Abstract
Background
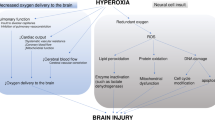
Cardiac arrest (CA) is a sudden event that is often characterized by hypoxic-ischemic brain injury (HIBI), leading to significant mortality and long-term disability. Brain tissue oxygenation (PbtO2) is an invasive tool for monitoring brain oxygen tension, but it is not routinely used in patients with CA because of the invasiveness and the absence of high-quality data on its effect on outcome. We conducted a systematic review of experimental and clinical evidence to understand the role of PbtO2 in monitoring brain oxygenation in HIBI after CA and the effect of targeted PbtO2 therapy on outcomes.
Methods
The search was conducted using four search engines (PubMed, Scopus, Embase, and Cochrane), using the Boolean operator to combine mesh terms such as PbtO2, CA, and HIBI.
Results
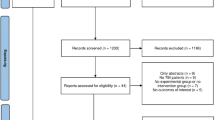
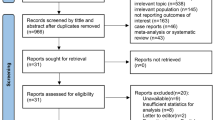
Among 1,077 records, 22 studies were included (16 experimental studies and six clinical studies). In experimental studies, PbtO2 was mainly adopted to assess the impact of gas exchanges, drugs, or systemic maneuvers on brain oxygenation. In human studies, PbtO2 was rarely used to monitor the brain oxygen tension in patients with CA and HIBI. PbtO2 values had no clear association with patients’ outcomes, but in the experimental studies, brain tissue hypoxia was associated with increased inflammation and neuronal damage.
Conclusions
Further studies are needed to validate the effect and the threshold of PbtO2 associated with outcome in patients with CA, as well as to understand the physiological mechanisms influencing PbtO2 induced by gas exchanges, drug administration, and changes in body positioning after CA.



Similar content being viewed by others
References
Gräsner JT, Lefering R, Koster RW, et al. EuReCa ONE 27 Nations, ONE Europe, ONE Registry. Resuscitation. 2016;105:188–95. https://doi.org/10.1016/j.resuscitation.2016.06.004.
Gräsner JT, Wnent J, Herlitz J, et al. Survival after out-of-hospital cardiac arrest in Europe-Results of the EuReCa TWO study. Resuscitation. 2020;148:218–26. https://doi.org/10.1016/j.resuscitation.2019.12.042.
Gill R, Teitcher M, Ruland S. Neurologic complications of cardiac arrest. Handb Clin Neurol. 2021;177:193–209. https://doi.org/10.1016/B978-0-12-819814-8.00029-9.
Lemiale V, Dumas F, Mongardon N, et al. Intensive care unit mortality after cardiac arrest: the relative contribution of shock and brain injury in a large cohort. Intensive Care Med. 2013;39(11):1972–80. https://doi.org/10.1007/s00134-013-3043-4.
Geocadin RG, Callaway CW, Fink EL, et al. Standards for studies of neurological prognostication in comatose survivors of cardiac arrest: a scientific statement from the American heart association. Circulation. 2019;140(9):e517-542. https://doi.org/10.1161/CIR.0000000000000702.
Marquez AM, Morgan RW, Ko T, et al. Oxygen exposure during cardiopulmonary resuscitation is associated with cerebral oxidative injury in a randomized, blinded, controlled, preclinical trial. J Am Heart Assoc. 2020;9(9):e015032. https://doi.org/10.1161/JAHA.119.015032.
Al-Kawaz MN, Canner J, Caturegli G, et al. Duration of hyperoxia and neurologic outcomes in patients undergoing extracorporeal membrane oxygenation. Crit Care Med. 2021;49(10):e968–77. https://doi.org/10.1097/CCM.0000000000005069.
SRLF Trial Group. Hypoxemia in the ICU: prevalence, treatment, and outcome. Ann Intensive Care. 2018;8(1):82. https://doi.org/10.1186/s13613-018-0424-4.
Robba C, Siwicka-Gieroba D, Sikter A, et al. Pathophysiology and clinical consequences of arterial blood gases and pH after cardiac arrest. Intensive Care Med Exp. 2020;8(S1):19. https://doi.org/10.1186/s40635-020-00307-1.
Maloney-Wilensky E, Le Roux P. The physiology behind direct brain oxygen monitors and practical aspects of their use. Child’s Nervous System. 2010;26(4):419–30. https://doi.org/10.1007/s00381-009-1037-x.
