfi et al. 2012). We hypothesized that TAT-bound PACAP and VIP (PACAP-TAT, VIP-TAT) could be more effective in exerting retinoprotective effects when given in eye drops, by increasing the traversing efficacy and enhancing the activation of the PAC1 receptor. The aim of the present study, therefore, was to investigate the potential retinoprotective effects of PACAP-TAT and VIP-TAT administered in eye drops following bilateral carotid artery occlusion (BCCAO)-induced retinopathy in rats.
Materials and Methods
Materials
The peptides PACAP38, VIP, PACAP-TAT (tagging TAT at the C-terminus of PACAP38) and VIP-TAT (tagging TAT at the C-terminus of VIP) were chemically synthesized by GL Biochem Ltd. (Shanghai, China).
Peptides Labeled with Fluorescein Isothiocyanate
In order to trace the drugs, peptides were labeled with fluorescein isothiocyanate (FITC) using a FITC Protein Labeling Kit from ChangRu Biotech Ltd. (Guangzhou, China) according to the manufacturer’s protocol. After the labeling reaction, gel filtration was used to remove the free FITC. In order to determine the amount of the residual free FITC, the peptides were submitted to ultrafiltration using Amicon Ultra – 0.5 mL (Millipore, USA) with a molecular sieve of 2000 Da. After centrifugation (1000×g, 10 min) the peptide was subjected to fluorescence measurements performed with the multi-wavelength scanner Victor 3 (GE, USA) at the excitation of 495 nm and the emission of 520 nm. Protein concentrations were determined using the K4000 Bradford Protein Quantification Kit (Innovative, Guangzhou, China). The labeling efficiency was calculated using the following formula: label efficiency (LE) = fluorescence value (FV)/peptide mass (mol) (PM), representing the fluorescence intensity (AU) per mol of peptide (mol).
The Efficiency of Reaching the Retina
Male rats with body weight from 160 to 180 g were purchased from the Medical and Experimental Animal Center (Guangdong, China). Rats were randomly assigned to one of the experimental groups (7 rats per group) and subjected to eye drops with FITC labeled peptides (100 nmol/kg) and PBS as control. Rats were sacrificed by anesthesia 2 h after the eye drop administration and the retina was separated, weighed, washed three times with PBS and divided into two parts. One part was prepared on the glass slide with glycerin and subjected to fluorescence microscopic observation of FITC with 495 nm excitation/520 nm emission. All images, focused on the left upper regions of the retina, were taken with 500 ms exposure time. The other part of the retina was subjected to grinding and ultrasonication in PBS at a concentration of 100 mg weight tissue per milliliter of PBS. The supernatant was collected by centrifugation and the fluorescence intensity in the supernatant (100 uL) was determined. The valid fluorescence intensity (FI) for each sample treated with FITC labeled peptide was corrected by subtracting the fluorescence value of the sample treated with PBS, which was used as a blank background. The Efficiency of Traversing Eye to Retina (EtE) was expressed as the percentages of the FITC labeled peptide mass in the retina to the total FITC labeled peptide mass. The EtE was calculated using the following formula: EtE = tFI/LE/PW × 100% (tFI presents the fluorescence intensity of retina (arbitration unit, AU); LE presents the label efficiency of each peptide which has been determined above; PW presents the peptide mass (mole)).
cAMP Accumulation Assay
PAC1-CHO cells cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) at 37 °C were scraped off the surface with rubber policeman, washed twice with PBS and the density of the cells was adjusted to 2 × 106 /mL. Peptides were added to 500 uL cells suspension with the corresponding varying working concentrations of the detected factor. After incubation at 37 °C for 5-10 min cells were harvested and the lysates were subjected to cAMP quantification using the enzyme immunoassay kit for cAMP (Biyuntian, Shanghai, China), following the manufacturer’s instructions. Protein concentration of each sample was determined using BCA assay, and the cAMP level of each sample was calculated following the formula: cAMP level (pmol/mg protein) = cAMP concentration (pmol/mL)/protein concentration (mg/mL). The cAMP level in each sample was plotted as the percentage (%) of the maximal cAMP level in cells treated with PACAP27 versus the logarithmic value of the peptide concentrations. All experiments were run with at least four parallel samples and were repeated three times.
Histological Procedure in the Retina
Adult male rat litters were housed in the animal facility in individual cages in a 12 h light-dark cycle with food and water ad libitum. Animal housing, care and application of experimental procedures were in accordance with institutional guidelines under approved protocols (No: BA02/2000–26/2017, University of Pecs). Under isoflurane anesthesia, common carotid arteries were exposed on both sides through a midline incision and then ligated with a 3–0 filament. A group of animals (sham group) underwent all steps of the operating procedure except ligation of the carotid arteries. Immediately following the operation, the right eye of the animals was treated with derivatives of PACAP (PACAP-TAT /n = 17/ or VIP-TAT /n = 17/) eye drops (1 μg/drop). Dose and schedule of the eye drop treatments were based on our previous experiments (Werling et al. 2016). In the experiment for histological analysis, the different derivatives were dissolved in benzalkonium solution for ophthalmic use (solutio ophthalmica cum benzalkonio (SOCB)). The left eye, serving as a control, was treated with vehicle containing neither PACAP-TAT nor VIP-TAT. Animals were treated for five consecutive days, twice a day with one drop of drug, under brief isoflurane anesthesia (max. 5 min).