Maloney-Wilensky E, Gracias V, Itkin A, et al. Brain tissue oxygen and outcome after severe traumatic brain injury: a systematic review*. Crit Care Med. 2009;37(6):2057–63. https://doi.org/10.1097/CCM.0b013e3181a009f8.
Okonkwo DO, Shutter LA, Moore C, et al. Brain oxygen optimization in severe traumatic brain injury phase-II. Crit Care Med. 2017;45(11):1907–14. https://doi.org/10.1097/CCM.0000000000002619.
Bernard F, Barsan W, Diaz-Arrastia R, Merck LH, Yeatts S, Shutter LA. Brain oxygen optimization in severe traumatic brain injury (BOOST-3): a multicentre, randomised, blinded-endpoint, comparative effectiveness study of brain tissue oxygen and intracranial pressure monitoring versus intracranial pressure alone. BMJ Open. 2022;12(3):1060188. https://doi.org/10.1136/bmjopen-2021-060188.
Moore J, Young P, Udy A, et al. Brain Oxygen Neuromonitoring in Australia and New Zealand Assessment (BONANZA). hrc nz. Published 2021. https://www.hrc.govt.nz/resources/research-repository/brain-oxygen-neuromonitoring-australia-and-new-zealand-assessment
Payen JF, Richard M, Francony G, et al. Comparison of strategies for monitoring and treating patients at the early phase of severe traumatic brain injury: the multicentre randomised controlled OXY-TC trial study protocol. BMJ Open. 2020;10(8):1040550. https://doi.org/10.1136/bmjopen-2020-040550.
Chesnut R, Aguilera S, Buki A, et al. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2020;46(5):919–29. https://doi.org/10.1007/s00134-019-05900-x.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Lo CKL, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14(1):45. https://doi.org/10.1186/1471-2288-14-45.
Hooijmans CR, Rovers MM, de Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. https://doi.org/10.1186/1471-2288-14-43.
Annoni F, Peluso L, Akira Hirai L, et al. A comprehensive neuromonitoring approach in a large animal model of cardiac arrest. Anim Models Exp Med. 2022;5:56–60. https://doi.org/10.1002/ame2.12200.
Elmer J, Flickinger KL, Anderson MW, et al. Effect of neuromonitor-guided titrated care on brain tissue hypoxia after opioid overdose cardiac arrest. Resuscitation. 2018;129:121–6. https://doi.org/10.1016/j.resuscitation.2018.04.013.
Jung YH, Shamsiev K, Mamadjonov N, et al. Relationship of common hemodynamic and respiratory target parameters with brain tissue oxygen tension in the absence of hypoxemia or hypotension after cardiac arrest: A post-hoc analysis of an experimental study using a pig model. PLoS ONE. 2021;16(2):10245931. https://doi.org/10.1371/journal.pone.0245931.
Linner R, Werner O, Perez-de-Sa V, Cunha-Goncalves AD. Circulatory recovery is as fast with air ventilation as with 100% oxygen after asphyxia-induced cardiac arrest in piglets. Pediatr Res. 2009;66:4.
Zhou D, Li Z, Zhang S, et al. Mild hypercapnia improves brain tissue oxygen tension but not diffusion limitation in asphyxial cardiac arrest: an experimental study in pigs. BMC Anesthesiol. 2020;20(1):252. https://doi.org/10.1186/s12871-020-01162-z.
Lee HY, Shamsiev K, Mamadjonov N, et al. Effect of epinephrine administered during cardiopulmonary resuscitation on cerebral oxygenation after restoration of spontaneous circulation in a swine model with a clinically relevant duration of untreated cardiac arrest. Int J Environ Res Public Health. 2021;18(11):5896. https://doi.org/10.3390/ijerph18115896.
Putzer G, Martini J, Spraider P, et al. Adrenaline improves regional cerebral blood flow, cerebral oxygenation and cerebral metabolism during CPR in a porcine cardiac arrest model using low-flow extracorporeal support. Resuscitation. 2021;168:151–9. https://doi.org/10.1016/j.resuscitation.2021.07.036.
Putzer G, Braun P, Strapazzon G, et al. Monitoring of brain oxygenation during hypothermic CPR: a prospective porcine study. Resuscitation. 2016;104:1–5. https://doi.org/10.1016/j.resuscitation.2016.03.027.