Fourteen days after the operation, rats (n = 10 SHAM and n = 24 BCCAO) were killed with anesthetic and the eyes were processed for histology. The eyes were removed and the retinas were solved in phosphate buffered saline (PBS), fixed in 4% paraformaldehyde dissolved in 0.1 M phosphate buffer (PB) (Sigma, Budapest, Hungary) and embedded in Durcupan ACM resin (Sigma, Budapest, Hungary). Retinas were cut at 2 μm and stained with toluidine blue dye (Sigma, Hungary). Sections were mounted in DPX medium (Sigma, Hungary) and photographs were taken with a digital CCD camera using the Spot program. Central retinal areas within 1 mm from the optic nerve were used (n = 5 measurements from one tissue block). The following parameters were measured: (i) cross-section of the retina from the outer limiting membrane (OLM) to the inner limiting membrane (ILM), (ii) the width of individual retinal layers (outer nuclear layer [ONL], outer plexiform layer [OPL], inner nuclear layer [INL], inner plexiform layer [IPL]), (iii) the number of cells/100 μm section length in the GCL, and the (iv) number of cells/1 μm2 in the OPL and in the IPL. Results are presented as mean ± SEM. Statistical comparisons were made using the two-way ANOVA test followed by Tukey’s post hoc analysis.
Results
TAT Tagging Enhances the Efficiency of Reaching Retina
The fluorescence imaging results of the retina after the treatment with eye drops of FITC labeled peptides (Fig. 1) showed that the FITC fluorescence density per area unit in the retina treated with eye drops of PACAP-TAT (Fig. 1a) and VIP-TAT (Fig. 1c) was much higher than in retinas treated with eye drops of PACAP/VIP, indicating that PACAP-TAT/VIP-TAT reached the retina more efficiently than PACAP/VIP. The calculation of the Efficiency for Traversing Eye to Retina (EtE) showed that the PACAP-TAT/VIP-TAT reached the retina with the efficiency (3.66 ± 0.67%, 3.05 ± 0.58%) about three-fold that of PACAP/VIP (1.23 ± 0.56%, 0.97 ± 0.47%), respectively.
The efficiency of FITC labeled PACAP/PACAP-TAT (a, b) and VIP/VIP-TAT (c, d) traversing to retina given in eye drops. The retina was separated 2 h after the eye drops and submitted to the fluorescence microscopic observation of FITC fluorescence signal (A, C) and the calculation of Efficiency of Traversing Eye to Retina (EtE) (b, d). The data are means ± SEM of four experiments
TAT-Tagging Enhanced the Activity of PACAP/VIP on the Activation of PAC1-R
The results of cAMP assay (Fig. 2) showed that PACAP-TAT had EC50 of 23.6 ± 4.4 pM significantly higher than PACAP38 with 11.7 ± 3.1 pM, whereas VIP-TAT had EC50 of 0.14 ± 0.02 nM about 1/200 of the EC50 of VIP 30.1 ± 4.1 nM. These results showed that TAT-tagging enhanced the activity of VIP on the activation of PAC1-R, but inhibited the activity of PACAP38.
cAMP assay results showing the effects of PACAP/PACAP-TAT (a) and VIP/VIP-TAT (b) on the activation of PAC1-R. The intracellular cAMP accumulation in PAC1-CHO cells induced by PACAP38( ), PACAP-TAT(
), PACAP-TAT( ), VIP(
), VIP( ) and VIP-TAT (
) and VIP-TAT ( ) in their respective effective working concentration was plotted as the percentage (%) of the maximum cAMP level induced by PACAP27. The data are means ± SEM of four experiments
) in their respective effective working concentration was plotted as the percentage (%) of the maximum cAMP level induced by PACAP27. The data are means ± SEM of four experiments
Morphological Analysis in the Retina after BCCAO
Carotid occlusion caused significant thickness reduction in all layers compared to sham animals. The most marked reduction in thickness was found in the outer and inner plexiform layers, and as a consequence, the total retinal thickness (OLM-ILM) was significantly less than in control retinas (Figs. 3 and 4). PACAP derivatives (PACAP-TAT, VIP-TAT) administration alone in sham animals did not cause any changes in the retinal thickness (Figs. 3 and 4). Eye drops containing PACAP-TAT or VIP-TAT caused significant amelioration in all retinal layers compared to the sham group. The thickness of the major retinal layers was significantly larger than that of the degenerated ones (Figs. 3 and 4). This was especially conspicuous in the OPL, which almost disappeared in several BCCAO-induced degenerated retinas and was preserved in PACAP-TAT or VIP-TAT-treated animals. The number of cells in different retinal layers also changed. BCCAO led to a significant cell loss in the ONL, INL and GCL. Eye drops with PACAP-TAT counteracted the effects of the BCCAO in all nuclear layers. The cell numbers in the GLC/100 μm, in the ONL/500 μm2 and in the INL/500 μm2 were significantly higher compared to the BCCAO-induced degenerated retinas. VIP-TAT administration also led to reduced cell loss in almost all nuclear layers, except in the ONL/500 μm2 (Figs. 5, 6 and 7).
Light microphotographs of retinal sections. Retinal tissue from BCCAO+SOCB (d) showed severe degeneration compared to SHAM+SOCB (a), SHAM + PACAP-TAT (b) or SHAM + VIP-TAT (c). The retinal layers of BCCAO+SOCB rats following treatment with eye drops containing PACAP-TAT (e) or VIP-TAT (f) showed only mild degeneration. Abbreviations: PL, photoreceptor layer; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar: 20 μm
Quantification of retinal layers in SHAM+SOCB, in SHAM+PACAP-TAT, in BCCAO+SOCB, and in BCCAO+PACAP-TAT animals; the right eye was treated with PACAP-TAT eye drops, the left eye served as controls receiving only SOCB. Comparison of all retinal layers (a–e). Morphometric analysis showed that treatment with PACAP-TAT eye drops improved the structure of all the retinal layers. Statistical significance (*p < 0.05 vs. SHAM+SOCB retinas, #p < 0.05 vs. BCCAO+SOCB retinas) was calculated by two-way ANOVA followed by Fischer’s post hoc test
Quantification of the number of cells/100 μm GCL length (a), the number of cells/500 μm2 ONL (b) and INL (c) areas in SHAM+SOCB, in SHAM+PACAP-TAT, in BCCAO+SOCB, and in BCCAO+PACAP-TAT animals. Statistical significance (*p < 0.05 vs. SHAM+SOCB retinas, #p < 0.05 vs. BCCAO+SOCB retinas) was calculated by two-way ANOVA followed by Bonferroni’s post hoc test
Quantification of retinal layers in SHAM+SOCB, in SHAM+VIP-TAT, in BCCAO+SOCB, and in BCCAO+VIP-TAT animals; the right eye was treated with VIP-TAT eye drops, the left eye served as control receiving only SOCB. Comparison of all retinal layers (a–e). Morphometric analysis showed that treatment with VIP-TAT eye drops improved the structure of all the retinal layers. Statistical significance (*p < 0.05 vs. SHAM+SOCB retinas, #p < 0.05 vs. BCCAO+SOCB retinas) was calculated by two-way ANOVA followed by Fischer’s post hoc test
Quantification of the number of cells/100 μm GCL length (a), the number of cells/500 μm2 ONL (b) and INL (c) areas in SHAM+SOCB, in SHAM+VIP-TAT, in BCCAO+SOCB, and in BCCAO+VIP-TAT animals. Statistical significance (*p < 0.05 vs. SHAM+SOCB retinas, #p < 0.05 vs. BCCAO+SOCB retinas) was calculated by two-way ANOVA followed by Bonferroni’s post hoc test
Discussion
In the present study we demonstrated the efficacy of TAT-bound PACAP and VIP peptides to reach the retina and exert a retinoprotective effect in a model of ischemic retinopathy in rats. The retinoprotective effects of PACAP are well-documented in models of many different retinopathies (Atlasz et al. 2011, 2016; Shioda et al. 2016). Intravitreal injections of PACAP have been shown to lead to robust retinoprotective effects in various models of retinal injuries (Atlasz et al. 2016). The protective effects have been demonstrated to affect all neuronal cell types, from ganglion cells (Atlasz et al. 2010; Shoge et al. 1999) to photoreceptors and bipolar neurons (Szabadfi et al. 2016), the two main interneuronal types, amacrine and horizontal cells (Szabadfi et al. 2012) and the main glial cells, Muller glial cells (Nakatani et al. 2006; Werling et al. 2016). Furthermore, PACAP is an endogenous regulator of retinal microglial cells/macrophages, important in certain pathological conditions (Wada et al. 2013). PACAP not only affects the neurons and glial cells of the retina leading to retinoprotection, but also helps to preserve the integrity of the blood-retinal barrier (Scuderi et al. 2013) and protects the retinal pigment epithelial cells against oxidative stress injury, a process important in preservation of the outer barrier of the retina (Fabian et al. 2012). Furthermore, PACAP influences retinal vasculogenesis, especially under pathological conditions (Kvarik et al. 2016).
VIP has also been shown to have effects in the visual system according to some studies, although most results point to its involvement in photic neuronal transmission rather than its trophic effects (Akrouh and Kerschensteiner 2015; Dragich et al. 2010; Pérez de Sevilla Müller et al. 2017; Webb et al. 2013). VIP is an important neuromodulator along the visual transmission pathways, not only in the retina, but all the way to the cortex where it influences visual information processing (Galletti and Fattori 2018; Wilson and Glickfeld 2014). Regarding retinoprotection, a few studies indicate that VIP may also exert trophic effects in certain retinal injuries. Among others, VIP has been shown to protect retinal ganglion cells against excitotoxic injury in vitro (Shoge et al. 1998). VIP also protected against ischemia-reperfusion injury induced by ophthalmic vessel ligation (Tunçel et al. 1996), where both systemic and intravitreal VIP decreased oxidative stress as shown by reduced malondialdehyde levels. This led to a more preserved histological structure, which is in accordance with our present findings. Our earlier study, using the same hypoperfusion model used in the present study, showed that intravitreal VIP administration led to retinal morphological amelioration, but only at doses ten times higher than PACAP (Szabadfi et al. 2012). In the present study, we show a similar degree of protection, using TAT-bound VIP. VIP’s actions include not only direct effects, but also indirect effects, through stimulation of activity-dependent neurotrophic protein (ADNP) and its short fragment NAP, with highly potent neuroprotective effects. Both ADNP and NAP exerted strong protection against a variety of stress factors (Steingart et al. 2000). In the retina, NAP protected against laser-induced retinal damage (Belokopytov et al. 2011), to decrease hypoxia-inducible factor levels in a model of diabetic retinopathy (D’Amico et al. 2017, 2018; Maugeri et al. 2017), to prevent apoptotic cell death (Scuderi et al. 2014) and to promote neuronal growth after hypoxia-induced injury (Zheng et al. 2010). VIP also affects autonomic reflexes and choroidal blood flow, which eventually affects retinal blood supply (Bill and Sperber 1990). Applying VIP on the ocular surface in the form of eye drops has so far been shown to exert local effects on the cornea.
Regarding ischemic injury, PACAP has been shown to be protective in most cell layers affected in BCCAO-induced retinal ischemia. VIP was previously proven to be ten times less effective: intravitreal 100 pmol VIP, in contrast to the same dose of PACAP, led to no ameliorating effect on the retinal structure. However, 1000 pmol intravitreal VIP produced a protective effect. As eye drops, VIP was not effective alone (not shown). However, in our present study, we confirm that VIP bound to TAT peptide could effectively traverse the ocular barriers and exert a neuroprotective effect in the retina. PACAP-TAT did not prove to have significantly higher retinoprotective efficacy than untagged PACAP, but VIP exerted much stronger retinoprotective effects when bound to TAT. These results were consistent with our previous report that TAT with similar structure with PACAP(28–38) endowed VIP with higher affinity for PAC1-R (Yu et al. 2014). As for PACAP38, the tagging with TAT at the C-terminus of PACAP38 would be redundant and interfere with the receptor binding. This may be the reason why TAT tagging had some negative effect on PACAP38’s activity on the activation of PAC1-R. Also, as VIP has been implicated in a variety of other ocular diseases as a possible therapeutic approach (Berger et al. 2010; Cakmak et al. 2017; Satitpitakul et al. 2018), our results with topical applications leading to retinoprotection may open new therapeutic approaches.