Putzer G, Martini J, Spraider P, et al. Effects of different adrenaline doses on cerebral oxygenation and cerebral metabolism during cardiopulmonary resuscitation in pigs. Resuscitation. 2020;156:223–9. https://doi.org/10.1016/j.resuscitation.2020.06.024.
Mavroudis CD, Ko TS, Morgan RW, et al. Epinephrine’s effects on cerebrovascular and systemic hemodynamics during cardiopulmonary resuscitation. Crit Care. 2020;24(1):583. https://doi.org/10.1186/s13054-020-03297-4.
Cavus E, Bein B, Dörges V, et al. Brain tissue oxygen pressure and cerebral metabolism in an animal model of cardiac arrest and cardiopulmonary resuscitation. Resuscitation. 2006;71(1):97–106. https://doi.org/10.1016/j.resuscitation.2006.03.007.
Cavus E, Dörges V, Wagner-Berger H, et al. Changes of local brain tissue oxygen pressure after vasopressin during spontaneous circulation. Acta Neurochir (Wien). 2005;147(3):283–90. https://doi.org/10.1007/s00701-004-0406-1.
García-Bardon A, Kamuf J, Ziebart A, et al. Levosimendan increases brain tissue oxygen levels after cardiopulmonary resuscitation independent of cardiac function and cerebral perfusion. Sci Rep. 2021;11(1):14220. https://doi.org/10.1038/s41598-021-93621-x.
Lee HY, Jung YH, Mamadjonov N, et al. Effects of sodium nitroprusside administered via a subdural intracranial catheter on the microcirculation, oxygenation, and electrocortical activity of the cerebral cortex in a pig cardiac arrest model. J Am Heart Assoc. 2022. https://doi.org/10.1161/JAHA.122.025400.
Putzer G, Braun P, Martini J, et al. Effects of head-up vs supine CPR on cerebral oxygenation and cerebral metabolism—a prospective, randomized porcine study. Resuscitation. 2018;128:51–5. https://doi.org/10.1016/j.resuscitation.2018.04.038.
Lurie KG, Yannopoulos D, McKnite SH, et al. Comparison of a 10-breaths-per-minute versus a 2-breaths-per-minute strategy during cardiopulmonary resuscitation in a porcine model of cardiac arrest. Respir Care. 2008;53(7):862–70.
Sekhon MS, Griesdale DE, Ainslie PN, et al. Intracranial pressure and compliance in hypoxic ischemic brain injury patients after cardiac arrest. Resuscitation. 2019;141:96–103. https://doi.org/10.1016/j.resuscitation.2019.05.036.
Sekhon MS, Gooderham P, Menon DK, et al. The burden of brain hypoxia and optimal mean arterial pressure in patients with hypoxic ischemic brain injury after cardiac arrest*. Crit Care Med. 2019;47(7):960–9. https://doi.org/10.1097/CCM.0000000000003745.
Sekhon MS, Ainslie PN, Menon DK, et al. Brain hypoxia secondary to diffusion limitation in hypoxic ischemic brain injury postcardiac arrest. Crit Care Med. 2020;48(3):378–84. https://doi.org/10.1097/CCM.0000000000004138.
Balu R, Rajagopalan S, Baghshomali S, et al. Cerebrovascular pressure reactivity and intracranial pressure are associated with neurologic outcome after hypoxic-ischemic brain injury. Resuscitation. 2021;164:114–21. https://doi.org/10.1016/j.resuscitation.2021.04.023.
Hoiland RL, Ainslie PN, Wellington CL, et al. Brain hypoxia is associated with neuroglial injury in humans post-cardiac arrest. Circ Res. 2021;129(5):583–97. https://doi.org/10.1161/CIRCRESAHA.121.319157.
Fergusson NA, Hoiland RL, Thiara S, et al. Goal-directed care using invasive neuromonitoring versus standard of care after cardiac arrest. Crit Care Med. 2021. https://doi.org/10.1097/CCM.0000000000004945.
Sekhon MS, Ainslie PN, Griesdale DE. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: a “two-hit” model. Crit Care. 2017;21(1):90. https://doi.org/10.1186/s13054-017-1670-9.
Giannì G, Minini A, Fratino S, et al. The impact of short-term hyperoxia on cerebral metabolism: a systematic review and meta-analysis. Neurocrit Care. 2022;37(2):547–57.