In summary, our present study provides evidence, for the first time, that topical administration of PACAP and VIP derivatives (PACAP-TAT and VIP-TAT) dissolved in SOCB attenuate ischemic retinal degeneration via the PAC1 receptor presumably due to a multifactorial protective mechanism.
References
Abad C, Tan YV (2018) Immunomodulatory roles of PACAP and VIP: lessons from knockout mice. J Mol Neurosci 66:102–113. https://doi.org/10.1007/s12031-018-1150-y
Akrouh A, Kerschensteiner D (2015) Morphology and function of three VIP-expressing amacrine cell types in the mouse retina. J Neurophysiol 114:2431–2438. https://doi.org/10.1152/jn.00526.2015
Atlasz T, Szabadfi K, Kiss P, Babai N, Koszegi Z, Tamas A, Reglodi D, Gabriel R (2008) PACAP-mediated neuroprotection of neurochemically identified cell types in MSG-induced retinal degeneration. J Mol Neurosci 36:97–104. https://doi.org/10.1007/s12031-008-9059-5
Atlasz T, Szabadfi K, Kiss P, Tamas A, Toth G, Reglodi D, Gabriel R (2010) Evaluation of the protective effects of PACAP with cell-specific markers in ischemia-induced retinal degeneration. Brain Res Bull 81:497–504. https://doi.org/10.1016/j.brainresbull.2009.09.004
Atlasz T, Szabadfi K, Kiss P, Marton Z, Griecs M, Hamza L, Gaal V, Biro Z, Tamas A, Hild G, Nyitrai M, Toth G, Reglodi D, Gabriel R (2011) Effects of PACAP in UV-A radiation-induced retinal degeneration models in rats. J Mol Neurosci 43:51–57. https://doi.org/10.1007/s12031-010-9392-3
Atlasz T, Vaczy A, Werling D, Kiss P, Tamas A, Kovacs K, Fabian E, Kvarik T, Mammel B, Danyadi B, Lokos E, Reglodi D (2016) Neuroprotective effects of PACAP in the retina. In: Reglodi, Tamas (eds) Pituitary adenylate cyclase activating polypeptide-PACAP, current topics in neurotoxicity 11. Springer Nature, New York, pp 501–527
Bardosi S, Bardosi A, Nagy Z, Reglodi D (2016) Expression of PACAP and PAC1 receptor in normal human thyroid gland and in thyroid papillary carcinoma. J Mol Neurosci 60:171–178. https://doi.org/10.1007/s12031-016-0823-7
Belokopytov M, Shulman S, Dubinsky G, Gozes I, Belkin M, Rosner M (2011) Ameliorative effect of NAP on laser-induced retinal damage. Acta Ophthalmol 89:e126–e131. https://doi.org/10.1111/j.1755-3768.2010.02041.x
Berger EA, McClellan SA, Barrett RP, Hazlett LD (2010) VIP promotes resistance in the Pseudomonas aeruginosa-infected cornea by modulating adhesion molecule expression. Invest Ophthalmol Vis Sci 51:5776–5782. https://doi.org/10.1167/iovs.09-4917
Bill A, Sperber GO (1990) Control of retinal and choroidal blood flow. Eye (Lond) 4:319–325. https://doi.org/10.1038/eye.1990.43
Brifault C, Vaudry D, Wurtz O (2016) The neuropeptide PACAP, a potent disease modifier candidate for brain stroke treatment. In: Reglodi, Tamas (eds) Pituitary adenylate cyclase activating polypeptide-PACAP, current topics in neurotoxicity 11. Springer Nature, New York, pp 583–606
Cakmak AI, Basmak H, Gursoy H, Ozkurt M, Yildirim N, Erkasap N, Bilgec MD, Tuncel N, Colak E (2017) Vasoactive intestinal peptide, a promising agent for myopia? Int J Ophthalmol 10:211–216. https://doi.org/10.18240/ijo.2017.02.05
Carrión M, Pérez-García S, Martínez C, Juarranz Y, Estrada-Capetillo L, Puig-Kröger A, Gomariz RP, Gutiérrez-Cañas I (2016) VIP impairs acquisition of the macrophage proinflammatory polarization profile. J Leukoc Biol 100:1385–1393. https://doi.org/10.1189/jlb.3A0116-032RR
D’Amico AG, Maugeri G, Bucolo C, Saccone S, Federico C, Cavallaro S, D’Agata V (2017) Nap interferes with hypoxia-inducible factors and VEGF expression in retina of diabetic rats. J Mol Neurosci 61:256–266. https://doi.org/10.1007/s12031-016-0869-6
D’Amico AG, Maugeri G, Rasà DM, La Cognata V, Saccone S, Federico C, Cavallaro S, D’Agata V (2018) NAP counteracts hyperglycemia/hypoxia induced retinal pigment epithelial barrier breakdown through modulation of HIFs and VEGF expression. J Cell Physiol 233:1120–1128. https://doi.org/10.1002/jcp.25971
Dietz GP, Bähr M (2004) Delivery of bioactive molecules into the cell: the Trojan horse approach. Mol Cell Neurosci 27:85–131. https://doi.org/10.1016/j.mcn.2004.03.005
Dragich JM, Loh DH, Wang LM, Vosko AM, Kudo T, Nakamura TJ, Odom IH, Tateyama S, Hagopian A, Waschek JA, Colwell CS (2010) The role of the neuropeptides PACAP and VIP in the photic regulation of gene expression in the suprachiasmatic nucleus. Eur J Neurosci 31:864–875. https://doi.org/10.1111/j.1460-9568.2010.07119.x
Egri P, Fekete C, Dénes Á, Reglődi D, Hashimoto H, Fülöp BD, Gereben B (2016) Pituitary adenylate cyclase-activating polypeptide (PACAP) regulates the hypothalamo-pituitary-thyroid (HPT) axis via type 2 deiodinase in male mice. Endocrinology 157:2356–2366. https://doi.org/10.1210/en.2016-1043