Jentzer JC, Chonde MD, Dezfulian C. Myocardial dysfunction and shock after cardiac arrest. Biomed Res Int. 2015;2015:1–14. https://doi.org/10.1155/2015/314796.
Sandroni C, Cronberg T, Sekhon M. Brain injury after cardiac arrest: pathophysiology, treatment, and prognosis. Intensive Care Med. 2021;47(12):1393–414. https://doi.org/10.1007/s00134-021-06548-2.
Sandroni C, D’Arrigo S, Cacciola S, et al. Prediction of poor neurological outcome in comatose survivors of cardiac arrest: a systematic review. Intensive Care Med. 2020;46(10):1803–51. https://doi.org/10.1007/s00134-020-06198-w.
Battaglini D, Pelosi P, Robba C. The importance of neuromonitoring in non brain injured patients. Crit Care. 2022;26(1):78. https://doi.org/10.1186/s13054-022-03914-4.
Nortje J, Gupta AK. The role of tissue oxygen monitoring in patients with acute brain injury. Br J Anaesth. 2006;97(1):95–106. https://doi.org/10.1093/bja/ael137.
Lang EW, Mulvey JM, Mudaliar Y, Dorsch NWC. Direct cerebral oxygenation monitoring—a systematic review of recent publications. Neurosurg Rev. 2007;30(2):99–107. https://doi.org/10.1007/s10143-006-0062-4.
Robba C, Taccone FS, Citerio G. Monitoring cerebral oxygenation in acute brain-injured patients. Intensive Care Med. 2022;48(10):1463–6.
Soar J, Nolan JP, Böttiger BW, et al. European resuscitation council guidelines for resuscitation 2015. Resuscitation. 2015;95:100–47. https://doi.org/10.1016/j.resuscitation.2015.07.016.
Johnson NJ, Carlbom DJ, Gaieski DF. Ventilator management and respiratory care after cardiac arrest. Chest. 2018;153(6):1466–77. https://doi.org/10.1016/j.chest.2017.11.012.
Kaur C, Ling E. Blood brain barrier in hypoxic-ischemic conditions. Curr Neurovasc Res. 2008;5(1):71–81. https://doi.org/10.2174/156720208783565645.
Ek CJ, D’Angelo B, Baburamani AA, et al. Brain barrier properties and cerebral blood flow in neonatal mice exposed to cerebral hypoxia-ischemia. J Cereb Blood Flow Metab. 2015;35(5):818–27. https://doi.org/10.1038/jcbfm.2014.255.
Kilgannon JH. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165. https://doi.org/10.1001/jama.2010.707.
Bellomo R, Bailey M, Eastwood GM, et al. Arterial hyperoxia and in-hospital mortality after resuscitation from cardiac arrest. Crit Care. 2011;15(2):R90. https://doi.org/10.1186/cc10090.
Wang HE, Prince DK, Drennan IR, et al. Post-resuscitation arterial oxygen and carbon dioxide and outcomes after out-of-hospital cardiac arrest. Resuscitation. 2017;120:113–8. https://doi.org/10.1016/j.resuscitation.2017.08.244.
Palmer E, Post B, Klapaukh R, et al. The association between supraphysiologic arterial oxygen levels and mortality in critically ill patients. A multicenter observational cohort study. Am J Respir Crit Care Med. 2019;200(11):1373–80. https://doi.org/10.1164/rccm.201904-0849OC.
Ni YN, Wang YM, Liang BM, Liang ZA. The effect of hyperoxia on mortality in critically ill patients: a systematic review and meta analysis. BMC Pulm Med. 2019;19(1):53. https://doi.org/10.1186/s12890-019-0810-1.
La Via L, Astuto M, Bignami EG, et al. The effects of exposure to severe hyperoxemia on neurological outcome and mortality after cardiac arrest. Minerva Anestesiol. 2022;88(10):853–63.
Nolan JP, Sandroni C, Böttiger BW, et al. European resuscitation council and European society of intensive care medicine guidelines 2021: post-resuscitation care. Intensive Care Med. 2021;47(4):369–421. https://doi.org/10.1007/s00134-021-06368-4.
Merchant RM, Topjian AA, Panchal AR, et al. Part 1: Executive summary: 2020 American heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020;142(162):S337–57. https://doi.org/10.1161/CIR.0000000000000918.