Endo K, Nakamachi T, Seki T, Kagami N, Wada Y, Nakamura K, Kishimoto K, Hori M, Tsuchikawa D, Shinntani N, Hashimoto H, Baba A, Koide R, Shioda S (2011) Neuroprotective effect of PACAP against NMDA-induced retinal damage in the mouse. J Mol Neurosci 43:22–29. https://doi.org/10.1007/s12031-010-9434-x
Fabian E, Reglodi D, Mester L, Szabo A, Szabadfi K, Tamas A, Toth G, Kovacs K (2012) Effects of PACAP on intracellular signaling pathways in human retinal pigment epithelial cells exposed to oxidative stress. J Mol Neurosci 48:493–500. https://doi.org/10.1007/s12031-012-9812-7
Farkas J, Kovács LÁ, Gáspár L, Nafz A, Gaszner T, Ujvári B, Kormos V, Csernus V, Hashimoto H, Reglődi D, Gaszner B (2017) Construct and face validity of a new model for the three-hit theory of depression using PACAP mutant mice on CD1 background. Neuroscience 354:11–29. https://doi.org/10.1016/j.neuroscience.2017.04.019
Fukiage C, Nakajima T, Takayama Y, Minagawa Y, Shearer TR, Azuma M (2007) PACAP induces neurite outgrowth in cultured trigeminal ganglion cells and recovery of corneal sensitivity after flap surgery in rabbits. Am J Ophthalmol 143:255–262. https://doi.org/10.1016/j.ajo.2006.10.034
Fulop BD, Sandor B, Szentleleky E, Karanyicz E, Reglodi D, Gaszner B, Zakany R, Hashimoto H, Juhasz T, Tamas A (2018) Altered notch signalling in developing molar teeth of pituitary adenylate cyclase activating polypeptide (PACAP)-deficient mice. J Mol Neurosci. https://doi.org/10.1007/s12031-018-1146-7
Gaal V, Mark L, Kiss P, Kustos I, Tamas A, Kocsis B, Lubics A, Nemeth V, Nemeth A, Lujber L, Pytel J, Toth G, Reglodi D (2008) Investigation of the effects of PACAP on the composition of tear and endolymph proteins. J Mol Neurosci 36:321–329. https://doi.org/10.1007/s12031-008-9067-5
Galletti C, Fattori P (2018) The dorsal visual stream revisited: stable circuits or dynamic pathways? Cortex 98:203–217. https://doi.org/10.1016/j.cortex.2017.01.009
Giladi E, Hill JM, Dresner E, Stack CM, Gozes I (2007) Vasoactive intestinal peptide (VIP) regulates activity-dependent neuroprotective protein (ADNP) expression in vivo. J Mol Neurosci 33:278–283. https://doi.org/10.1007/s12031-007-9003-0
Gozes I (2008) VIP, from gene to behavior and back: summarizing my 25 years of research. J Mol Neurosci 36:115–124. https://doi.org/10.1007/s12031-008-9105-3
Gupta A, Gargiulo AT, Curtis GR, Badve PS, Pandey S, Barson JR (2018) Pituitary adenylate cyclase-activating polypeptide-27 (PACAP-27) in the thalamic paraventricular nucleus is stimulated by ethanol drinking. Alcohol Clin Exp Res 42:1650–1660. https://doi.org/10.1111/acer.13826
Han P, Tang Z, Yin J, Maalouf M, Beach TG, Reiman EM, Shi J (2014) Pituitary adenylate cyclase-activating polypeptide protects against β-amyloid toxicity. Neurobiol Aging 35:2064–2071. https://doi.org/10.1016/j.neurobiolaging.2014.03.022
Heppner TJ, Hennig GW, Nelson MT, May V, Vizzard MA (2018) PACAP38-mediated bladder afferent nerve activity hyperexcitability and Ca2+ activity in urothelial cells from mice. J Mol Neurosci. https://doi.org/10.1007/s12031-018-1119-x
Hill JM, Hauser JM, Sheppard LM, Abebe D, Spivak-Pohis I, Kushnir M, Deitch I, Gozes I (2007) Blockage of VIP during mouse embryogenesis modifies adult behavior and results in permanent changes in brain chemistry. J Mol Neurosci 31:183–200
Jimeno R, Leceta J, Martínez C, Gutiérrez-Cañas I, Carrión M, Pérez-García S, Garín M, Mellado M, Gomariz RP, Juarranz Y (2014) Vasoactive intestinal peptide maintains the nonpathogenic profile of human th17-polarized cells. J Mol Neurosci 54:512–525. https://doi.org/10.1007/s12031-014-0318-3
King SB, Lezak KR, O’Reilly M, Toufexis DJ, Falls WA, Braas K, May V, Hammack SE (2017) The effects of prior stress on anxiety-like responding to intra-BNST pituitary adenylate cyclase activating polypeptide in male and female rats. Neuropsychopharmacology 42:1679–1687. https://doi.org/10.1038/npp.2017.16
Koh SM, Coll T, Gloria D, Sprehe N (2017) Corneal endothelial cell integrity in precut human donor corneas enhanced by autocrine vasoactive intestinal peptide. Cornea 36:476–483. https://doi.org/10.1097/ICO.0000000000001136
Kvarik T, Mammel B, Reglodi D, Kovacs K, Werling D, Bede B, Vaczy A, Fabian E, Toth G, Kiss P, Tamas A, Ertl T, Gyarmati J, Atlasz T (2016) PACAP is protective in a rat model of retinopathy of prematurity. J Mol Neurosci 60:179–185. https://doi.org/10.1007/s12031-016-0797-5
Lajko A, Meggyes M, Fulop BD, Gede N, Reglodi D, Szereday L (2018) Comparative analysis of decidual and peripheral immune cells and immune-checkpoint molecules during pregnancy in wild-type and PACAP-deficient mice. Am J Reprod Immunol 9:e13035. https://doi.org/10.1111/aji.13035
Ma Y, Zhao S, Wang X, Shen S, Ma M, Xu W, Hong A (2015) A new recombinant PACAP-derived peptide efficiently promotes corneal wound repairing and lacrimal secretion. Invest Ophthalmol Vis Sci 56:4336–4349. https://doi.org/10.1167/iovs.15-17088
Maugeri G, D’Amico AG, Saccone S, Federico C, Cavallaro S, D’Agata V (2017) PACAP and VIP inhibit HIF-1α-mediated VEGF expression in a model of diabetic macular edema. J Cell Physiol 232:1209–1215. https://doi.org/10.1002/jcp.25616
Maugeri G, D’Amico AG, Rasa DM, Saccone S, Federico C, Cavallaro S, D’Agata V (2018a) PACAP and VIP regulate hypoxia-inducible factors in neuroblastoma cells exposed to hypoxia. Neuropeptides 69:84–91. https://doi.org/10.1016/j.npep.2018.04.009
Maugeri G, Longo A, D’Amico AG, Rasà DM, Reibaldi M, Russo A, Bonfiglio V, Avitabile T, D’Agata V (2018b) Trophic effect of PACAP on human corneal endothelium. Peptides 99:20–26. https://doi.org/10.1016/j.peptides.2017.11.003
Moody TW, Gozes I (2007) Vasoactive intestinal peptide receptors: a molecular target in breast and lung cancer. Curr Pharm Des 13:1099–1104. https://doi.org/10.2174/138161207780619000
Morell M, Souza-Moreira L, González-Rey E (2012) VIP in neurological diseases: more than a neuropeptide. Endocr Metab Immune Disord Drug Targets 12:323–332
Nakamachi T, Ohtaki H, Seki T, Yofu S, Kagami N, Hashimoto H, Shintani N, Baba A, Mark L, Lanekoff I, Kiss P, Farkas J, Reglodi D, Shioda S (2016) PACAP suppresses dry eye signs by stimulating tear secretion. Nat Commun 7:12034. https://doi.org/10.1038/ncomms12034
Nakatani M, Seki T, Shinohara Y, Taki C, Nishimura S, Takaki A, Shioda S (2006) Pituitary adenylate cyclase-activating peptide (PACAP) stimulates production of interleukin-6 in rat Müller cells. Peptides 27:1871–1876. https://doi.org/10.1016/j.peptides.2005.12.011
Olson KE, Kosloski-Bilek LM, Anderson KM, Diggs BJ, Clark BE, Gledhill JM Jr, Shandler SJ, Mosley RL, Gendelman HE (2015) Selective VIP receptor agonists facilitate immune transformation for dopaminergic neuroprotection in MPTP-intoxicated mice. J Neurosci 35:16463–16478. https://doi.org/10.1523/JNEUROSCI.2131-15.2015
Parsons RL, May V (2018) PACAP-induced PAC1 receptor internalization and recruitment of endosomal signaling regulate cardiac neuron excitability. J Mol Neurosci. https://doi.org/10.1007/s12031-018-1127-x
Pérez de Sevilla Müller L, Solomon A, Sheets K, Hapukino H, Rodriguez AR, Brecha NC (2017) Multiple cell types form the VIP amacrine cell population. J Comp Neurol. https://doi.org/10.1002/cne.24234
Prevost G, Arabo A, Jian L, Quelennec E, Cartier D, Hassan S, Falluel-Morel A, Tanguy Y, Gargani S, Lihrmann I, Kerr-Conte J, Lefebvre H, Pattou F, Anouar Y (2013) The PACAP-regulated gene selenoprotein T is abundantly expressed in mouse and human β-cells and its targeted inactivation impairs glucose tolerance. Endocrinology 154:3796–3806. https://doi.org/10.1210/en.2013-1167
Reglodi D, Tamas A (2016) Pituitary adenylate cyclase activating polypeptide – PACAP. Springer Nature, New York
Reglodi D, Kiss P, Lubics A, Tamas A (2011) Review of the protective effects of PACAP in models of neurodegenerative diseases in vitro and in vivo. Curr Pharm Des 17:962–972. https://doi.org/10.2174/138161211795589355
Reglodi D, Tamas A, Koppan M, Szogyi D, Welke L (2012) Role of PACAP in female fertility and reproduction at gonadal level – recent advances. Front Endocrinol (Lausanne) 3:155. https://doi.org/10.3389/fendo.2012.00155
Reglodi D, Illes A, Opper B, Schafer E, Tamas A, Nemeth J, Horvath G (2018a) Presence and effects of pituitary adenylate cyclase activating polypeptide under physiological and pathological conditions in the stomach. Front Endocrinol (Lausanne) 9:90. https://doi.org/10.3389/fendo.2018.00090
Reglodi D, Jungling A, Longuespée R, Kriegsmann J, Casadonte R, Kriegsmann M, Juhasz T, Bardosi A, Tamas A, Fulop BD, Kovacs K, Nagy Z, Sparks J, Miseta A, Mazzucchelli G, Hashimoto H, Bardosi A (2018b) Accelerated pre-senile systemic amyloidosis in PACAP knockout mice – a protective role of PACAP in age-related degenerative processes. J Pathol 245:478–490. https://doi.org/10.1002/path.5100
Reglodi D, Tamas A, Jungling A, Vaczy A, Rivnyak A, Fulop BD, Szabo E, Lubics A, Atlasz T (2018c) Protective effects of pituitary adenylate cyclase activating polypeptide against neurotoxic agents. Neurotoxicology 66:185–194. https://doi.org/10.1016/j.neuro.2018.03.010