Hare GMT, Kavanagh BP, Mazer CD, et al. Hypercapnia increases cerebral tissue oxygen tension in anesthetized rats. Can J Anesth. 2003;50(10):1061–8. https://doi.org/10.1007/BF03018375.
Eastwood G, Nichol A, Bellomo R, Arabi Y. TAME cardiac arrest: a phase III multicenter randomized trial of targeted therapeutic mild hypercapnia after resuscitated cardiac arrest. Saudi Critical Care Journal. 2017;1(6):10. https://doi.org/10.4103/sccj.sccj_23_17.
Perkins GD, Ji C, Deakin CD, et al. A randomized trial of epinephrine in out-of-hospital cardiac arrest. N Engl J Med. 2018;379(8):711–21. https://doi.org/10.1056/NEJMoa1806842.
Lin S, Callaway CW, Shah PS, et al. Adrenaline for out-of-hospital cardiac arrest resuscitation: a systematic review and meta-analysis of randomized controlled trials. Resuscitation. 2014;85(6):732–40. https://doi.org/10.1016/j.resuscitation.2014.03.008.
Ditchey RV, Lindenfeld J. Failure of epinephrine to improve the balance between myocardial oxygen supply and demand during closed-chest resuscitation in dogs. Circulation. 1988;78(2):382–9. https://doi.org/10.1161/01.CIR.78.2.382.
Tang W, Weil MH, Gazmuri RJ, Sun S, Duggal C, Bisera J. Pulmonary ventilation/perfusion defects induced by epinephrine during cardiopulmonary resuscitation. Circulation. 1991;84(5):2101–7. https://doi.org/10.1161/01.CIR.84.5.2101.
Tang W, Weil MH, Sun S, Noc M, Yang L, Gazmuri RJ. Epinephrine increases the severity of postresuscitation myocardial dysfunction. Circulation. 1995;92(10):3089–93. https://doi.org/10.1161/01.CIR.92.10.3089.
Ilicki J, Bruchfeld S, Djärv T. Can epinephrine therapy be detrimental to patients with hypertrophic cardiomyopathy with hypotension or cardiac arrest? A systematic review. Eur J Emerg Med. 2019;26(3):150–7. https://doi.org/10.1097/MEJ.0000000000000573.
Ristagno G, Sun S, Tang W, Castillo C, Weil MH. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardiopulmonary resuscitation*. Crit Care Med. 2007;35(9):2145–9. https://doi.org/10.1097/01.CCM.0000280427.76175.D2.
Hardebo JE, Owman C. Barrier mechanisms for neurotransmitter monoamines and their precursors at the blood-brain interface. Ann Neurol. 1980;8(1):1–11. https://doi.org/10.1002/ana.410080102.
Lott C, Truhlář A, Alfonzo A, et al. European Resuscitation Council Guidelines 2021: Cardiac arrest in special circumstances. Resuscitation. 2021;161:152–219. https://doi.org/10.1016/j.resuscitation.2021.02.011.
Faraci FM. Effects of endothelin and vasopressin on cerebral blood vessels. Am J Physiol-Heart Circulat Physiol. 1989;257(3):H799–803. https://doi.org/10.1152/ajpheart.1989.257.3.H799.
Oyama H, Suzuki Y, Satoh SI, et al. Role of nitric oxide in the cerebral vasodilatory responses to vasopressin and oxytocin in dogs. J Cereb Blood Flow Metab. 1993;13(2):285–90. https://doi.org/10.1038/jcbfm.1993.35.
Katircioglu SF, Seren M, Parlar AI, et al. Levosimendan effect on spinal cord ischemia-reperfusion injury following aortic clamping. J Card Surg. 2008;23(1):44–8. https://doi.org/10.1111/j.1540-8191.2007.00486.x.
Lafci B, Yasa H, Ilhan G, et al. Protection of the spinal cord from ischemia: comparative effects of levosimendan and iloprost. Eur Surg Res. 2008;41(1):1–7. https://doi.org/10.1159/000121394.
Feldman Z, Kanter MJ, Robertson CS, et al. Effect of head elevation on intracranial pressure, cerebral perfusion pressure, and cerebral blood flow in head-injured patients. J Neurosurg. 1992;76(2):207–11. https://doi.org/10.3171/jns.1992.76.2.0207.