Ross RA, Leon S, Madara JC, Schafer D, Fergani C, Maguire CA, Verstegen AM, Brengle E, Kong D, Herbison AE, Kaiser UB, Lowell BB, Navarro VM (2018) PACAP neurons in the ventral premammillary nucleus regulate reproductive function in the female mouse. Elife 7. https://doi.org/10.7554/eLife.35960.001
Sasaki S, Watanabe J, Ohtaki H, Matsumoto M, Murai N, Nakamachi T, Hannibal J, Fahrenkrug J, Hashimoto H, Watanabe H, Sueki H, Honda K, Miyazaki A, Shioda S (2017) Pituitary adenylate cyclase-activating polypeptide promotes eccrine gland sweat secretion. Br J Dermatol 176:413–422. https://doi.org/10.1111/bjd.14885
Satitpitakul V, Sun Z, Suri K, Amouzegar A, Katikireddy KR, Jurkunas UV, Kheirkhah A, Dana R (2018) Vasoactive intestinal peptide promotes corneal allograft survival. Am J Pathol 188:2016–2024. https://doi.org/10.1016/j.ajpath.2018.05.010
Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF (1999) In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285:1569–1572. https://doi.org/10.1126/science.285.5433.1569
Scuderi S, D’Amico AG, Castorina A, Imbesi R, Carnazza ML, D’Agata V (2013) Ameliorative effect of PACAP and VIP against increased permeability in a model of outer blood retinal barrier dysfunction. Peptides 39:119–124. https://doi.org/10.1016/j.peptides.2012.11.015
Scuderi S, D’Amico AG, Castorina A, Federico C, Marrazzo G, Drago F, Bucolo C, D’Agata V (2014) Davunetide (NAP) protects the retina against early diabetic injury by reducing apoptotic death. J Mol Neurosci 54:395–404. https://doi.org/10.1007/s12031-014-0244-4
Shi H, Carion TW, Jiang Y, Steinle JJ, Berger EA (2016) VIP protects human retinal microvascular endothelial cells against high glucose-induced increases in TNF-α and enhances RvD1. Prostaglandins Other Lipid Mediat 123:28–32. https://doi.org/10.1016/j.prostaglandins.2016.03.001
Shioda S, Gozes I (2011) VIP and PACAP: novel approaches to brain functions and neuroprotection. Curr Pharm Des 17:961. https://doi.org/10.2174/138161211795589391
Shioda S, Takenoya F, Wada N, Hirabayashi T, Seki T, Nakamachi T (2016) Pleiotropic and retinoprotective functions of PACAP. Anat Sci Int 91:313–324. https://doi.org/10.1007/s12565-016-0351-0
Shioda S, Takenoya F, Hirabayashi T, Wada N, Seki T, Nonaka N, Nakamachi T (2018) Effects of PACAP on dry eye symptoms, and possible use for therapeutic application. J Mol Neurosci. https://doi.org/10.1007/s12031-018-1087-1
Shoge K, Mishima HK, Saitoh T, Ishihara K, Tamura Y, Shiomi H et al (1998) Protective effects of vasoactive intestinal peptide against delayed glutamate neurotoxicity in cultured retina. Brain Res 809:127–136
Shoge K, Mishima HK, Saitoh T, Ishihara K, Tamura Y, Shiomi H, Sasa M (1999) Attenuation by PACAP of glutamate-induced neurotoxicity in cultured retinal neurons. Brain Res 839:66–73. https://doi.org/10.1016/S0006-8993(99)01690-X
Steingart RA, Solomon B, Brenneman DE, Fridkin M, Gozes I (2000) VIP and peptides related to activity-dependent neurotrophic factor protect PC12 cells against oxidative stress. J Mol Neurosci 15:137–145. https://doi.org/10.1385/JMN:15:3:137
Szabadfi K, Danyadi B, Kiss P, Tamas A, Fabian E, Gabriel R, Reglodi D (2012) Protective effects of vasoactive intestinal peptide (VIP) in ischemic retinal degeneration. J Mol Neurosci 48:501–507. https://doi.org/10.1007/s12031-012-9774-9
Szabadfi K, Reglodi D, Szabo A, Szalontai B, Valasek A, Setalo G Jr, Kiss P, Tamas A, Wilhelm M, Gabriel R (2016) Pituitary adenylate cyclase activating polypeptide, a potential therapeutic agent for diabetic retinopathy in rats: focus on the vertical information processing pathway. Neurotox Res 29:432–446. https://doi.org/10.1007/s12640-015-9593-1
Tunçel N, Başmak H, Uzuner K, Tunçel M, Altiokka G, Zaimoğlu V, Ozer A, Gürer F (1996) Protection of rat retina from ischemia-reperfusion injury by vasoactive intestinal peptide (VIP): the effect of VIP on lipid peroxidation andF antioxidant enzyme activity of retina and choroid. Ann NY Acad Sci 805:489–498. https://doi.org/10.1111/j.1749-6632.1996.tb17509.x
Tuncel N, Yildirim N, Gurer F, Basmak H, Uzuner K, Sahinturk V, Gursoy H (2016) Effect of vasoactive intestinal peptide on the wound healing of alkali-burned corneas. Int J Ophthalmol 9:204–210. https://doi.org/10.18240/ijo.2016.02.04
Vaczy A, Reglodi D, Somoskeoy T, Kovacs K, Lokos E, Szabo E, Tamas A, Atlasz T (2016) The protective role of PAC1-receptor agonist maxadilan in BCCAO-induced retinal degeneration. J Mol Neurosci 60:186–194. https://doi.org/10.1007/s12031-016-0818-4
Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H (2009) Pituitary adenylate cyclase activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 61:283–357. https://doi.org/10.1124/pr.109.001370
Vu JP, Larauche M, Flores M, Luong L, Norris J, Oh S, Liang LJ, Waschek J, Pisegna JR, Germano PM (2015) Regulation of appetite, body composition, and metabolic hormones by vasoactive intestinal polypeptide (VIP). J Mol Neurosci 56:377–387. https://doi.org/10.1007/s12031-015-0556-z
Wada Y, Nakamachi T, Endo K, Seki T, Ohtaki H, Tsuchikawa D, Hori M, Tsuchida M, Yoshikawa A, Matkovits A, Kagami N, Imai N, Fujisaka S, Usui I, Tobe K, Koide R, Takahashi H, Shioda S (2013) PACAP attenuates NMDA-induced retinal damage in association with modulation of the microglia/macrophage status into an acquired deactivation subtype. J Mol Neurosci 51:493–502. https://doi.org/10.1007/s12031-013-0017-5
Wang ZY, Alm P, Håkanson R (1996) PACAP occurs in sensory nerve fibers and participates in ocular inflammation in the rabbit. Ann NY Acad Sci 805:779–783. https://doi.org/10.1111/j.1749-6632.1996.tb17556.x
Watanabe J, Nakamachi T, Matsuno R, Hayashi D, Nakamura M, Kikuyama S, Nakajo S, Shioda S (2007) Localization, characterization and function of pituitary adenylate cyclase-activating polypeptide during brain development. Peptides 28:1713–1719. https://doi.org/10.1016/j.peptides.2007.06.029
Webb IC, Coolen LM, Lehman MN (2013) NMDA and PACAP receptor signaling interact to mediate retinal-induced scn cellular rhythmicity in the absence of light. PLoS One 8:e76365. https://doi.org/10.1371/journal.pone.0076365
Werling D, Reglodi D, Banks WA, Salameh TS, Kovacs K, Kvarik T, Vaczy A, Kovacs L, Mayer F, Danyadi B, Lokos E, Tamas A, Toth G, Zs B, Tamas A, Atlasz T (2016) Ocular delivery of PACAP1-27 protects the retina from ischemic damage in rodents. Invest Ophthalmol Vis Sci 57:6683–6691. https://doi.org/10.1167/iovs.16-20630
Werling D, Banks WA, Salameh TS, Kvarik T, Kovacs LA, Vaczy A, Szabo E, Mayer F, Varga R, Tamas A, Toth G, Zs B, Atlasz T, Reglodi D (2017) Passage through the ocular barriers and beneficial effects in retinal ischemia of topical application of PACAP1-38 in rodents. Int J Mol Sci 18. https://doi.org/10.3390/ijms18030675
Wilson AM, Glickfeld LL (2014) Visual circuits get the VIP treatment. Cell 156:1123–1124. https://doi.org/10.1016/j.cell.2014.02.043
Yoshitomi T, Yamaji K, Ishikawa H, Ohnishi Y (2002) Effect of pituitary adenylate cyclase-activating peptide on isolated rabbit iris sphincter and dilator muscles. Invest Ophthalmol Vis Sci 43:780–783
Yu R, Guo X, Huang L, Zeng Z, Zhang H (2012a) The novel peptide PACAP-TAT with enhanced traversing ability attenuates the severe lung injury induced by repeated smoke inhalation. Peptides 38:142–149. https://doi.org/10.1016/j.peptides.2012.09.005
Yu R, Zeng Z, Guo X, Zhang H, Liu X, Ding Y, Chen J (2012b) The TAT peptide endows PACAP with an enhanced ability to traverse bio-barriers. Neurosci Lett 527:1–5. https://doi.org/10.1016/j.neulet.2012.08.005
Yu R, Yang Y, Cui Z, Zheng L, Zeng Z, Zhang H (2014) Novel peptide VIP-TAT with higher affinity for PAC1 inhibited scopolamine induced amnesia. Peptides 60:41–50. https://doi.org/10.1016/j.peptides.2014.07.018
Zheng Y, Zeng H, She H, Liu H, Sun N (2010) Expression of peptide NAP in rat retinal Müller cells prevents hypoxia-induced retinal injuries and promotes retinal neurons growth. Biomed Pharmacother 64:417–423. https://doi.org/10.1016/j.biopha.2010.01.016
Acknowledgements
This work was supported by NKFIH FK129190, K119759, NAP 2017-1.2.1-NKP-2017-00002, National Natural Science Foundation of China (31670848), Natural Science Foundation of Guangdong Province (2016A030313087), Bolyai Scholarship, GINOP-2.3.2-15-2016-00050 “PEPSYS”, MTA-TKI 14016, EFOP-3.6.3-VEKOP-16-15 2017-00008 “The role of neuro-inflammation in neurodegeneration: from molecules to clinics”, EFOP 3.6.3-VEKOP-2017-00009, EFOP-3.6.1.-16-2016-00004 “Comprehensive Development for Implementing Smart Specialization Strategies at the University of Pécs”, ÚNKP-18-4-PTE-364, ÚNKP-18-2-I-PTE-199, ÚNKP-16-4, ÚNKP-17-2-II New National Excellence Program of the Ministry of Human Capacities, Centre for Neuroscience, PTE AOK Research Grant KA-2017-15, Higher Education Institutional Excellence Programme of the Ministry of Human Capacities in Hungary, within the framework of the 20765-3/2018/FEKUTSTRAT.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Atlasz, T., Werling, D., Song, S. et al. Retinoprotective Effects of TAT-Bound Vasoactive Intestinal Peptide and Pituitary Adenylate Cyclase Activating Polypeptide. J Mol Neurosci 68, 397–407 (2019). https://doi.org/10.1007/s12031-018-1229-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-018-1229-5