Meixensberger J, Baunach S, Amschler J, Dings J, Roosen K. Influence of body position on tissue- p O 2, cerebral perfusion pressure and intracranial pressure in patients with acute brain injury. Neurol Res. 1997;19(3):249–53. https://doi.org/10.1080/01616412.1997.11740808.
Ng I, Lim J, Wong HB. Effects of head posture on cerebral hemodynamics: its influences on intracranial pressure, cerebral perfusion pressure, and cerebral oxygenation. Neurosurgery. 2004;54(3):593–8. https://doi.org/10.1227/01.NEU.0000108639.16783.39.
Schwarz S, Georgiadis D, Aschoff A, Schwab S. Effects of body position on intracranial pressure and cerebral perfusion in patients with large hemispheric stroke. Stroke. 2002;33(2):497–501. https://doi.org/10.1161/hs0202.102376.
Burnol L, Payen JF, Francony G, et al. Impact of head-of-bed posture on brain oxygenation in patients with acute brain injury: a prospective cohort study. Neurocrit Care. 2021;35(3):662–8. https://doi.org/10.1007/s12028-021-01240-1.
Stiefel MF, Spiotta A, Gracias VH, et al. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J Neurosurg. 2005;103(5):805–11. https://doi.org/10.3171/jns.2005.103.5.0805.
Spiotta AM, Stiefel MF, Gracias VH, et al. Brain tissue oxygen–directed management and outcome in patients with severe traumatic brain injury. J Neurosurg. 2010;113(3):571–80. https://doi.org/10.3171/2010.1.JNS09506.
van den Brink WA, van Santbrink H, Steyerberg EW, et al. Brain oxygen tension in severe head injury. Neurosurgery. 2000;46(4):868–78. https://doi.org/10.1097/00006123-200004000-00018.
Elmer J, Scutella M, Pullalarevu R, et al. The association between hyperoxia and patient outcomes after cardiac arrest: analysis of a high-resolution database. Intensive Care Med. 2015;41(1):49–57. https://doi.org/10.1007/s00134-014-3555-6.
Gouvea Bogossian E, Battaglini D, Fratino S, et al. The role of brain tissue oxygenation monitoring in the management of subarachnoid hemorrhage: a scoping review. Neurocrit Care. 2023. https://doi.org/10.1007/s12028-023-01680-x.
Foreman B, Ngwenya LB, Stoddard E, Hinzman JM, Andaluz N, Hartings JA. Safety and reliability of bedside, single burr hole technique for intracranial multimodality monitoring in severe traumatic brain injury. Neurocrit Care. 2018;29(3):469–80. https://doi.org/10.1007/s12028-018-0551-7.
Anania P, Battaglini D, Miller JP, et al. Escalation therapy in severe traumatic brain injury: how long is intracranial pressure monitoring necessary? Neurosurg Rev. 2021;44(5):2415–23. https://doi.org/10.1007/s10143-020-01438-5.
Tas J, Czosnyka M, van der Horst ICC, et al. Cerebral multimodality monitoring in adult neurocritical care patients with acute brain injury: a narrative review. Front Physiol. 2022;13:1071161. https://doi.org/10.3389/fphys.2022.1071161.
Francoeur C, Landis WP, Winters M, et al. Near-infrared spectroscopy during cardiopulmonary resuscitation for pediatric cardiac arrest: A prospective, observational study. Resuscitation. 2022;174:35–41. https://doi.org/10.1016/j.resuscitation.2022.03.014.
Author information
Authors and Affiliations
Contributions
All authors: conception and design, analysis and interpretation of literature, drafting the article and revising it critically for important intellectual content, final approval of the version to be published, agreement on accuracy and integrity of any part of the work.
Corresponding author
Ethics declarations
Conflicts of Interest
Chiara Robba is Associate Editor of the journal Neurocritical Care.
Ethical approval/informed consent
The authors confirm that for this work, ethical guidelines, ethical approvals (institutional review board), and use of informed consent were not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Battaglini, D., Bogossian, E.G., Anania, P. et al. Monitoring of Brain Tissue Oxygen Tension in Cardiac Arrest: a Translational Systematic Review from Experimental to Clinical Evidence. Neurocrit Care 40, 349–363 (2024). https://doi.org/10.1007/s12028-023-01721-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-023-01721-5